Accuracy of Preoperative Endoscopy in Determining Tumor Location Required for Surgical Planning for Esophagogastric Junction Cancer
Abstract
:1. Introduction
2. Materials and Methods
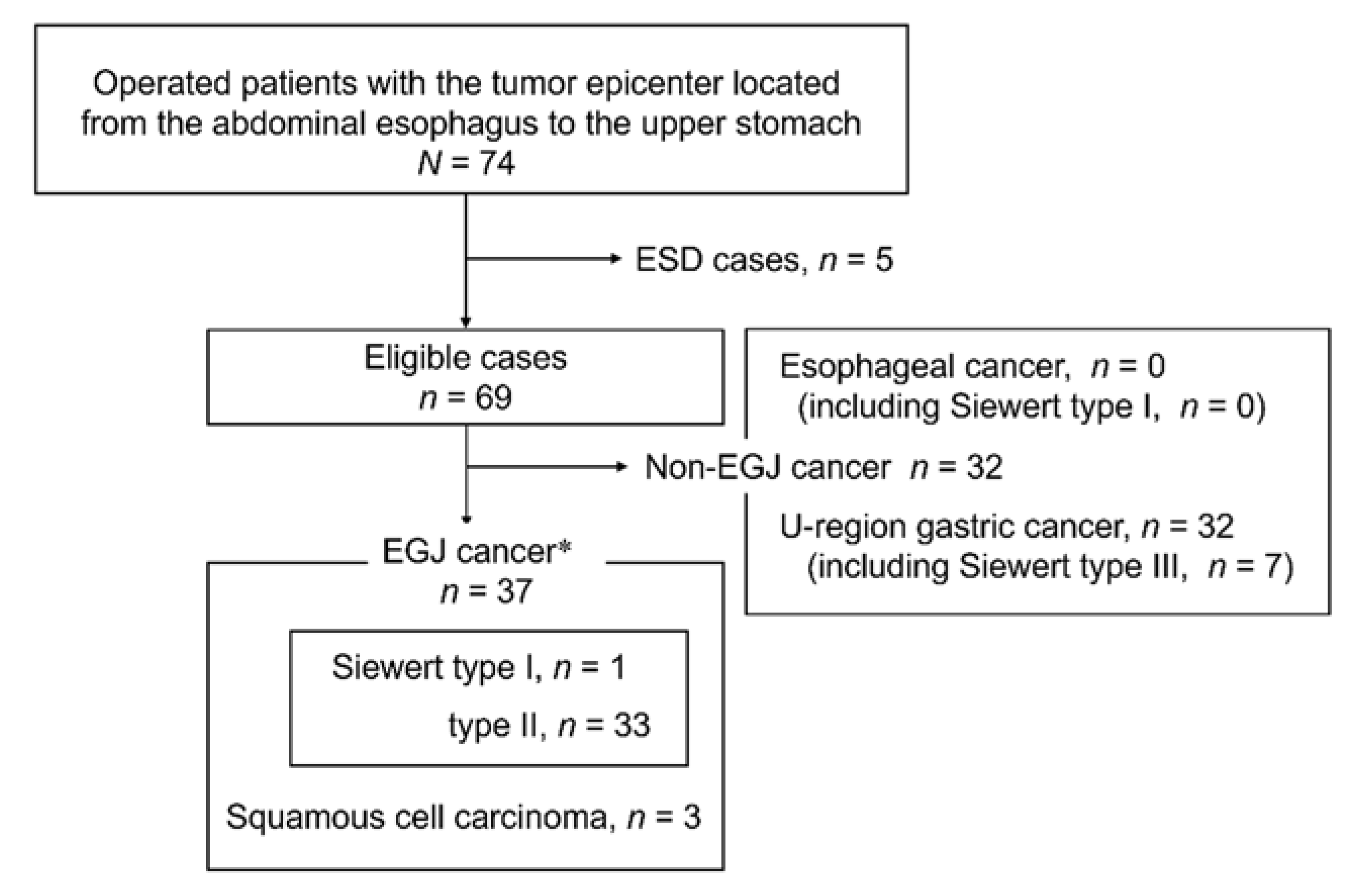
2.1. Patients
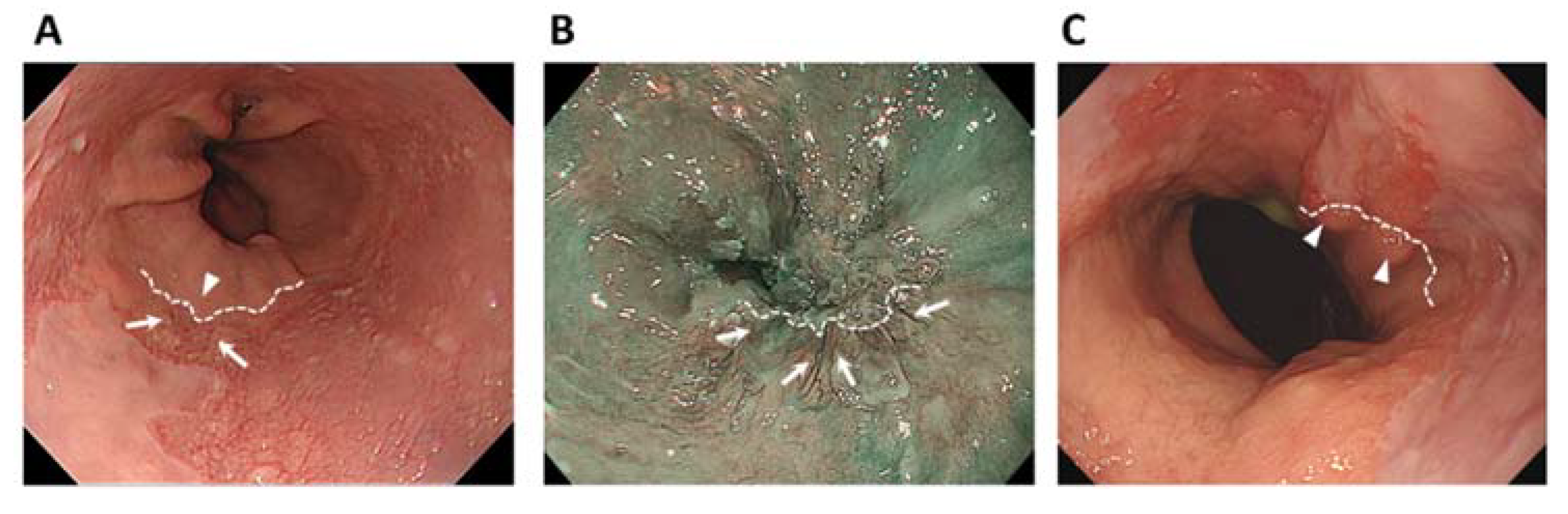
2.2. Endoscopic Examination
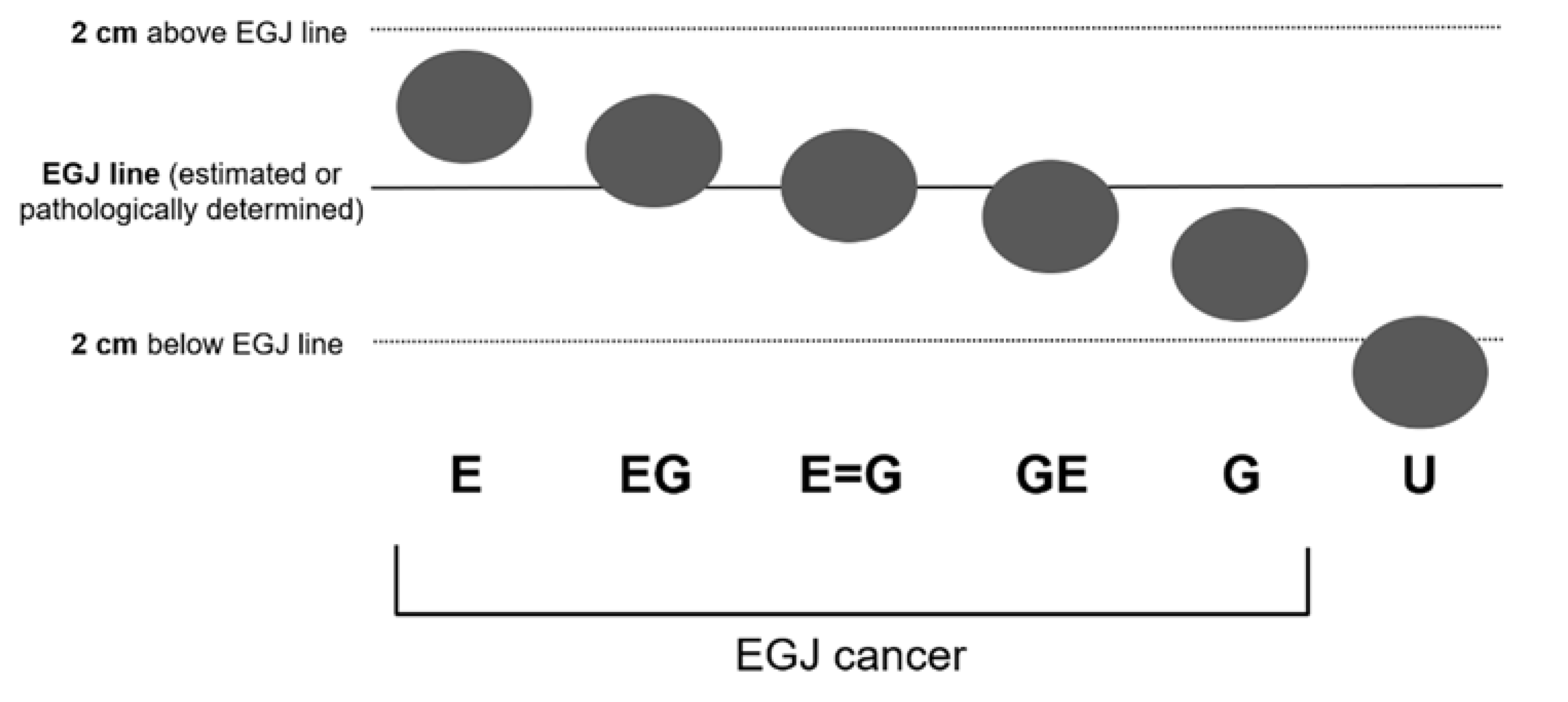
2.3. Image Assessment
2.4. Evaluation of Accuracy
2.5. Statistics
2.6. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagergren, J.; Lagergren, P. Recent developments in esophageal adenocarcinoma. CA Cancer J. Clin. 2013, 63, 232–248. [Google Scholar] [CrossRef]
- Bollschweiler, E.; Wolfgarten, E.; Gutschow, C.; Hölscher, A.H. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001, 92, 549–555. [Google Scholar] [CrossRef]
- Wu, H.; Rusiecki, J.A.; Zhu, K.; Potter, K.; Devesa, S.S. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Buas, M.F.; Vaughan, T.L. Epidemiology and risk factors for gastroesophageal junction tumors: Understanding the rising incidence of this disease. Semin. Radiat. Oncol. 2013, 23, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Makuuchi, H.; Ozawa, S.; Shimada, H.; Chino, O. The present status and future of Barrett’s esophageal adenocarcinoma in Japan. Digestion 2019, 99, 185–190. [Google Scholar] [CrossRef]
- Kusano, C.; Gotoda, T.; Khor, C.J.; Katai, H.; Kato, H.; Taniguchi, H.; Shimoda, T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J. Gastroenterol. Hepatol 2008, 23, 1662–1665. [Google Scholar] [CrossRef]
- Chow, W.H.; Blaser, M.J.; Blot, W.J.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Perez-Perez, G.I.; Schoenberg, J.B.; Stanford, J.L.; Rotterdam, H.; et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998, 58, 588–590. [Google Scholar] [PubMed]
- Lagergren, J.; Bergström, R.; Lindgren, A.; Nyrén, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999, 340, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric cancer, 3rd English ed. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: Part I. Esophagus 2015, 14, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Siewert, J.R.; Stein, H.J. Carcinoma of the gastroesophageal junction-classification, pathology and extent of resection. Dis. Esophagus 1996, 9, 173–182. [Google Scholar] [CrossRef]
- Siewert, J.R.; Stein, H.J.; Sendler, A.; Fink, U. Surgical resection for cancer of the cardia. Semin. Surg. Oncol. 1999, 17, 125–131. [Google Scholar] [CrossRef]
- Stein, H.J.; Feith, M.; Siewert, J.R. Cancer of the esophagogastric junction. Surg. Oncol. 2000, 9, 35–41. [Google Scholar] [CrossRef]
- Kumamoto, T.; Kurahashi, Y.; Niwa, H.; Nakanishi, Y.; Okumura, K.; Ozawa, R.; Ishida, Y.; Shinohara, H. True esophagogastric junction adenocarcinoma: Background of its definition and current surgical trends. Surg. Today 2020, 50, 809–814. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Kato, Y.; Daiko, H.; Nishimura, M.; Katsumata, K.; Sugiyama, Y.; et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: Experiences at a single institution in Japan. Ann. Surg. Oncol. 2012, 19, 677–683. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Hiki, N.; Yoshikawa, T.; Kishi, K.; Ito, Y.; Ohi, M.; Wada, N.; Takiguchi, S.; Mine, S.; Hasegawa, S.; et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery 2015, 157, 551–555. [Google Scholar] [CrossRef]
- Koyanagi, K.; Kato, F.; Kanamori, J.; Daiko, H.; Ozawa, S.; Tachimori, Y. Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: A retrospective single-institution study. Ann. Gastroenterol. Surg. 2018, 2, 187–196. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Takeuchi, H.; Doki, Y.; Mine, S.; Terashima, M.; Yasuda, T.; Yoshida, K.; Daiko, H.; Sakuramoto, S.; Yoshikawa, T.; et al. Mapping of lymph node metastasis from esophagogastric junction tumors: A prospective nationwide multicenter study. Ann. Surg. 2021, 274, 120–127. [Google Scholar] [CrossRef]
- Shiraishi, O.; Yasuda, T.; Kato, H.; Iwama, M.; Hiraki, Y.; Yasuda, A.; Shinkai, M.; Kimura, Y.; Imano, M. Risk factors and prognostic impact of mediastinal lymph node metastases in patients with esophagogastric junction cancer. Ann. Surg. Oncol. 2020, 27, 4433–4440. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, B.A.; Wijnhoven, B.P.; Poley, J.W.; Hermans, J.J.; Biermann, K.; Spaander, M.C.W.; Bruno, M.J.; Tilanus, H.W.; van Lanschot, J.J.B. Preoperative assessment of tumor location and station-specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World J. Surg. 2013, 37, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, C.; Bernini, M.; Giacopuzzi, S.; Pugliese, R.; Catalano, F.; Festini, M.; Rodella, L.; de Manzoni, G. Evaluation of Siewert classification in gastro-esophageal junction adenocarcinoma: What is the role of endoscopic ultrasonography? J. Surg. Oncol. 2005, 91, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011, 140, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Kato, Y.; Matsuura, M.; Gagner, M. Significance of palisading longitudinal esophagus vessels: Identification of the true esophagogastric junction has histopathological and oncological considerations. Dig. Dis. Sci. 2010, 55, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.; Inoue, H.; Ikeda, H.; Onimaru, M.; Yoshida, A.; Santi, E.G.; Sato, H.; Hayee, B.; Kudo, S.-E. Microvasculature of the esophagus and gastroesophageal junction: Lesson learned from submucosal endoscopy. World J. Gastrointest Endosc. 2016, 8, 690–696. [Google Scholar] [CrossRef]
- van Soest, E.M.; Dieleman, J.P.; Siersema, P.D.; Sturkenboomm, M.C.J.M.; Kuipersm, E.J. Increasing incidence of Barrett’s oesophagus in the general population. Gut 2005, 54, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Dent, J.; Armstrong, D.; Bergman, J.J.G.H.M.; Gossner, L.; Hoshihara, Y.; Jankowski, J.A.; Junghard, O.; Lundell, L.; Tytgat, G.N.J.; et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology 2006, 131, 1392–1399. [Google Scholar] [CrossRef]
- Kumagai, Y.; Yagi, M.; Aida, J.; Ishida, H.; Suzuki, S.; Hashimoto, T.; Amanuma, Y.; Kusano, M.; Mukai, S.; Yamazaki, S.; et al. Detailed features of palisade vessels as a marker of the esophageal mucosa revealed by magnifying endoscopy with narrow band imaging. Dis. Esophagus 2012, 25, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Schölvinck, D.W.; Goto, O.; Seldenrijk, C.A.; Bisschops, R.; Horii, J.; Ochiai, Y.; Schoon, E.J.; Schenk, B.E.; Uraoka, T.; van Oijen, M.G.H.; et al. Detection of palisade vessels as a landmark for Barrett’s esophagus in a Western population. J. Gastroenterol. 2016, 51, 682–690. [Google Scholar] [CrossRef]
- Naini, B.V.; Souza, R.F.; Odze, R.D. Barrett’s esophagus: A comprehensive and contemporary review for pathologists. Am. J. Surg. Pathol. 2016, 40, 45–66. [Google Scholar] [CrossRef]
- Andrés, A.M.; Marzo, P.F. Chance-corrected measures of reliability and validity in K x K tables. Stat. Methods Med. Res. 2005, 14, 473–492. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Endo, T.; Nosho, K.; Arimura, Y.; Sato, M.; Imai, K. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J. Gastroenterol. 2004, 39, 14–20. [Google Scholar] [CrossRef]
- Goda, K.; Tajiri, H.; Ikegami, M.; Urashima, M.; Nakayoshi, T.; Kaise, M. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma. Gastrointest Endosc. 2007, 65, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kallo, A.; Mizumami, K.; Murakami, K.; Sawa, A. Endoscopy and Barrett’s esophagus: Current perspectives in the US and Japan. Intern. Med. 2021, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
| Pathological Category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| E 1 | EG 1 | E = G 1 | GE 1 | G 1 | U | Concordance | |||
| Total | |||||||||
| Preoperative category | E 1 | 02 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| EG 1 | 0 | 5 2 | 0 | 0 | 0 | 0 | 5 | 1.00 | |
| E = G 1 | 0 | 0 | 9 2 | 1 3 | 1 3 | 0 | 11 | 0.82 | |
| GE 1 | 0 | 1 4 | 1 4 | 12 2 | 0 | 1 | 15 | 0.80 | |
| G1 | 0 | 0 | 0 | 1 4 | 3 2 | 0 | 4 | 0.75 | |
| U | 0 | 0 | 0 | 1 4 | 2 4 | 31 2 | 34 | 0.91 | |
| Total | 0 | 6 | 10 | 15 | 6 | 32 | 69 | ||
| n (%) | |
|---|---|
| Age (years) 1 | 67 ± 13 |
| Sex (M/F) | 27/10 |
| BMI | 21.8 ± 3.0 |
| Tumor size (mm, major axis) 1 | 40 ± 19 |
| Barrett’s esophagus | 15 (41) |
| Esophageal hiatus hernia | 16 (43) |
| Histology | |
| Squamous cell carcinoma | 3 (8) |
| Adenocarcinoma | 33 (89) |
| pap, tub1 | 8 (22) |
| tub2 | 17 (46) |
| por, muc | 8 (22) |
| cT | |
| 1b | 11 (30) |
| 2 | 6 (16) |
| 3 | 14 (38) |
| 4a | 6 (16) |
| cN | |
| 0 | 18 (49) |
| 1 | 7 (19) |
| 2 | 8 (22) |
| 3 | 4 (11) |
| Operative procedure | |
| Total Gastrectomy | 19 (51) |
| Proximal Gastrectomy | 10 (27) |
| Esophagectomy | 8 (22) |
| Operative time (min) 2 | 450 (202–751) |
| Blood loss (mL) 2 | 80 (0–850) |
| Factor | RR | (95%CI) | p-Value |
|---|---|---|---|
| Sex M vs. F | 2.59 | (0.363–18.5) | 0.404 |
| Age ≥75 vs. <75 | 3.47 | (0.990–12.2) | 0.080 |
| Barrett’s esophagus | 4.40 | (1.022–18.9) | 0.042 |
| Esophageal hiatus hernia | 2.19 | (0.611–7.83) | 0.254 |
| Major axis of tumor ≥4 vs. <4 cm | 1.60 | (0.423–6.06) | 0.674 |
| Pathological Category | ||||||||
|---|---|---|---|---|---|---|---|---|
| E 1 | EG 1 | E 1 = G | GE 1 | G 1 | Predictive Value | |||
| Total | ||||||||
| With Barrett’s Esophagus | ||||||||
| Preoperative category | E 1 | 02 | 0 | 0 | 0 | 0 | 0 | NA |
| EG 1 | 0 | 42 | 0 | 0 | 0 | 4 | 1.00 | |
| E = G 1 | 0 | 0 | 2 2 | 0 | 13 | 3 | 0.67 | |
| GE 1 | 0 | 14 | 1 4 | 12 | 0 | 3 | 0.33 | |
| G1 | 0 | 0 | 0 | 0 4 | 2 2 | 2 | 1.00 | |
| U | 0 | 0 | 0 | 1 4 | 2 4 | 3 | 0.00 | |
| Total | 0 | 5 | 3 | 2 | 5 | 15 | ||
| Without Barrett’s Esophagus | ||||||||
| Preoperative category | E 1 | 0 2 | 0 | 0 | 0 | 0 | 0 | NA |
| EG 1 | 0 | 1 2 | 0 | 0 | 0 | 1 | 1.00 | |
| E = G 1 | 0 | 0 | 7 2 | 1 3 | 0 | 8 | 0.88 | |
| GE 1 | 0 | 0 | 0 | 11 2 | 0 | 11 | 1.00 | |
| G 1 | 0 | 0 | 0 | 1 4 | 1 2 | 2 | 0.50 | |
| U | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| Total | 0 | 1 | 7 | 13 | 1 | 22 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumura, K.; Hojo, Y.; Tomita, T.; Kumamoto, T.; Nakamura, T.; Kurahashi, Y.; Ishida, Y.; Hirota, S.; Miwa, H.; Shinohara, H. Accuracy of Preoperative Endoscopy in Determining Tumor Location Required for Surgical Planning for Esophagogastric Junction Cancer. J. Clin. Med. 2021, 10, 3371. https://doi.org/10.3390/jcm10153371
Okumura K, Hojo Y, Tomita T, Kumamoto T, Nakamura T, Kurahashi Y, Ishida Y, Hirota S, Miwa H, Shinohara H. Accuracy of Preoperative Endoscopy in Determining Tumor Location Required for Surgical Planning for Esophagogastric Junction Cancer. Journal of Clinical Medicine. 2021; 10(15):3371. https://doi.org/10.3390/jcm10153371
Chicago/Turabian StyleOkumura, Koichi, Yudai Hojo, Toshihiko Tomita, Tsutomu Kumamoto, Tatsuro Nakamura, Yasunori Kurahashi, Yoshinori Ishida, Seiichi Hirota, Hiroto Miwa, and Hisashi Shinohara. 2021. "Accuracy of Preoperative Endoscopy in Determining Tumor Location Required for Surgical Planning for Esophagogastric Junction Cancer" Journal of Clinical Medicine 10, no. 15: 3371. https://doi.org/10.3390/jcm10153371
APA StyleOkumura, K., Hojo, Y., Tomita, T., Kumamoto, T., Nakamura, T., Kurahashi, Y., Ishida, Y., Hirota, S., Miwa, H., & Shinohara, H. (2021). Accuracy of Preoperative Endoscopy in Determining Tumor Location Required for Surgical Planning for Esophagogastric Junction Cancer. Journal of Clinical Medicine, 10(15), 3371. https://doi.org/10.3390/jcm10153371








