Liposomal Inhalation after Tracheostomy—A Randomized Controlled Trial
Abstract
1. Introduction
2. Study Design and Methods
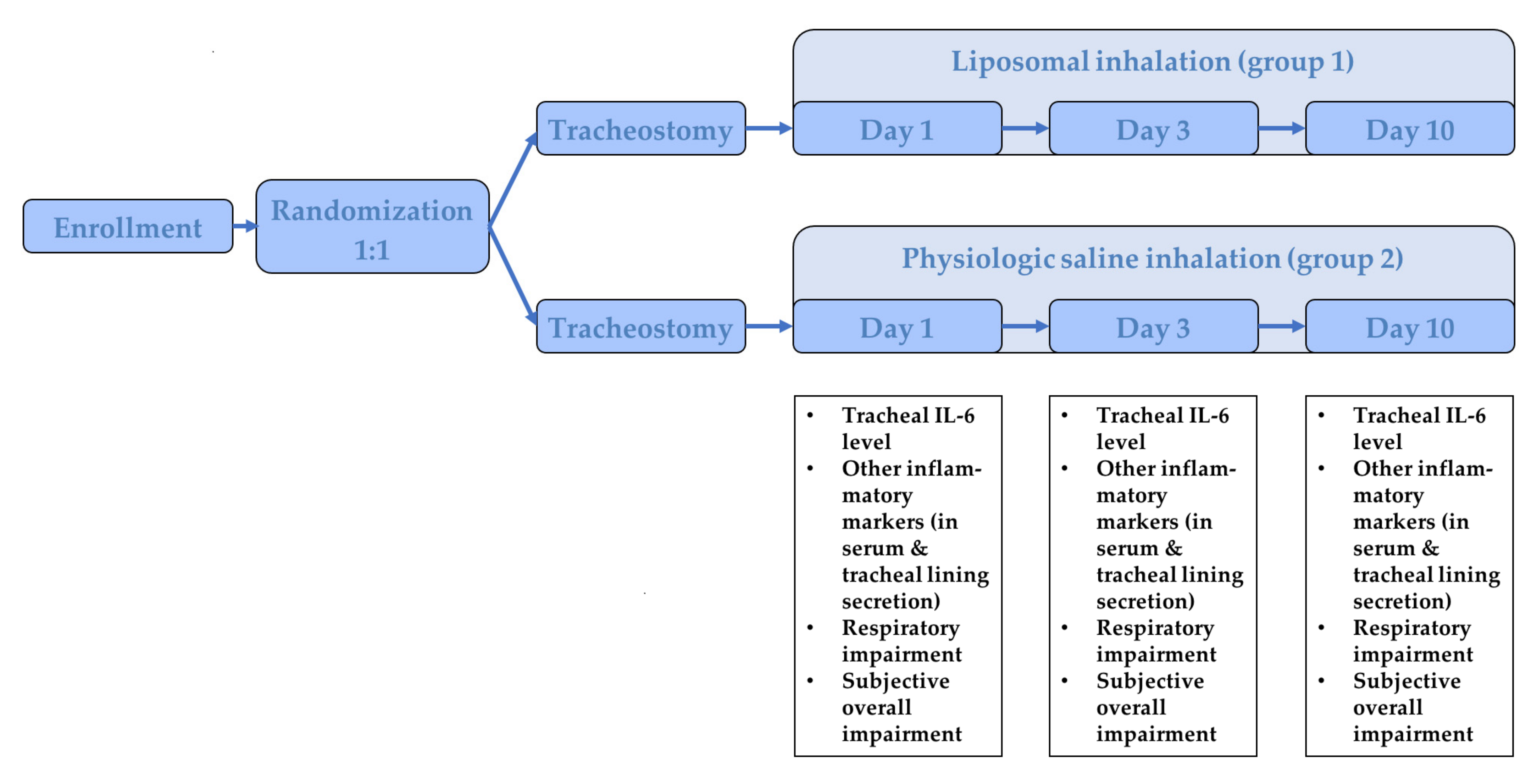
2.1. Study Population
2.2. Study Protocol
2.3. Outcome Parameter
2.4. Statistical Analysis
3. Results
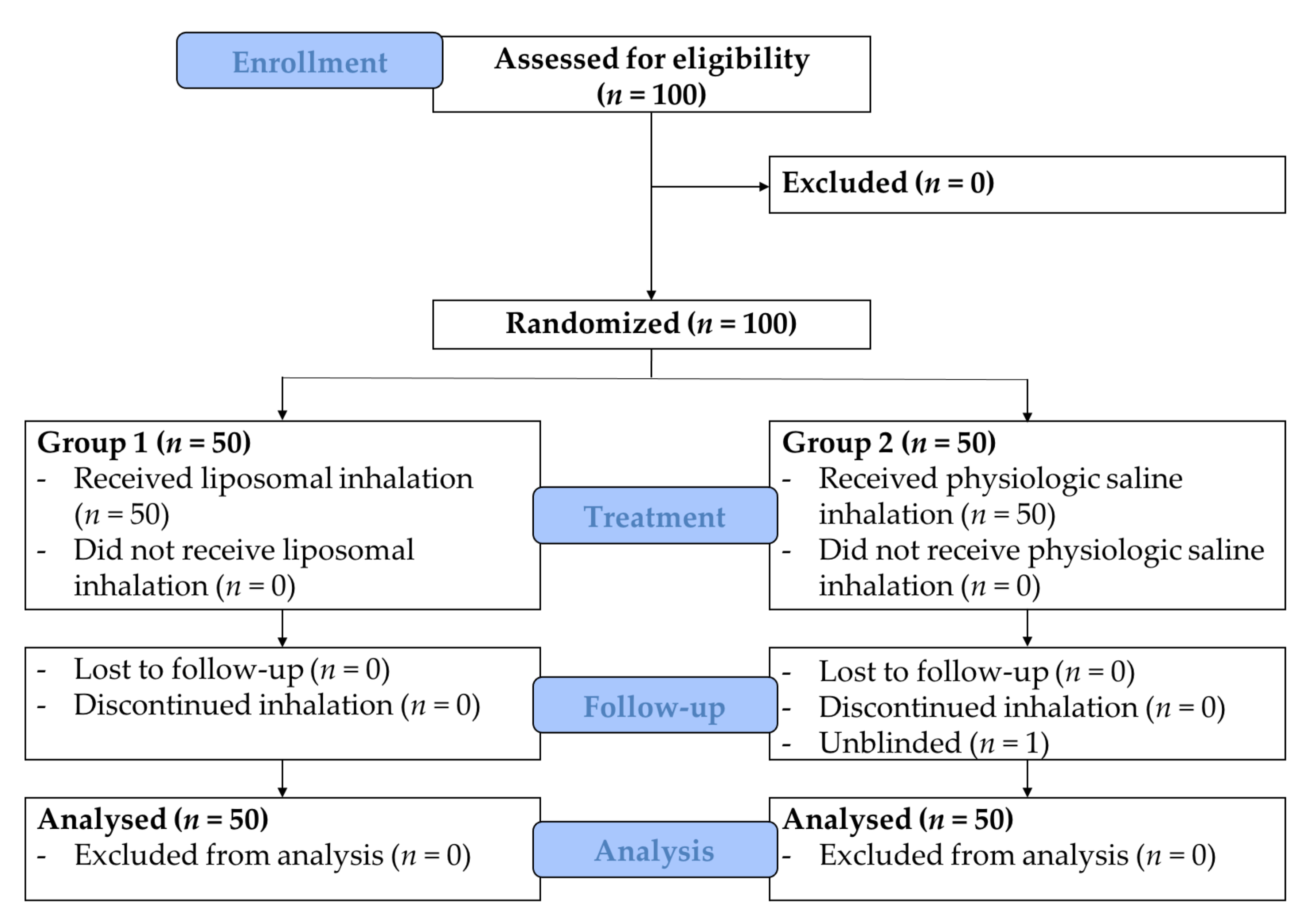
3.1. Study Population
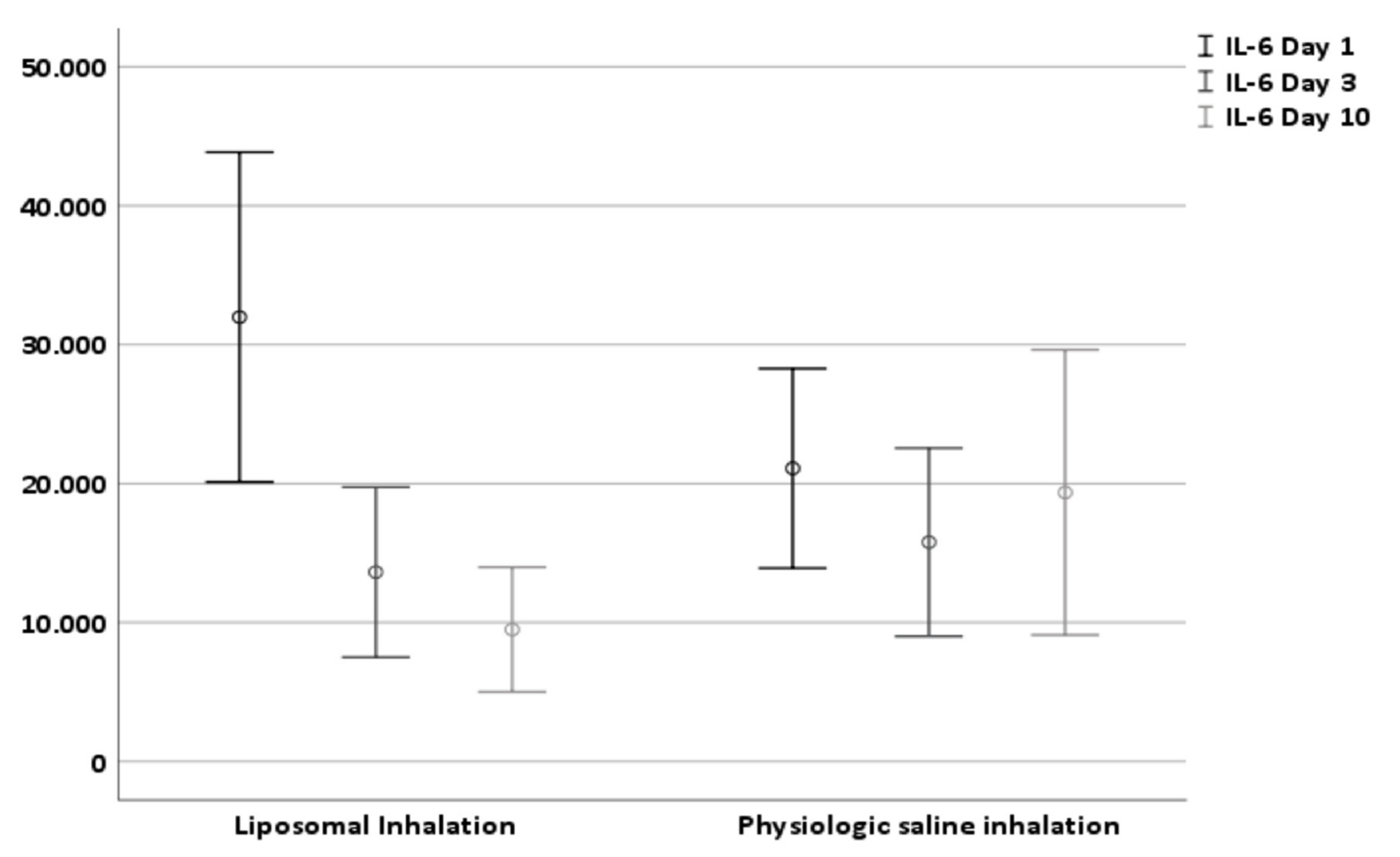
3.2. Effect on Tracheal IL-6 Level (Primary Endpoint)
3.3. Effect on Respiratory Impairment (Secondary Endpoint)
3.4. Effect on Further Inflammatory Parameter (Other Endpoint)
3.5. Effect on Subjective Overall Impairment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pignatti, P.; Balestrino, A.; Herr, C.; Bals, R.; Moretto, D.; Corradi, M.; Alinovi, R.; Delmastro, M.; Vogelmeier, C.; Nava, S.; et al. Tracheostomy and related host-pathogen interaction are associated with airway inflammation as characterized by tracheal aspirate analysis. Respir. Med. 2009, 103, 201–208. [Google Scholar] [CrossRef]
- Klemm, E.; Nowak, A.K. Tracheotomy-Related Deaths. Dtsch. Arztebl. Int. 2017, 114, 273–279. [Google Scholar] [PubMed]
- Hill, N.S. Neuromuscular disease in respiratory and critical care medicine. Respir. Care 2006, 51, 1065–1071. [Google Scholar] [PubMed]
- Kanna, B.; Ayman, H.A.; Soni, A. Early tracheostomy in intensive care trauma patient improves resource utilization: A cohort study and literature review. Lett. Crit. Care. 2005, 9, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Carson, S.S.; Holmes, G.M.; Howard, A.; Carey, T.S. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993–2002. Crit. Care Med. 2004, 32, 2219–2226. [Google Scholar] [CrossRef]
- McGrath, B.A.; Wallace, S. The UK National Tracheostomy Safety Project and the role of speech and language therapists. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 181–187. [Google Scholar] [CrossRef]
- Yu, M. Tracheostomy patients on the ward: Multiple benefits from a multidisciplinary team. Crit. Care 2010, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Langerman, A.; Patel, R.M.; Cohen, E.E.; Blair, E.A.; Stenson, K.M. Airway management before chemoradiation for advanced head and neck cancer. Head Neck 2012, 34, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kramp, B.; Donat, M.; Dommerich, S.; Pau, H.W.; Podbielski, A. Prospective controlled study of microbial colonization of the trachea in tracheotomized and laryngectomized patients with HME (heat and moisture exchanger). Acta Otolaryngol. 2009, 129, 1136–1144. [Google Scholar] [CrossRef]
- Griese, M.; Felber, J.; Reiter, K.; Strong, P.; Reid, K.; Belohradsky, B.H.; Jäger, G.; Nicolai, T. Airway inflammation in children with tracheostomy. Pediatr. Pulmonol. 2004, 37, 356–361. [Google Scholar] [CrossRef]
- Birk, R.; Händel, A.; Wenzel, A.; Kramer, B.; Aderhold, C.; Hörmann, K.; Stuck, B.A.; Sommer, J.U. Heated air humidification versus cold air nebulization in newly tracheostomized patients. Head Neck 2017, 39, 2481–2487. [Google Scholar] [CrossRef]
- Rosso, M.; Prgomet, D.; Marjanović, K.; Pušeljić, S.; Kraljik, N. Pathohistological changes of tracheal epithelium in laryngectomized patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 3539–3544. [Google Scholar] [CrossRef]
- Epstein, S.K. Anatomy and physiology of tracheostomy. Respir. Care 2005, 50, 476–482. [Google Scholar]
- O’Dwyer, D.N.; Dickson, R.P.; Moore, B.B. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol. 2016, 196, 4839–4847. [Google Scholar] [CrossRef] [PubMed]
- Bień, S.; Okła, S.; van As-Brooks, C.J.; Ackerstaff, A.H. The effect of a Heat and Moisture Exchanger (Provox HME) on pulmonary protection after total laryngectomy: A randomized controlled study. Eur. Arch. Otorhinolaryngol. 2010, 267, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Gehr, P.; Im Hof, V.; Geiser, M.; Schürch, S. Der mukoziliäre Apparat der Lunge—Die Rolle des Surfactant. Schweiz. Med. Wochenschr. 2000, 130, 691–698. [Google Scholar]
- Braun, A.; Steinecker, M.; Schumacher, S.; Griese, M. Surfactant function in children with chronic airway inflammation. J. Appl. Physiol. (1985) 2004, 97, 2160–2165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asada, K.; Ichiyama, T.; Okuda, Y.; Okino, F.; Hashimoto, K.; Nishikawa, M.; Sugio, Y.; Furukawa, S. Cytokine levels in sputum of patients with tracheostomy and profound multiple disabilities. Cytokine 2008, 42, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Beubler, E.; Fischer, R.; Untersteiner, G.; Strohmaier, W. Influence of the Surfactant Tyloxapol on Mucociliary Clearance in Human Respiratory Cystic Fibrosis Cells. Pharmacology 2016, 98, 1–3. [Google Scholar] [CrossRef]
- De Sanctis, G.T.; Tomkiewicz, R.P.; Rubin, B.K.; Schürch, S.; King, M. Exogenous surfactant enhances mucociliary clearance in the anaesthetized dog. Eur. Respir. J. 1994, 7, 1616–1621. [Google Scholar] [CrossRef]
- Nadya, S.; Kazzi, J.; Romero, R.; McLaughlin, K. Surfactant Therapy Modulates Levels of Interleukin-6 (IL-6) and Interleukin-1B (IL-1B) in Tracheal Aspirates of Premature Infants with Respiratory Distress Syndrome (RDS). Pediatr. Res. 1999, 45, 204. [Google Scholar] [CrossRef][Green Version]
- Daneshina, R.; Wittmann, M. Positiver Effekt inhalativer Substitution von Phosphatidylcholin bei trockenem Reizhusten in einem Fall von medikamentös induzierter Lungenfribrose. Atemwegs Lungenerkrankungen 2013, 39, 323–326. [Google Scholar] [CrossRef]
| Variables | Liposomal Inhalation | Physiologic Saline Solution Inhalation | p-Value |
|---|---|---|---|
| Number | 50 | 50 | |
| Age (years ± SD) | 62.46 ± 11.28 | 60.86 ± 11.03 | 0.219 |
| Gender (m/f) | 0.587 | ||
| Female | 7 (14%) | 9 (18%) | |
| Male | 43 (86%) | 41 (82%) | |
| Comorbidities | 0.88 | ||
| Multimorbidity | 15 (30%) | 15 (30%) | |
| Neuronal | 1 (2%) | 1 (2%) | |
| Cardiovascular | 21 (42%) | 19 (38%) | |
| Hepatorenal | 2 (4%) | 1 (2%) | |
| Endocrine-metabol | 14 (28%) | 12 (24%) | |
| Pulmonal | 1 (2%) | 2 (4%) | |
| Diagnosis | 1 | ||
| Malignoma | 48 (96%) | 48 (96%) | |
| Inflammation | 0 | 0 | |
| Others | 2 (4%) | 2 (4%) | |
| Location | 0.226 | ||
| Oral cavity | 1 (2%) | 3 (6%) | |
| Nasopharynx | 1 (2%) | 0 | |
| Oropharynx | 19 (38%) | 25 (50%) | |
| Hypopharynx | 5 (10%) | 6 (12%) | |
| Larynx | 24 (48%) | 16 (32%) | |
| Therapy | 0.427 | ||
| Tumor surgery | 40 (80%) | 43 (86%) | |
| Tracheostomy alone | 10 (20%) | 7 (14%) | |
| Alcohol | 0.95 | ||
| Never | 21 (42%) | 18 (36%) | |
| Occasionally | 12 (24%) | 16 (32%) | |
| Daily | 10 (20%) | 12 (24%) | |
| Former abuse | 7 (14%) | 3 (6%) | |
| Information missing | 0 | 1 (2%) | |
| Nicotine | 0.142 | ||
| Non-smoker | 8 (16%) | 12 (24%) | |
| Smoker | 29 (58%) | 27 (54%) | |
| Former smoker | 12 (24%) | 7 (14%) | |
| Information missing | 1 (2%) | 4 (8%) | |
| Packyears (n ± SD) | 31.37 ± 21.82 | 28.37 ± 19.85 | 0.67 |
| Antibiotics prior to tracheostomy | 1/50 | 1/50 | 1 |
| Variables | Liposomal Inhalation | Physiologic Saline Inhalation | p-Value |
|---|---|---|---|
| IL-6 Level Day 1 (pg/mL ± SD) | 31,975.81 ± 41,771.89 | 21,093.45 ± 25,280.55 | 0.118 |
| IL-6 Level Day 3 | 13,622.90 ± 21,538.70 | 15,779.50 ± 23,807.35 | 0.636 |
| IL-6 Level Day 10 | 9491.67 ± 15,809.79 | 19,358.50 ± 36,130.19 | 0.080 |
| Delta Day 1–Day 10 | 22,484.14 ± 35,172.53 | 1734.94 ± 26,311.94 | 0.001 |
| Variables | Liposomal Inhalation | Physiologic Saline Inhalation | p-Value |
|---|---|---|---|
| Respiratory impairment Day 1 | 3.2 ± 1.2 | 3.1 ± 1.2 | 0.806 |
| Respiratory impairment Day 3 | 2.7 ± 1.2 | 3.2 ± 1.0 | 0.016 |
| Respiratory impairment Day 10 | 1.8 ± 1.6 | 2.4 ± 1.4 | 0.051 |
| Delta Day 1–Day 10 | 1.4 ± 1.8 | 0.7 ± 1.5 | 0.040 |
| Variables | Liposomal Inhalation | Physiologic Saline Inhalation | p-Value |
|---|---|---|---|
| White blood cell count (WBCC) Day 1 (103/µL) | 11.19 ± 3.79 | 11.36 ± 3.04 | 0.809 |
| WBCC Day 3 | 9.86 ± 3.12 | 10.11 ± 2.71 | 0.695 |
| WBCC Day 10 | 8.25 ± 2.57 | 8.48 ± 3.22 | 0.698 |
| WBCC Delta Day 1–Day 10 | 3.61 ± 4.38 | 3.51 ± 4.16 | 0.911 |
| CRP Day 1 (mg/dL) | 8.57 ± 5.13 | 13.00 ± 7.24 | 0.674 |
| CRP Day 3 | 13.00 ± 7.24 | 12.06 ± 5.91 | 0.508 |
| CRP Day 10 | 3.50 ± 3.16 | 4.48 ± 5.90 | 0.325 |
| CRP Delta Day 1–Day 10 | 5.27 ± 4.88 | 4.11 ± 7.40 | 0.357 |
| Variables | Liposomal Inhalation | Physiologic Saline Inhalation | p-Value |
|---|---|---|---|
| Subjective overall impairment Day 1 | 26.64 ± 8.81 | 23.33 ± 7.41 | 0.045 |
| Subjective overall impairment Day 3 | 24.67 ± 8.26 | 24.14 ± 9.02 | 0.759 |
| Subjective overall impairment Day 10 | 19.72 ± 9.66 | 23.04 ± 10.26 | 0.100 |
| Delta Day 1–Day 10 | 6.93 ± 12.18 | 0.29 ± 8.00 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofauer, B.; Straßen, U.; Chaker, A.; Schossow, B.; Wirth, M.; Wirth, M.; Bas, M.; Knopf, A. Liposomal Inhalation after Tracheostomy—A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 3312. https://doi.org/10.3390/jcm10153312
Hofauer B, Straßen U, Chaker A, Schossow B, Wirth M, Wirth M, Bas M, Knopf A. Liposomal Inhalation after Tracheostomy—A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(15):3312. https://doi.org/10.3390/jcm10153312
Chicago/Turabian StyleHofauer, Benedikt, Ulrich Straßen, Adam Chaker, Beate Schossow, Magdalena Wirth, Markus Wirth, Murat Bas, and Andreas Knopf. 2021. "Liposomal Inhalation after Tracheostomy—A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 15: 3312. https://doi.org/10.3390/jcm10153312
APA StyleHofauer, B., Straßen, U., Chaker, A., Schossow, B., Wirth, M., Wirth, M., Bas, M., & Knopf, A. (2021). Liposomal Inhalation after Tracheostomy—A Randomized Controlled Trial. Journal of Clinical Medicine, 10(15), 3312. https://doi.org/10.3390/jcm10153312






