Effect of Significant Coronary Artery Stenosis on Prognosis in Patients with Vasospastic Angina: A Propensity Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Invasive CAG and EG Provocation Test
2.4. Study Outcomes
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
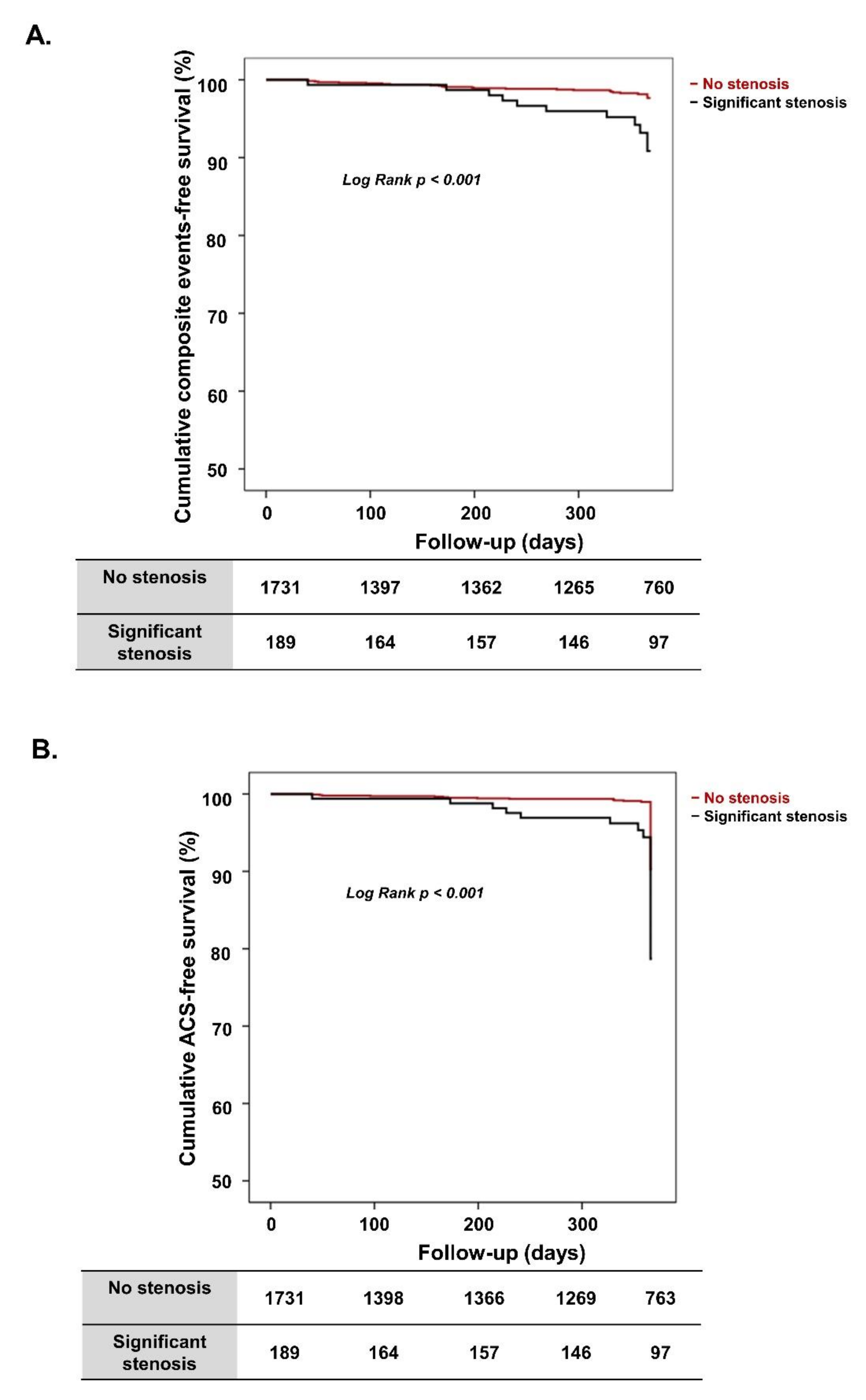
3.2. Clinical Outcomes According to Significant Coronary Artery Stenosis
3.3. Clinical Outcomes in Propensity Score-Matched Population
3.4. Effect of Significant Coronary Artery Stenosis on 1-Year ACS Rate in VA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef] [Green Version]
- MacAlpin, R.N. Relation of coronary arterial spasm to sites of organic stenosis. Am. J. Cardiol. 1980, 46, 143–153. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J. The pathophysiology of acute coronary syndromes. Heart 2000, 83, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.I.; Baek, S.H.; Her, S.H.; Han, S.H.; Ahn, Y.; Park, K.H.; Kim, D.S.; Yang, T.H.; Choi, D.J.; Suh, J.W.; et al. The 24-Month Prognosis of Patients with Positive or Intermediate Results in the Intracoronary Ergonovine Provocation Test. JACC Cardiovasc. Interv. 2015, 8, 914–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Takeshita, A.; Nose, Y. Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina. Circulation 1987, 75, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, Y.; Yasuda, S.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; Sueda, S.; et al. Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: Multicenter registry study of the Japanese Coronary Spasm Association. Circ. Arrhythm. Electrophysiol. 2011, 4, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Waters, D.D.; Miller, D.D.; Szlachcic, J.; Bouchard, A.; Methe, M.; Kreeft, J.; Theroux, P. Factors influencing the long-term prognosis of treated patients with variant angina. Circulation 1983, 68, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Lanza, G.A.; Sestito, A.; Sgueglia, G.A.; Infusino, F.; Manolfi, M.; Crea, F.; Maseri, A. Current clinical features, diagnostic assessment and prognostic determinants of patients with variant angina. Int. J. Cardiol. 2007, 118, 41–47. [Google Scholar] [CrossRef]
- Bory, M.; Pierron, F.; Panagides, D.; Bonnet, J.L.; Yvorra, S.; Desfossez, L. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur. Heart J. 1996, 17, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Scholl, J.M.; Veau, P.; Benacerraf, A.; Brau, J.; Hennetier, G.; Achard, F. Long-term prognosis of medically treated patients with vasospastic angina and no fixed significant coronary atherosclerosis. Am. Heart J. 1988, 115, 559–564. [Google Scholar] [CrossRef]
- Heupler, F.A., Jr. Syndrome of symptomatic coronary arterial spasm with nearly normal coronary arteriograms. Am. J. Cardiol. 1980, 45, 873–881. [Google Scholar] [CrossRef]
- Lee, M.H.; Jo, S.H.; Kwon, S.; Park, B.W.; Bang, D.W.; Hyon, M.S.; Baek, S.H.; Han, S.H.; Her, S.H.; Shin, D.I.; et al. Impact of Overweight/Obesity on Clinical Outcomes of Patient with Vasospastic Angina: From the Vasospastic Angina in Korea Registry. Sci. Rep. 2020, 10, 4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.S.; Jo, S.H.; Han, S.H.; Lee, K.Y.; Her, S.H.; Lee, M.H.; Seo, W.W.; Kim, S.E.; Yang, T.H.; Park, K.H.; et al. Clopidogrel plus Aspirin Use is Associated with Worse Long-Term Outcomes, but Aspirin Use Alone is Safe in Patients with Vasospastic Angina: Results from the VA-Korea Registry, A Prospective Multi-Center Cohort. Sci. Rep. 2019, 9, 17783. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Mahrholdt, H.; Kaski, J.C.; Sechtem, U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J. Am. Coll. Cardiol. 2012, 59, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, H.; Nagasawa, K.; Irie, T.; Egashira, S.; Egashira, K.; Sagara, T.; Kikuchi, Y.; Nakamura, M. Clinical characteristics and long-term prognosis of patients with variant angina. A comparative study between western and Japanese populations. Int. J. Cardiol. 1988, 18, 331–349. [Google Scholar] [CrossRef]
- Maseri, A.; Beltrame, J.F.; Shimokawa, H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ. J. 2009, 73, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Kaikita, K.; Sato, K.; Tanaka, T.; Sugamura, K.; Sakamoto, K.; Izumiya, Y.; Yamamoto, E.; Tsujita, K.; Yamamuro, M.; et al. Acetylcholine-Provoked Coronary Spasm at Site of Significant Organic Stenosis Predicts Poor Prognosis in Patients With Coronary Vasospastic Angina. J. Am. Coll. Cardiol. 2015, 66, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, M.; Ishida, T.; Kobayashi, T.; Nitta, N.; Sonoda, A.; Yamada, S.; Koike, T.; Kuniyoshi, N.; Murata, K.; Hirata, K.; et al. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction-prone strain of the Watanabe heritable hyperlipidemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2518–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsu, F.; Watarai, M. Mild stenosis makes prognosis of vasospastic angina worse. Coron. Artery Dis. 2011, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nobuyoshi, M.; Abe, M.; Nosaka, H.; Kimura, T.; Yokoi, H.; Hamasaki, N.; Shindo, T.; Kimura, K.; Nakamura, T.; Nakagawa, Y.; et al. Statistical analysis of clinical risk factors for coronary artery spasm: Identification of the most important determinant. Am. Heart J. 1992, 124, 32–38. [Google Scholar] [CrossRef]

| All (n = 1920) | No Significant Stenosis (n = 1731) | Significant Stenosis (n = 189) | p-Value | |
|---|---|---|---|---|
| Age, years | 55.1 ± 11.3 | 54.7 ± 11.3 | 58.7 ± 10.6 | <0.001 |
| Male, n (%) | 1185 (61.7) | 1046 (60.4) | 139 (73.5) | <0.001 |
| BMI, kg/m2 | 24.7 ± 3.4 | 24.8 ± 3.3 | 24.7 ± 3.8 | 0.676 |
| SBP, mmHg | 126.2 ± 18.4 | 126.1 ± 18.2 | 127.0 ± 19.7 | 0.506 |
| DBP, mmHg | 77.0 ± 12.3 | 77.0 ± 12.1 | 76.3 ± 13.8 | 0.427 |
| Previous CAD *, n (%) | 247 (12.9) | 215 (12.4) | 32 (16.9) | 0.080 |
| Diabetes mellitus, n (%) | 180 (9.4) | 146 (8.4) | 34 (18.0) | <0.001 |
| Hypertension, n (%) | 731 (73.1) | 9\636 (36.8) | 95 (50.3) | <0.001 |
| Dyslipidemia, n (%) | 313 (16.4) | 283 (16.4) | 30 (15.9) | 0.848 |
| Alcohol drinking, n (%) | 794 (41.4) | 702 (40.6) | 92 (48.7) | 0.031 |
| Current smoking, n (%) | 524 (27.7) | 458 (26.8) | 66 (35.3) | 0.014 |
| Laboratory findings | ||||
| Hemoglobin, g/dL | 13.9 ± 1.9 | 13.9 ± 1.9 | 13.9 ± 1.5 | 0.949 |
| Creatinine, mg/dL | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.8 ± 0.2 | 0.593 |
| Glucose, mg/dL | 111.4 ± 37.7 | 111.3 ± 38.3 | 113.0 ± 31.9 | 0.573 |
| hs-CRP, mg/dL | 0.7 ± 4.4 | 0.7 ± 4.5 | 0.6 ± 3.1 | 0.810 |
| Total cholesterol, mg/dL | 174.2 ± 36.3 | 174.8 ± 36.0 | 168.5 ± 38.6 | 0.029 |
| LDL cholesterol, mg/dL | 103.7 ± 31.5 | 104.3 ± 31.3 | 98.6 ± 33.5 | 0.030 |
| Triglyceride, mg/dL | 141.9 ± 104.3 | 140.2 ± 103.2 | 157.0 ± 113.0 | 0.049 |
| HDL cholesterol, mg/dL | 46.8 ± 12.8 | 47.1 ± 12.9 | 44.1 ± 11.2 | 0.001 |
| LV EF, % | 64.5 ± 6.6 | 64.6 ± 6.4 | 63.5 ± 7.8 | 0.108 |
| Previous cardiovascular medications | ||||
| Antiplatelet, n (%) | 424 (22.1) | 371 (21.4) | 53 (28.0) | 0.060 |
| Statin, n (%) | 296 (15.4) | 258 (14.9) | 38 (20.1) | 0.132 |
| CCB, n (%) | 375 (19.5) | 327 (18.9) | 48 (25.4) | 0.075 |
| Clinical diagnosis before ergonovine | ||||
| Angina, n (%) | 1740 (90.6) | 1572 (90.8) | 168 (88.9) | 0.388 |
| Myocardial infarction, n (%) | 35 (1.8) | 29 (1.7) | 6 (3.2) | 0.146 |
| Cardiac arrest, n (%) | 27 (1.4) | 25 (1.4) | 2 (1.1) | 1.000 |
| Syncope, n (%) | 24 (1.3) | 22 (1.3) | 2 (1.1) | 1.000 |
| VT or VF, n (%) | 12 (0.6) | 11 (0.6) | 1 (0.5) | 1.000 |
| AV block, n (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1.000 |
| All (n = 1920) | No Significant Stenosis (n =1731) | Significant Stenosis (n = 189) | p-Value | |
|---|---|---|---|---|
| Composite events | 36 (1.9) | 25 (1.4) | 11 (5.8) | <0.001 |
| ACS | 25 (1.3) | 16 (0.9) | 9 (4.8) | <0.001 |
| Cardiac death | 2 (0.1) | 2 (0.1) | 0 (0.0) | 1.000 |
| VT or VF | 3 (0.2) | 2 (0.1) | 1 (0.5) | 0.267 |
| AV block | 6 (0.3) | 5 (0.3) | 1 (0.5) | 0.463 |
| All-cause death | 8 (0.4) | 8 (0.5) | 0 (0.0) | 1.000 |
| All (n = 1920) | No Significant Stenosis (n =1731) | Significant Stenosis (n = 189) | p-Value | |
|---|---|---|---|---|
| Composite events | 75 (3.9) | 58 (3.4) | 17 (9.0) | 0.001 |
| ACS | 59 (3.1) | 45 (2.6) | 14 (7.4) | 0.001 |
| Cardiac death | 3 (0.2) | 3 (0.2) | 0 (0.0) | 1.000 |
| VT or VF | 8 (0.4) | 6 (0.3) | 2 (1.1) | 0.182 |
| AV block | 7 (0.4) | 6 (0.3) | 1 (0.5) | 0.516 |
| All-cause death | 12 (0.6) | 12 (0.7) | 0 (0.0) | 0.621 |
| One-Year | All (n = 546) | No Significant Stenosis (n = 364) | Significant Stenosis (n = 182) | p-Value |
|---|---|---|---|---|
| Composite events | 19 (4.5) | 8 (3.0) | 11 (7.4) | 0.035 |
| ACS | 14 (2.6) | 5 (1.4) | 9 (4.9) | 0.019 |
| Cardiac death | 1 (0.2) | 1 (0.3) | 0 (0.0) | 1.000 |
| VT or VF | 2 (0.4) | 1 (0.3) | 1 (0.5) | 1.000 |
| AV block | 2 (0.4) | 1 (0.3) | 1 (0.5) | 1.000 |
| All-cause death | 2 (0.4) | 2 (0.5) | 0 (0.0) | 0.555 |
| Total Period | All (n = 546) | No Significant Stenosis (n = 364) | Significant Stenosis (n = 182) | p-Value |
| Composite events | 31 (5.7) | 14 (3.8) | 17 (9.3) | 0.009 |
| ACS | 24 (4.4) | 10 (2.7) | 14 (7.7) | 0.008 |
| Cardiac death | 2 (0.4) | 2 (0.5) | 0 (0.0) | 0.555 |
| VT or VF | 4 (0.7) | 2 (0.5) | 2 (1.1) | 0.604 |
| AV block | 2 (0.4) | 1 (0.3) | 1 (0.5) | 1.000 |
| All-cause death | 3 (0.5) | 3 (0.8) | 0 (0.0) | 0.554 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Significant coronary artery stenosis | 5.36 | 2.335–12.303 | <0.001 | 6.67 | 2.798–15.908 | <0.001 |
| Age | 0.97 | 0.939–1.006 | 0.101 | 0.96 | 0.924–0.994 | 0.023 |
| Male | 0.91 | 0.398–2.060 | 0.813 | 1.20 | 0.514–2.820 | 0.670 |
| Hypertension | 0.91 | 0.401–2.077 | 0.828 | - | - | - |
| Diabetes | 0.84 | 0.193–3.581 | 0.811 | - | - | - |
| Dyslipidemia | 2.93 | 1.283–6.694 | 0.011 | 3.14 | 1.358–7.277 | 0.007 |
| Current smoking | 1.48 | 0.649–3.368 | 0.351 | - | - | - |
| Alcohol drinking | 0.95 | 0.422–2.114 | 0.890 | - | - | - |
| Hemoglobin, g/dL | 1.01 | 0.819–1.235 | 0.958 | - | - | - |
| Creatinine, mg/dL | 0.69 | 0.116–4.144 | 0.689 | - | - | - |
| hs-CRP, mg/dL | 1.02 | 0.959–1.075 | 0.599 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, M.-H.; Jo, S.-H.; Seo, W.-W.; Kim, H.-L.; Lee, K.-Y.; Yang, T.-H.; Her, S.-H.; Han, S.-H.; Lee, B.-K.; et al. Effect of Significant Coronary Artery Stenosis on Prognosis in Patients with Vasospastic Angina: A Propensity Score-Matched Analysis. J. Clin. Med. 2021, 10, 3341. https://doi.org/10.3390/jcm10153341
Kim H-J, Lee M-H, Jo S-H, Seo W-W, Kim H-L, Lee K-Y, Yang T-H, Her S-H, Han S-H, Lee B-K, et al. Effect of Significant Coronary Artery Stenosis on Prognosis in Patients with Vasospastic Angina: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2021; 10(15):3341. https://doi.org/10.3390/jcm10153341
Chicago/Turabian StyleKim, Hyun-Jin, Min-Ho Lee, Sang-Ho Jo, Won-Woo Seo, Hack-Lyoung Kim, Kwan-Yong Lee, Tae-Hyun Yang, Sung-Ho Her, Seung-Hwan Han, Byoung-Kwon Lee, and et al. 2021. "Effect of Significant Coronary Artery Stenosis on Prognosis in Patients with Vasospastic Angina: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 10, no. 15: 3341. https://doi.org/10.3390/jcm10153341
APA StyleKim, H.-J., Lee, M.-H., Jo, S.-H., Seo, W.-W., Kim, H.-L., Lee, K.-Y., Yang, T.-H., Her, S.-H., Han, S.-H., Lee, B.-K., Park, K.-H., Rha, S.-W., Gwon, H.-C., Choi, D.-J., & Baek, S.-H. (2021). Effect of Significant Coronary Artery Stenosis on Prognosis in Patients with Vasospastic Angina: A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 10(15), 3341. https://doi.org/10.3390/jcm10153341






