Lesion Geometry as Assessed by Optical Coherence Tomography Is Related to Myocardial Ischemia as Determined by Cardiac Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. OCT Image Acquisition and Analysis
2.3. CMR Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
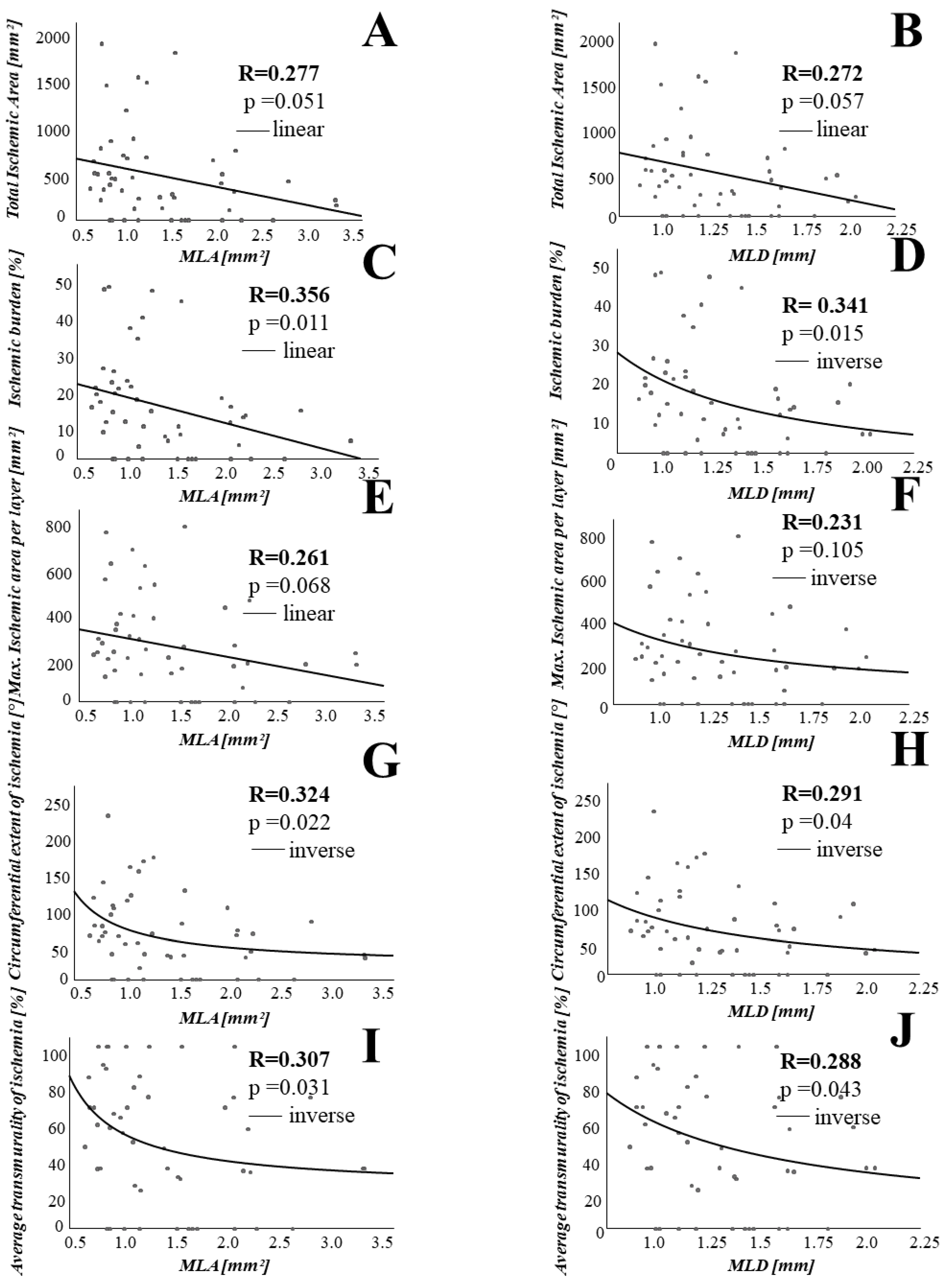
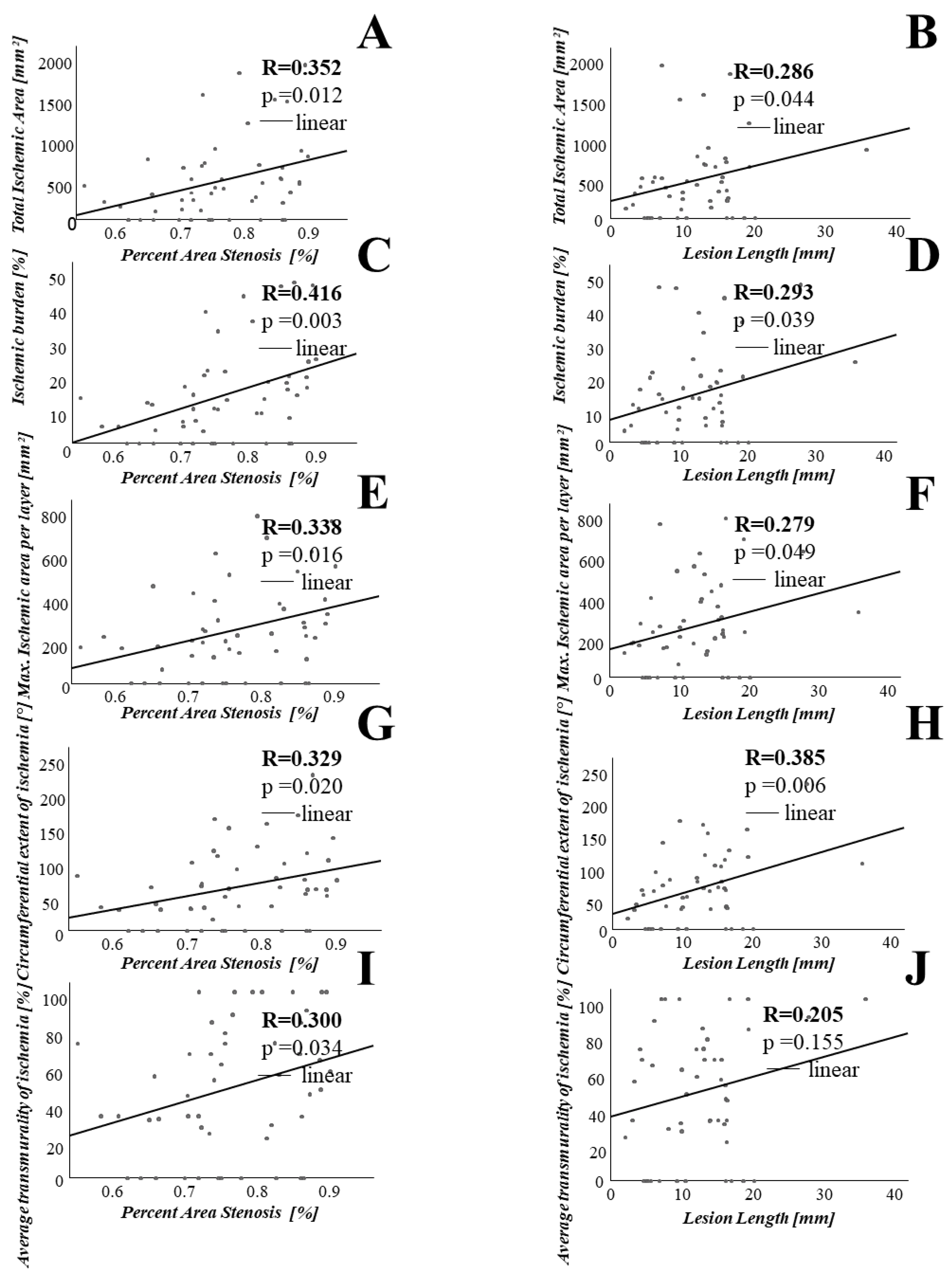
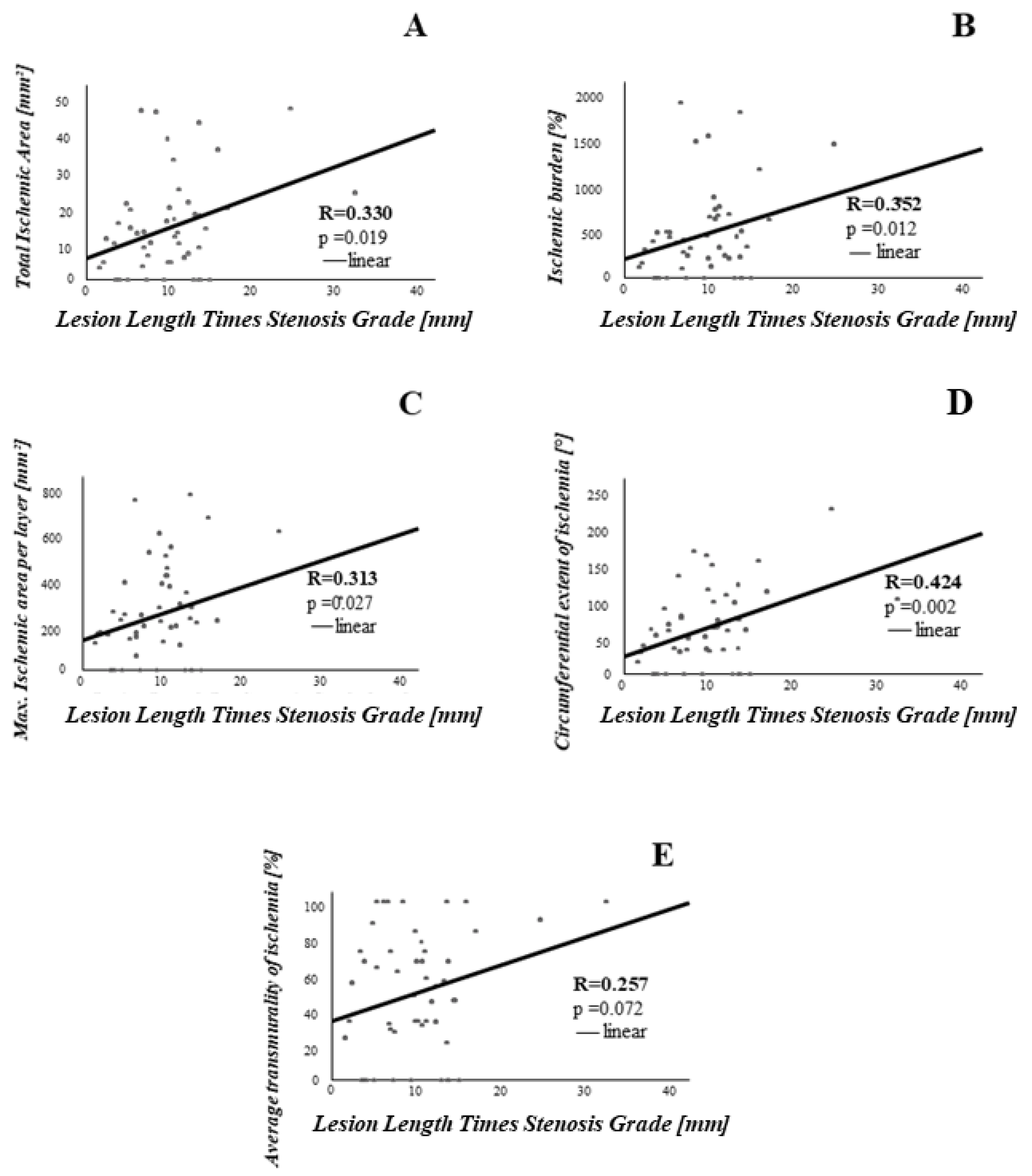
3.2. Association between Coronary Lesion Characteristics and Ischemia
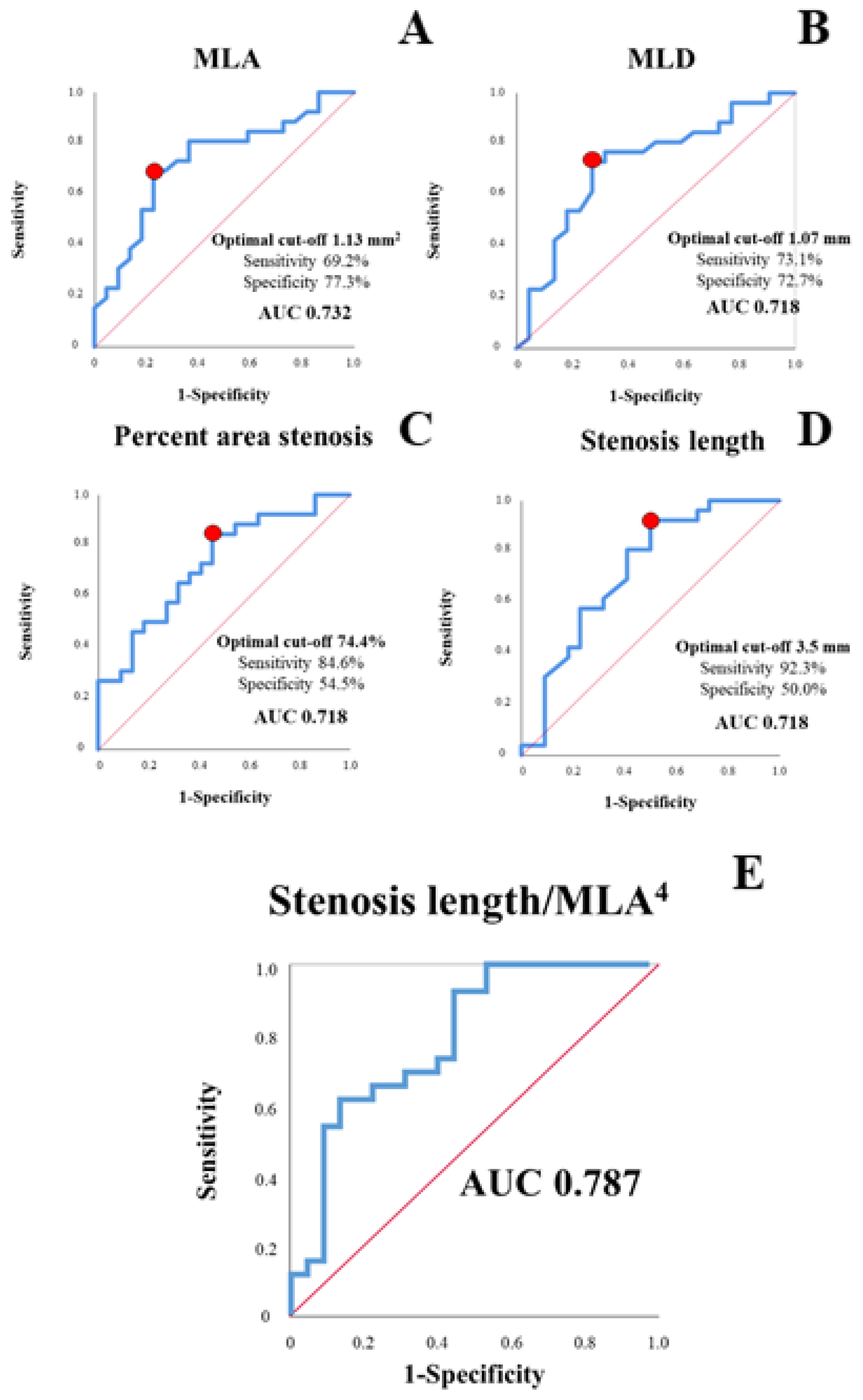
3.3. Lesion-Derived Predictors of Clinically Relevant Ischemia
4. Discussion
- Intraluminal stenosis parameters assessing lesion severity are associated with the extent of myocardial ischemia as determined by CMR.
- Plaque composition is not significantly related to extent of ischemia as determined by CMR.
- OCT-derived stenosis parameters, alone and in combination, predicted ischemic area >10% of the myocardium with good diagnostic efficiency.
4.1. Stenosis Geometry as Determined by OCT Is Associated with CMR Derived Ischemia
4.2. OCT-Only Based Prediction of Clinically Relevant Ischemia
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Omerovic, E.; Fournier, S.; Kelbæk, H.; Johnson, N.P.; Rothenbühler, M.; Xaplanteris, P.; Abdel-Wahab, M.; Barbato, E.; Høfsten, D.E.; et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: Meta-analysis of individual patient data. Eur. Heart J. 2019, 40, 180–186. [Google Scholar] [CrossRef]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.; Fearon, W.F.; Barbato, E.; Tonino, P.A.; Engstrøm, T.; Kääb, S.; Dambrink, J.-H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Adamson, P.D.; Newby, D.E. Non-invasive imaging of the coronary arteries. Eur. Heart J. 2019, 40, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.-Y.; Mikolich, J.R.; et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients with Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef]
- Steel, K.; Broderick, R.; Gandla, V.; Larose, E.; Resnic, F.; Jerosch-Herold, M.; Brown, K.A.; Kwong, R.Y. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation 2009, 120, 1390–1400. [Google Scholar] [CrossRef] [Green Version]
- Nagel, E.; Berry, C. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. Reply. N. Engl. J. Med. 2019, 381, 2277–2278. [Google Scholar] [PubMed] [Green Version]
- Shaw, L.J.; Berman, D.S.; Picard, M.; Friedrich, M.G.; Kwong, R.Y.; Stone, G.W.; Senior, R.; Min, J.K.; Hachamovitch, R.; Scherrer-Crosbie, M.; et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc. Imaging 2014, 7, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo, N.; Escaned, J.; Alfonso, F.; Nolte, C.; Rodriguez, V.; Jimenez-Quevedo, P.; Bañuelos, C.; Fernández-Ortiz, A.; Garcia, E.; Hernandez-Antolin, R.; et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: A comparison with fractional flow reserve and intravascular ultrasound. J. Am. Coll. Cardiol. 2012, 59, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Kanno, Y.; Sugiyama, T.; Hoshino, M.; Usui, E.; Hamaya, R.; Kanaji, Y.; Murai, T.; Lee, T.; Yonetsu, T.; Kakuta, T. Optical coherence tomography-defined plaque vulnerability in relation to functional stenosis severity stratified by fractional flow reserve and quantitative flow ratio. Catheter. Cardiovasc. Interv. 2020, 96, E238–E247. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, T.; Prati, F.; Kulawik, T.; Ficarra, E.; Bil, J.; Gil, R. Optical coherence tomography criteria for defining functional severity of intermediate lesions: A comparative study with FFR. Int. J. Cardiovasc. Imaging 2013, 29, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Pyxaras, S.A.; Tu, S.; Barbato, E.; Barbati, G.; Di Serafino, L.; De Vroey, F.; Toth, G.; Mangiacapra, F.; Sinagra, G.; De Bruyne, B.; et al. Quantitative angiography and optical coherence tomography for the functional assessment of nonobstructive coronary stenoses: Comparison with fractional flow reserve. Am. Heart J. 2013, 166, 1010–1018.e1. [Google Scholar] [CrossRef] [PubMed]
- Reith, S.B.; Battermann, S.; Jaskolka, A.; Lehmacher, W.; Hoffmann, R.; Marx, N.; Burgmaier, M. Relationship between optical coherence tomography derived intraluminal and intramural criteria and haemodynamic relevance as determined by fractional flow reserve in intermediate coronary stenoses of patients with type 2 diabetes. Heart 2013, 99, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Kitabata, H.; Kubo, T.; Masuno, T.; Ohta, S.; Ozaki, Y.; Sougawa, H.; Orii, M.; Shimamura, K.; Ishibashi, K.; et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ. J. 2012, 76, 2218–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reith, S.; Battermann, S.; Hellmich, M.; Marx, N.; Burgmaier, M. Correlation between optical coherence tomography-derived intraluminal parameters and fractional flow reserve measurements in intermediate grade coronary lesions: A comparison between diabetic and non-diabetic patients. Clin. Res. Cardiol. 2015, 104, 59–70. [Google Scholar] [CrossRef]
- Reith, S.; Battermann, S.; Hellmich, M.; Marx, N.; Burgmaier, M. Correlation between OCT-derived intrastent dimensions and fractional flow reserve measurements after coronary stent implantation and impact on clinical outcome. J. Invasive Cardiol. 2015, 27, 222–228. [Google Scholar] [PubMed]
- Reith, S.; Battermann, S.; Hellmich, M.; Marx, N.; Burgmaier, M. Impact of type 2 diabetes mellitus and glucose control on fractional flow reserve measurements in intermediate grade coronary lesions. Clin. Res. Cardiol. 2014, 103, 191–201. [Google Scholar] [CrossRef]
- Dettori, R.; Milzi, A.; Burgmaier, K.; Almalla, M.; Hellmich, M.; Marx, N.; Reith, S.; Burgmaier, M. Prognostic irrelevance of plaque vulnerability following plaque sealing in high-risk patients with type 2 diabetes: An optical coherence tomography study. Cardiovasc. Diabetol. 2020, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Battermann, S.; Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Burgmaier, M.; Reith, S. High cardiovascular risk of patients with type 2 diabetes is only partially attributed to angiographic burden of atherosclerosis. Diab. Vasc. Dis. Res. 2020, 17, 1479164120953612. [Google Scholar] [CrossRef]
- Burgmaier, M.; Milzi, A.; Dettori, R.; Burgmaier, K.; Hellmich, M.; Almalla, M.; Marx, N.; Reith, S. Colocalization of plaque macrophages and calcification in coronary plaques as detected by optical coherence tomography predicts cardiovascular outcome. Cardiol. J. 2020, 27, 303–306. [Google Scholar] [CrossRef]
- Reith, S.; Milzi, A.; Lemma, E.D.; Dettori, R.; Burgmaier, K.; Marx, N.; Burgmaier, M. Intrinsic calcification angle: A novel feature of the vulnerable coronary plaque in patients with type 2 diabetes: An optical coherence tomography study. Cardiovasc. Diabetol. 2019, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Burgmaier, M.; Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S. Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS ONE 2018, 13, e0205984. [Google Scholar] [CrossRef] [PubMed]
- Reith, S.; Milzi, A.; Dettori, R.; Marx, N.; Burgmaier, M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: An optical coherence tomography study. Clin. Res. Cardiol. 2018, 107, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Milzi, A.; Burgmaier, M.; Burgmaier, K.; Hellmich, M.; Marx, N.; Reith, S. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: An intracoronary optical coherence tomography study. Cardiovasc. Diabetol. 2017, 16, 152. [Google Scholar] [CrossRef]
- Reith, S.; Battermann, S.; Hoffmann, R.; Marx, N.; Burgmaier, M. Optical coherence tomography derived differences of plaque characteristics in coronary culprit lesions between type 2 diabetic patients with and without acute coronary syndrome. Catheter. Cardiovasc. Interv. 2014, 84, 700–707. [Google Scholar] [CrossRef]
- Burgmaier, M.; Hellmich, M.; Marx, N.; Reith, S. A score to quantify coronary plaque vulnerability in high-risk patients with type 2 diabetes: An optical coherence tomography study. Cardiovasc. Diabetol. 2014, 13, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reith, S.; Battermann, S.; Jaskolka, A.; Lehmacher, W.; Hoffmann, R.; Marx, N.; Burgmaier, M. Predictors and incidence of stent edge dissections in patients with type 2 diabetes as determined by optical coherence tomography. Int. J. Cardiovasc. Imaging 2013, 29, 1237–1247. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, V.; Cho, J.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonetsu, T.; Kakuta, T.; Lee, T.; Takahashi, K.; Kawaguchi, N.; Yamamoto, G.; Koura, K.; Hishikari, K.; Iesaka, Y.; Fujiwara, H.; et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur. Heart J. 2011, 32, 1251–1259. [Google Scholar] [CrossRef]
- Kato, K.; Yonetsu, T.; Kim, S.J.; Xing, L.; Lee, H.; McNulty, I.; Yeh, R.W.; Sakhuja, R.; Zhang, S.; Uemura, S.; et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: A 3-vessel optical coherence tomography study. Circ. Cardiovasc. Imaging. 2012, 5, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, Y.; Watanabe, M.; Kamon, D.; Ueda, T.; Soeda, T.; Okayama, S.; Ishigami, K.; Kawata, H.; Horii, M.; Inoue, F.; et al. Effects of Fatty Acid Therapy in Addition to Strong Statin on Coronary Plaques in Acute Coronary Syndrome: An Optical Coherence Tomography Study. J. Am. Heart Assoc. 2020, 9, e015593. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed] [Green Version]
- Morton, G.; Schuster, A.; Perera, D.; Nagel, E. Cardiac magnetic resonance imaging to guide complex revascularization in stable coronary artery disease. Eur. Heart J. 2010, 31, 2209–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [Green Version]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Faes, T.J.C.; Meer, R.; Heyndrickx, G.R.; Kerkhof, P.L.M. Fractional Flow Reserve Evaluated as Metric of Coronary Stenosis—A Mathematical Model Study. Front. Cardiovasc. Med. 2019, 6, 189. [Google Scholar] [CrossRef] [Green Version]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2020, 41, 383–391. [Google Scholar] [PubMed]


| n = 51 | |
|---|---|
| Age (years) | 70.7 ± 8.0 |
| Male sex (n, %) | 39 (70.9) |
| CV Risk profile | |
| Diabetes mellitus (n, %) | 43 (78.2) |
| BMI (kg/m²) | 29.8 ± 3.8 |
| Hypertension (n, %) | 52 (94.5) |
| Dyslipidemia (n, %) | 35 (63.6) |
| Current smoking (n, %) | 7 (12.7) |
| Pack Years (PY) | 18.3 ± 22.6 |
| Family history of CAD (n, %) | 22 (40.0) |
| Lab values | |
| Cholesterol (mg/dL) | 194.0 ± 43.5 |
| LDLc (mg/dL) | 121.2 ± 34.8 |
| HDLc (mg/dL) | 44.4 ± 10.1 |
| Triglycerides (mg/dL) | 178.6 ± 84.2 |
| hsCRP (mg/dL) | 9.9 ± 11.6 |
| HbA1c (%) | 6.7 ± 1.0 |
| Medications | |
| ACEi/ARB (n,%) | 42 (77.8) |
| ß-Blocker (n,%) | 48 (87.3) |
| ASA (n,%) | 51 (92.7) |
| Statin (n,%) | 37 (68.5) |
| Metformin (n,%) | 31 (60.8) |
| Insulin (n,%) | 17 (33.3) |
| Incretin-based therapy (n,%) | 8 (15.7) |
| n = 55 | |
|---|---|
| OCT-derived stenosis parameters | |
| MLA (mm²) | 1.4 ± 0.6 |
| MLD (mm) | 1.1 ± 0.2 |
| Percent area stenosis (%) | 75.0 ± 9.4 |
| Lesion length (mm) | 13.7 ± 7.4 |
| Reference area (mm2) | 5.8 ± 1.7 |
| OCT-derived plaque composition | |
| Calcified plaque (n, %) | 37 (62.7) |
| Fibrous plaque (n, %) | 49 (83.1) |
| Lipid plaque (n, %) | 25 (42.4) |
| TCFA (n, %) | 10 (16.9) |
| Minimal FCT (µm) | 82.4 ± 28.2 |
| Mean FCT (µm) | 125.8 ± 29.5 |
| Mean lipid arc (°) | 139.0 ± 51.3 |
| LVI (mm *°) | 617.2 ± 458.0 |
| Presence of macrophages (n, %) | 23 (41.8) |
| Mean macrophage arc (°) | 16.1 ± 26.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettori, R.; Milzi, A.; Frick, M.; Burgmaier, K.; Almalla, M.; Lubberich, R.K.; Marx, N.; Reith, S.; Burgmaier, M. Lesion Geometry as Assessed by Optical Coherence Tomography Is Related to Myocardial Ischemia as Determined by Cardiac Magnetic Resonance Imaging. J. Clin. Med. 2021, 10, 3342. https://doi.org/10.3390/jcm10153342
Dettori R, Milzi A, Frick M, Burgmaier K, Almalla M, Lubberich RK, Marx N, Reith S, Burgmaier M. Lesion Geometry as Assessed by Optical Coherence Tomography Is Related to Myocardial Ischemia as Determined by Cardiac Magnetic Resonance Imaging. Journal of Clinical Medicine. 2021; 10(15):3342. https://doi.org/10.3390/jcm10153342
Chicago/Turabian StyleDettori, Rosalia, Andrea Milzi, Michael Frick, Kathrin Burgmaier, Mohammad Almalla, Richard Karl Lubberich, Nikolaus Marx, Sebastian Reith, and Mathias Burgmaier. 2021. "Lesion Geometry as Assessed by Optical Coherence Tomography Is Related to Myocardial Ischemia as Determined by Cardiac Magnetic Resonance Imaging" Journal of Clinical Medicine 10, no. 15: 3342. https://doi.org/10.3390/jcm10153342
APA StyleDettori, R., Milzi, A., Frick, M., Burgmaier, K., Almalla, M., Lubberich, R. K., Marx, N., Reith, S., & Burgmaier, M. (2021). Lesion Geometry as Assessed by Optical Coherence Tomography Is Related to Myocardial Ischemia as Determined by Cardiac Magnetic Resonance Imaging. Journal of Clinical Medicine, 10(15), 3342. https://doi.org/10.3390/jcm10153342






