Outcomes and Timing of Bedside Percutaneous Tracheostomy of COVID-19 Patients over a Year in the Intensive Care Unit
Abstract
:1. Introduction
- −
- The benefits of percutaneous dilatational tracheostomy (PDT) on respiratory functions and the weaning process
- −
- The timing of tracheostomy and its association with ICU length of stay and mechanical ventilation
- −
- Risk of SARS-CoV-2 infection in HCWs during the PDT procedure
2. Materials and Methods
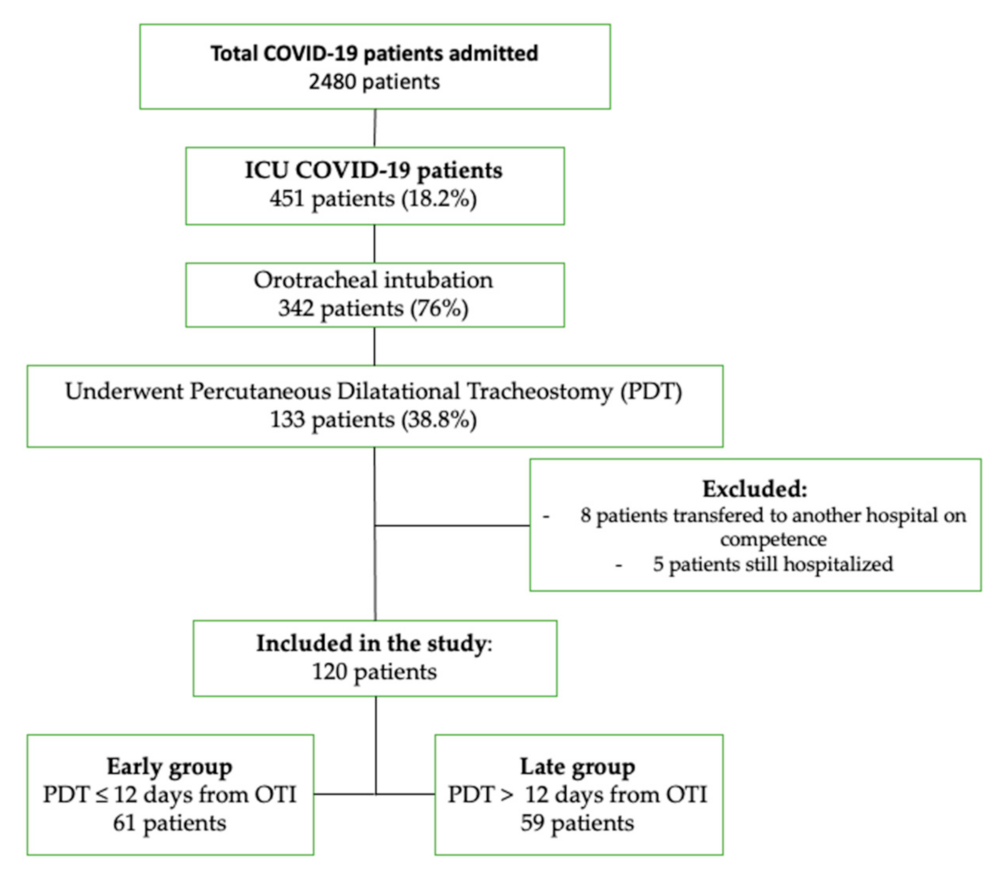
2.1. Study Design and Participants
- (1)
- The early group, which included patients who underwent PDT within the first 12 days of orotracheal intubation (OTI)
- (2)
- The late group, which included patients in whom the procedure was performed more than 12 days after OTI.
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Outcome 7 Days after PDT in COVID-19 ICU Patients
3.2. PDT Complications
3.3. Early versus Late PDT
3.4. SARS-CoV-2 Transmission in HCWs Who Performed PDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Infection Prevention and Control Guidance for Long-Term Care Facilities in the Context of COVID-19: Interim Guidance, 21 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Privitera, D.; Angaroni, L.; Capsoni, N.; Forni, E.; Pierotti, F.; Vincenti, F.; Bellone, A. Flowchart for Non-Invasive Ventilation Support in COVID-19 Patients from a Northern Italy Emergency Department. Intern. Emerg. Med. 2020, 15, 767–771. [Google Scholar] [CrossRef]
- Dullemond, K.; Renschler, C.; Trojanowski, J.; Scheuermeyer, F.; Stenstrom, R.; Griesdale, D.; MacRedmond, R.; Nattress, E.; Farina, L.; Yoo, J. Success and Complications of Endotracheal Intubation in Critical Care Settings under COVID-19 Protocols. Can. J. Emerg. Med. 2021, 23, 512–517. [Google Scholar] [CrossRef]
- Durbin, C.G. Indications for and Timing of Tracheostomy. Respir. Care 2005, 50, 483–487. [Google Scholar] [PubMed]
- Trouillet, J.L.; Collange, O.; Belafia, F.; Blot, F.; Capellier, G.; Cesareo, E.; Constantin, J.M.; Demoule, A.; Diehl, J.L.; Guinot, P.G.; et al. Tracheotomy in the Intensive Care Unit: Guidelines from a French Expert Panel: The French Intensive Care Society and the French Society of Anaesthesia and Intensive Care Medicine. Anaesth. Crit. Care Pain Med. 2018, 37, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Botti, C.; Lusetti, F.; Peroni, S.; Neri, T.; Castellucci, A.; Salsi, P.; Ghidini, A. The Role of Tracheotomy and Timing of Weaning and Decannulation in Patients Affected by Severe COVID-19. Ear Nose Throat J. 2021, 100, 116S–119S. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Cimon, K.; Severn, M.; Pessoa-Silva, C.L.; Conly, J. Aerosol Generating Procedures and Risk of Transmission of Acute Respiratory Infections to Healthcare Workers: A Systematic Review. PLoS ONE 2012, 7, e35797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, B.A.; Ashby, N.; Birchall, M.; Dean, P.; Doherty, C.; Ferguson, K.; Gimblett, J.; Grocott, M.; Jacob, T.; Kerawala, C.; et al. Multidisciplinary Guidance for Safe Tracheostomy Care during the COVID-19 Pandemic: The NHS National Patient Safety Improvement Programme (NatPatSIP). Anaesthesia 2020, 75, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Frova, G.; Quintel, M. A New Simple Method for Percutaneous Tracheostomy: Controlled Rotating Dilation—A Preliminary Report. Intensive Care Med. 2002, 28, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Byhahn, C.; Lischke, V.; Halbig, S.; Scheifler, G.; Westphal, K. Ciaglia blue rhino: A modified technique for percutaneous dilatation tracheostomy. Technique and early clinical results. Der Anaesthesist 2000, 49, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Mehta, Y. Percutaneous Tracheostomy. Ann. Card. Anaesth. 2017, 20, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; di Caro, A.; Petrosillo, N. Incidence of Bacterial and Fungal Bloodstream Infections in COVID-19 Patients in Intensive Care: An Alarming “Collateral Effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Trouillet, J.L.; Collange, O.; Belafia, F.; Blot, F.; Capellier, G.; Cesareo, E.; Constantin, J.M.; Demoule, A.; Diehl, J.L.; Guinot, P.G.; et al. Tracheotomy in the Intensive Care Unit: Guidelines from a French Expert Panel. Ann. Intensive Care 2018, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hibino, M.; Falcione, B.A.; Eichinger, K.M.; Patel, R.; Empey, K.M. Immunosuppressive Aspects of Analgesics and Sedatives Used in Mechanically Ventilated Patients: An Underappreciated Risk Factor for the Development of Ventilator-Associated Pneumonia in Critically Ill Patients. Ann. Pharmacother. 2014, 48, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Dossow, V.; Luetz, A.; Haas, A.; Sawitzki, B.; Wernecke, K.D.; Volk, H.D.; Spies, C.D. Effects of Remifentanil and Fentanyl on the Cell-Mediated Immune Response in Patients Undergoing Elective Coronary Artery Bypass Graft Surgery. J. Int. Med. Res. 2008, 36, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.; Youssef, T.A.; El-Begermy, M.M.; Younis, H.M. Timing of Tracheostomy in Patients with Prolonged Endotracheal Intubation: A Systematic Review. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 679–690. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 120) | Early Group (n = 61) | Late Group (n = 59) | p-Value a | |
|---|---|---|---|---|
| Age, median (IQR) | 68.5 (61–75) | 70 (64–77) | 65 (69–73) | 0.056 |
| Male, n (%) | 80 (66.7%) | 42 (68.9%) | 38 (64.4%) | 0.606 |
| Female, n (%) | 40 (33.3%) | 19 (31.1%) | 21 (35.5%) | |
| BMI, kg/m2, median (IQR) | 27.8 (25–31) | 27.6 (25–31) | 28.4 (26–31) | 0.357 |
| SOFA score, median (IQR) | 5 (3–7) | 5 (4–7) | 5 (3–8) | 0.817 |
| APACHE II score, median (IQR) | 12.5 (10–17) | 13 (10–17) | 12 (9–17) | 0.646 |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 80 (66.7%) | 45 (73.7%) | 35 (59.3%) | 0.093 |
| Other cardiopathies | 24 (20.0%) | 15 (24.6%) | 9 (15.2%) | 0.201 |
| Diabetes | 28 (23.3%) | 15 (24.6%) | 13 (22.0%) | 0.741 |
| Obesity | 54 (45.0%) | 25 (41%) | 29 (49.1%) | 0.369 |
| Kidney disease (stage 3–5 of CKD) | 8 (6.7%) | 5 (8.2%) | 3 (5.1%) | 0.494 |
| Moderate to severe chronic liver disease | 1 (0.8%) | 1 (1.6%) | 0 (0.0%) | 0.323 |
| COPD/Bronchial asthma | 21 (17.5%) | 12 (19.7%) | 9 (15.2%) | 0.524 |
| Previous neoplasia (solid neoplasia or hematological malignancy in the last 5 years) | 14 (11.7%) | 7 (11.4%) | 7 (11.8%) | 0.621 |
| Previous surgery in last month | 4 (3.3%) | 2 (3.2%) | 2 (3.4%) | 0.973 |
| Previous hospitalization last six months | 8 (6.7%) | 3 (4.9%) | 5 (8.5%) | 0.435 |
| Chronic neurological disorders | 20 (16.7%) | 10 (16.4%) | 10 (16.9%) | 0.935 |
| Autoimmune diseases | 15 (12.5%) | 6 (9.8%) | 9 (15.2%) | 0.370 |
| Other chronical diseases | 32 (26.6%) | 23 (37.7%) | 9 (15.2%) | 0.005 |
| Total 1 (n = 110), Mean ± SD | Day of OTI in ICU | Day of PDT | Day 7 after PDT | p-Value a |
|---|---|---|---|---|
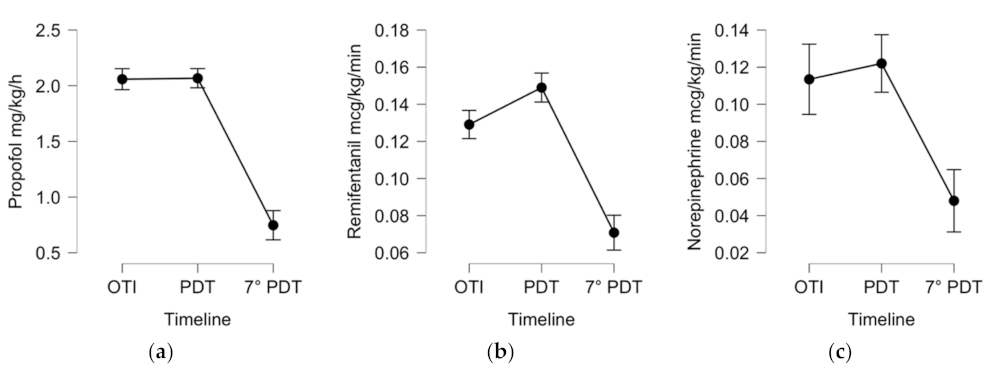
| Sedative, analgesic, and inotropic therapy by intravenous continuous infusion | ||||
| Propofol, mg/kg/h | 2.0 (±0.39) | 2.01 (±0.53) | 0.7 (±0.87) | p < 0.001 |
| Remifentanil, mcg/kg/min | 0.13 (±0.03) | 0.15 (±0.05) | 0.07 (±0.07) | p < 0.001 |
| Norepinephrine, mcg/kg/min | 0.11 (±0.12) | 0.12 (±0.13) | 0.05 (±0.09) | p < 0.001 |
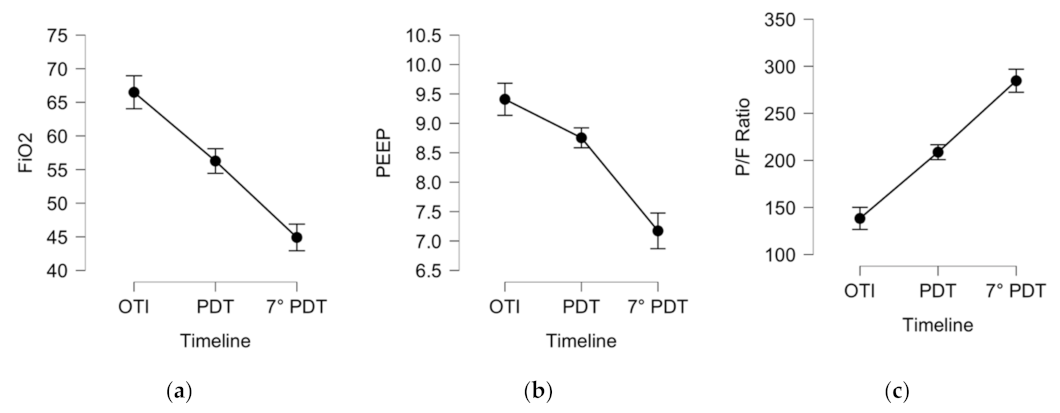
| Ventilation parameters and respiratory exchange ratio | ||||
| P/F Ratio, mmHg | 138 (±43) | 208 (±64) | 243 (±95) | p < 0.001 |
| FiO2, % | 66.5 (±15) | 56 (±14) | 45 (±14) | p < 0.001 |
| PEEP, cmH2O | 9.4 (±1.9) | 8.7 (±1.5) | 7.1 (±1.6) | p < 0.001 |
| Total 1 (n = 120) Mean ± SD | Early Group (n = 61), Mean ± SD | Late Group (n = 59), Mean ± SD | p-Value a | p-Value b Adjusted | |
|---|---|---|---|---|---|
| Pre-ICU hospitalization, days | 5.9 (±8.0) | 5.6 (±6.5) | 6.2 (±9.4) | 0.819 | |
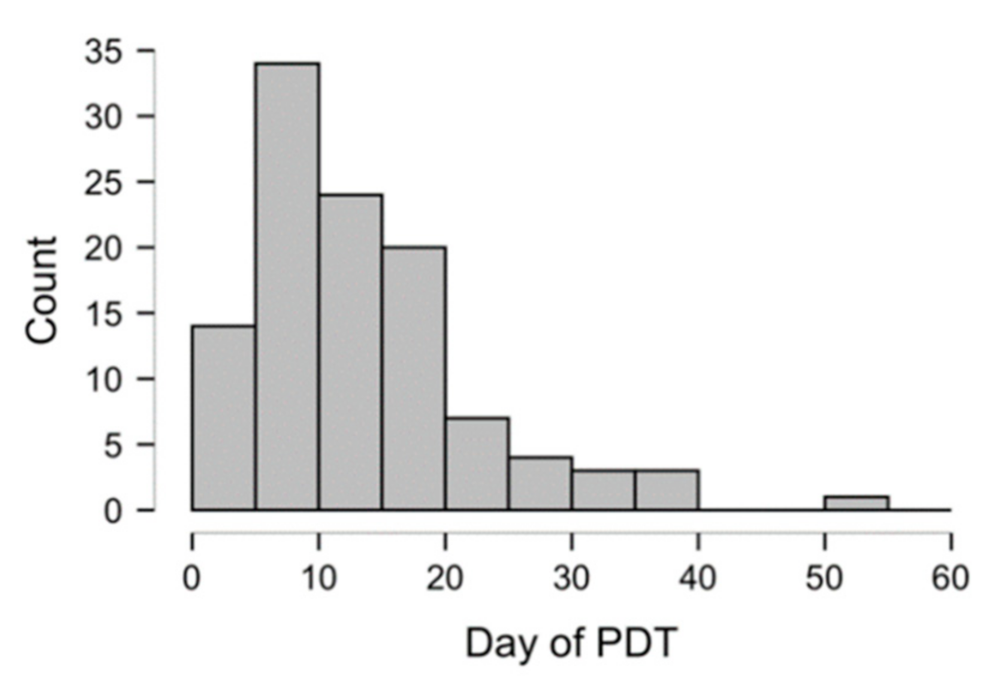
| Day of PDT, days from OTI to PDT | 13.9 (±8.7) | 7.6 (±2.6) | 20.4 (±8.1) | <0.001 | |
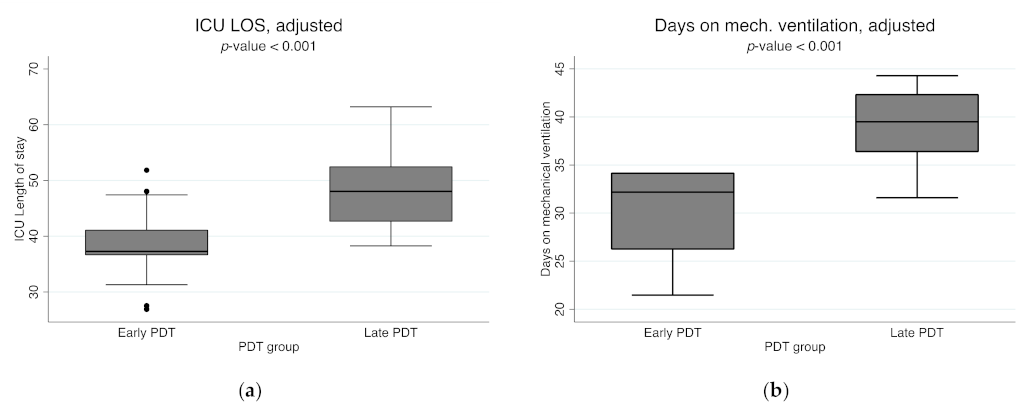
| Mechanical ventilation, days | 34.5 (±15.8) | ||||
| Mean | 30.3 (±14.1) | 38.9 (±16.3) | <0.001 | ||
| Adjusted mean b | 30.3 (±4.2) b | 38.9 (±4.3) b | <0.001 | ||
| Spontaneous breathing from weaning to decannulation, days | 6.1 (±5.5) | 5.2 (±4.9) | 6.9 (±6.1) | 0.085 | |
| Days on tracheostomy, from PDT to decannulation | 26.7 (±16.3) | 27.3 (±16.2) | 25.9 (±16.7) | 0.650 | |
| ICU length of stay, days | 42.3 (±18.9) | ||||
| Mean | 36.8 (±17.1) | 48.4 (±19.4) | <0.001 | ||
| Adjusted mean b | 37.7 (±6.6) b | 47.7 (±6.5) b | <0.001 | ||
| Outcome | |||||
| ICU discharged, patients (%) | 78 (65%) | 38 (62.3%) | 40 (67.8%) | ||
| ICU mortality, patients (%) | 42 (35%) | 23 (37.7%) | 19 (32.2%) | 0.165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetaj, N.; Maritti, M.; Stazi, G.; Marini, M.C.; Centanni, D.; Garotto, G.; Caravella, I.; Dantimi, C.; Fusetti, M.; Santagata, C.; et al. Outcomes and Timing of Bedside Percutaneous Tracheostomy of COVID-19 Patients over a Year in the Intensive Care Unit. J. Clin. Med. 2021, 10, 3335. https://doi.org/10.3390/jcm10153335
Tetaj N, Maritti M, Stazi G, Marini MC, Centanni D, Garotto G, Caravella I, Dantimi C, Fusetti M, Santagata C, et al. Outcomes and Timing of Bedside Percutaneous Tracheostomy of COVID-19 Patients over a Year in the Intensive Care Unit. Journal of Clinical Medicine. 2021; 10(15):3335. https://doi.org/10.3390/jcm10153335
Chicago/Turabian StyleTetaj, Nardi, Micaela Maritti, Giulia Stazi, Maria Cristina Marini, Daniele Centanni, Gabriele Garotto, Ilaria Caravella, Cristina Dantimi, Matteo Fusetti, Carmen Santagata, and et al. 2021. "Outcomes and Timing of Bedside Percutaneous Tracheostomy of COVID-19 Patients over a Year in the Intensive Care Unit" Journal of Clinical Medicine 10, no. 15: 3335. https://doi.org/10.3390/jcm10153335
APA StyleTetaj, N., Maritti, M., Stazi, G., Marini, M. C., Centanni, D., Garotto, G., Caravella, I., Dantimi, C., Fusetti, M., Santagata, C., Macchione, M., De Angelis, G., Giansante, F., Busso, D., Di Lorenzo, R., Scarcia, S., Carucci, A., Cabas, R., Gaviano, I., ... ICU COVID-19 Study Group. (2021). Outcomes and Timing of Bedside Percutaneous Tracheostomy of COVID-19 Patients over a Year in the Intensive Care Unit. Journal of Clinical Medicine, 10(15), 3335. https://doi.org/10.3390/jcm10153335








