Quantitative Breast Density in Contrast-Enhanced Mammography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
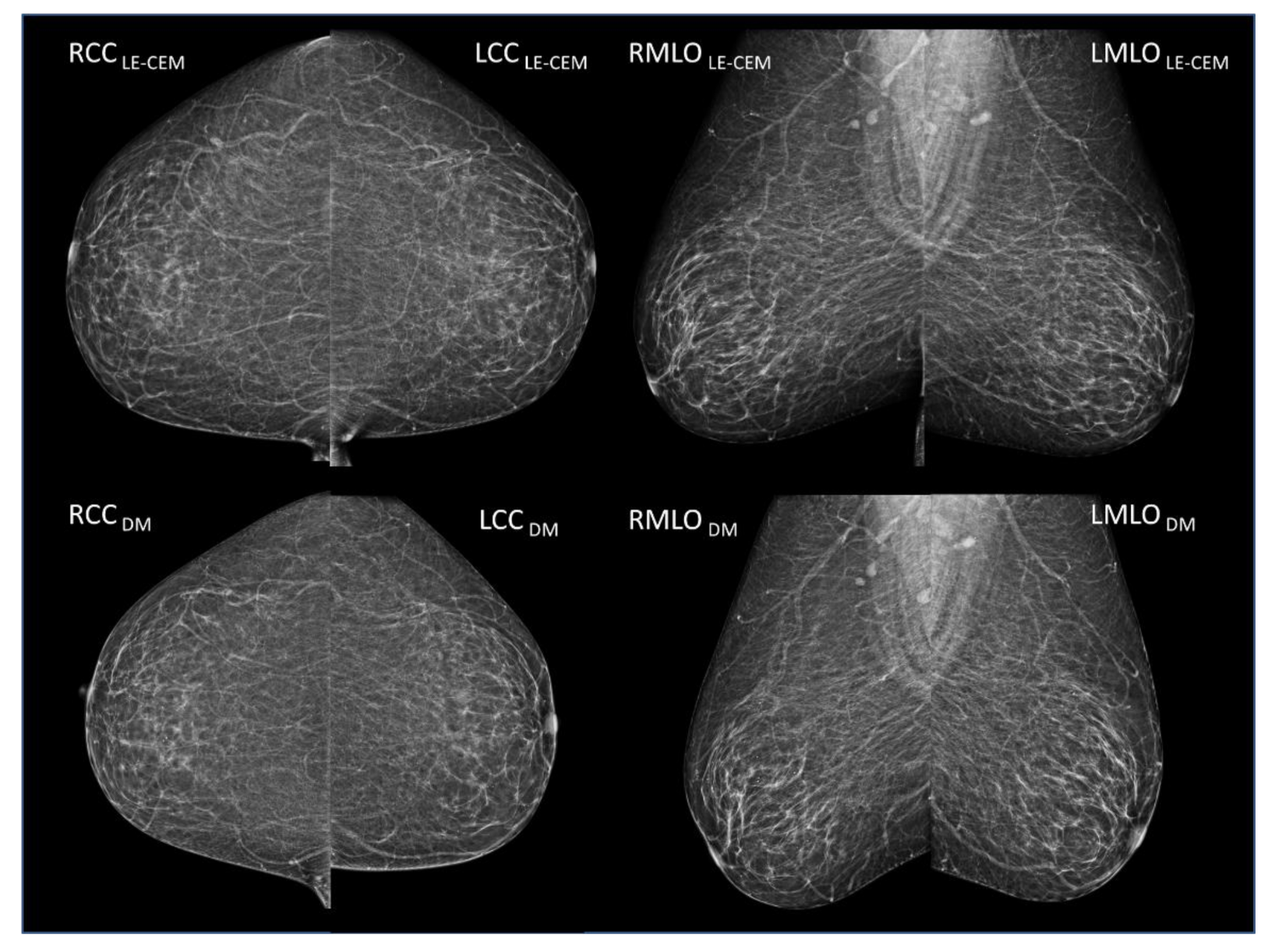
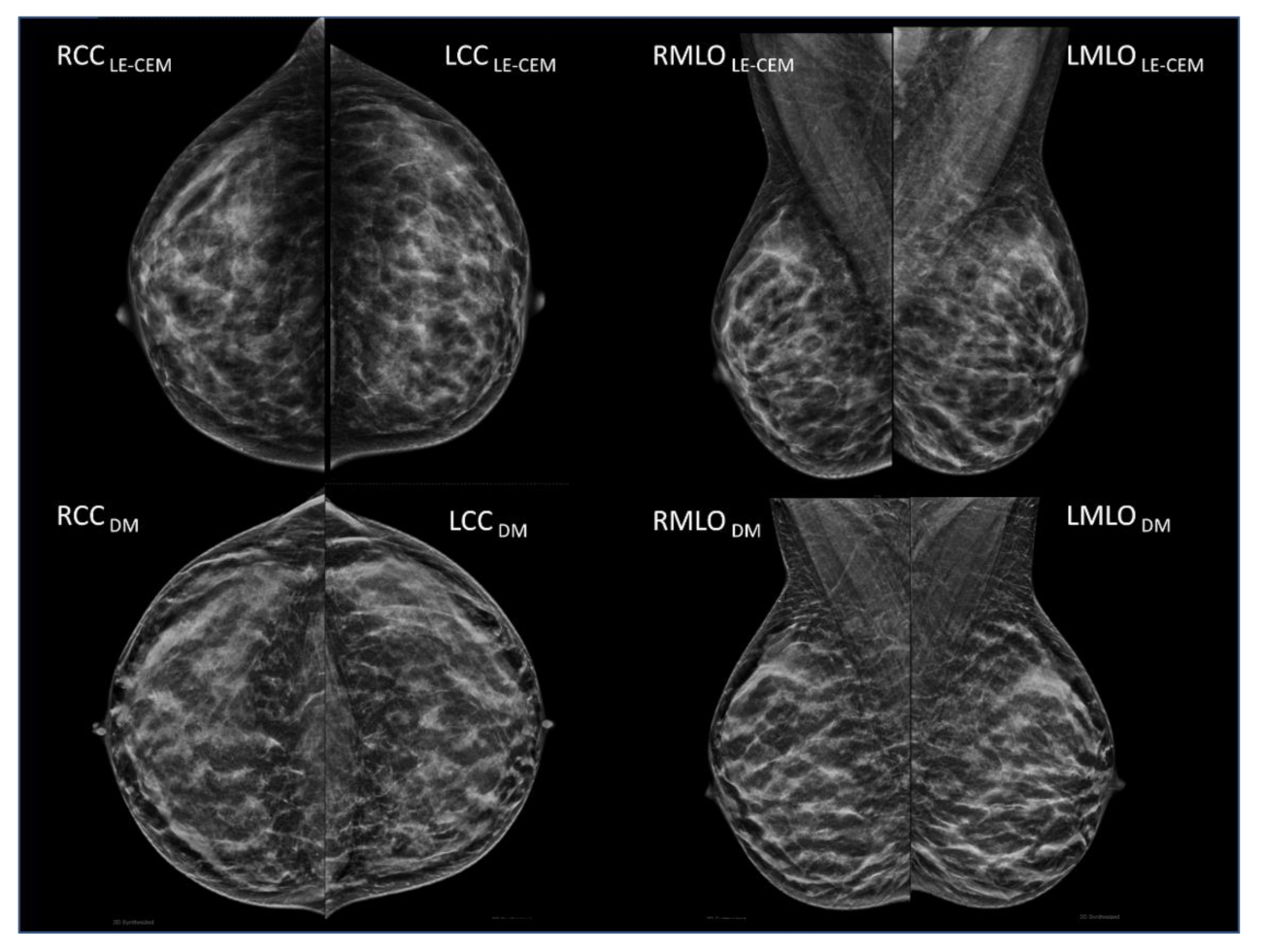
2.2. CEM and Mammography/Tomosynthesis Imaging
2.3. Breast Density
2.4. Statistical Analysis
3. Results
3.1. Study Population
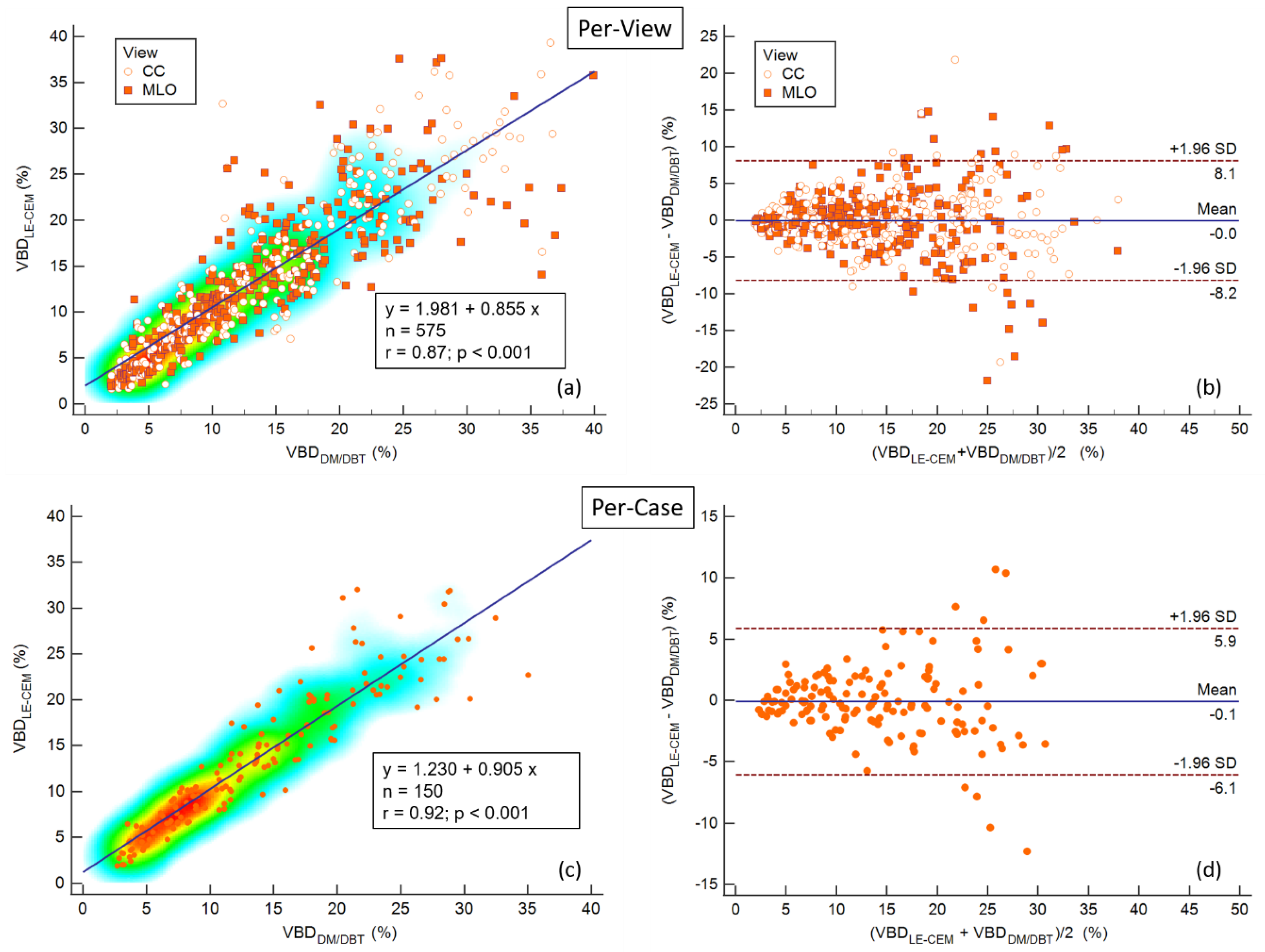
3.2. Volumetric Breast Density
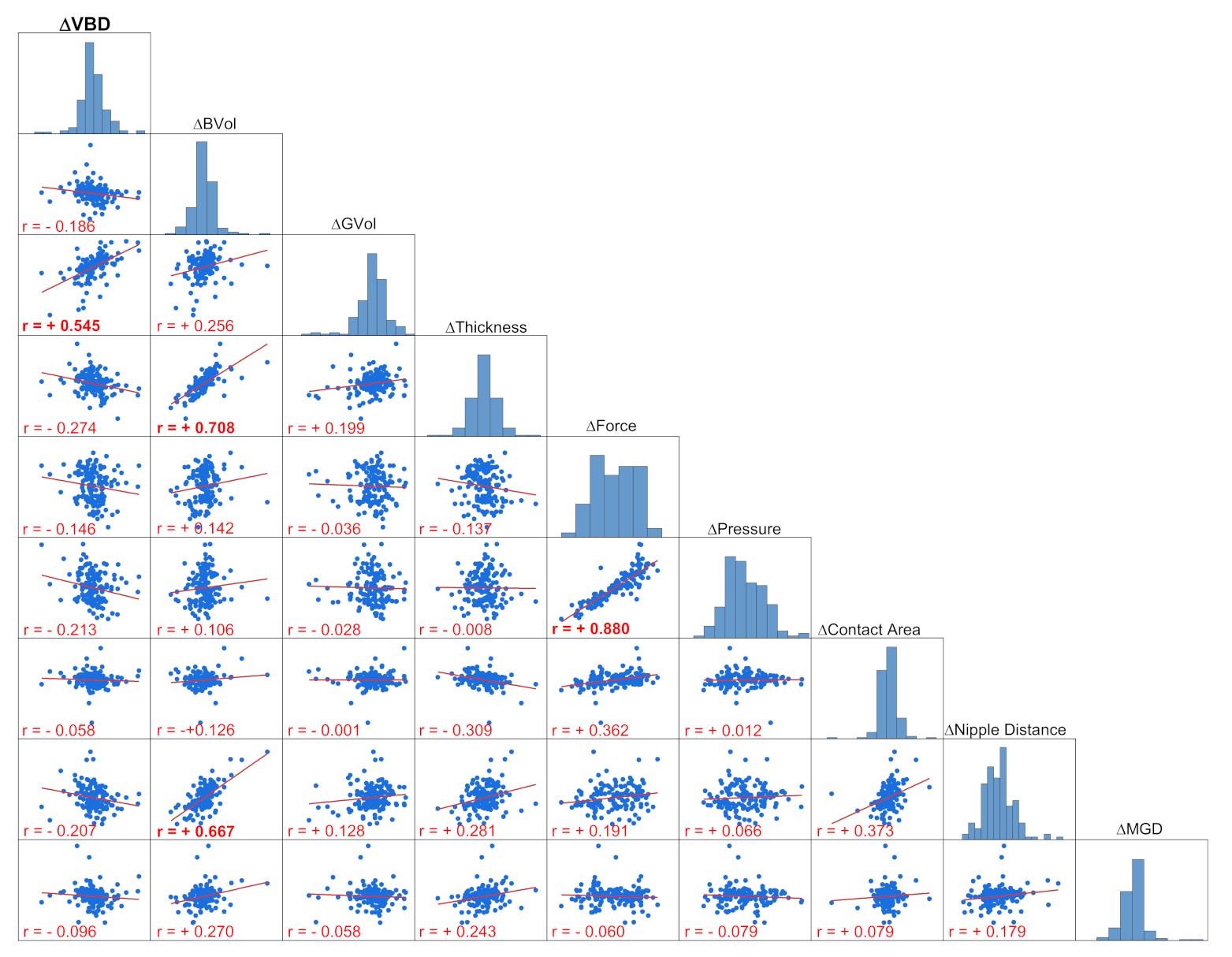
3.3. Multiple Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, N.; Warren, R. Mammographic Screening and Mammographic Patterns. Breast Cancer Res. 2000, 2, 247–251. [Google Scholar] [CrossRef][Green Version]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast Density as a Predictor of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic Density and Breast Cancer Risk: Current Understanding and Future Prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Soguel, L.; Durocher, F.; Tchernof, A.; Diorio, C. Adiposity, Breast Density, and Breast Cancer Risk: Epidemiological and Biological Considerations. Eur. J. Cancer Prev. 2017, 26, 511–520. [Google Scholar] [CrossRef]
- Posso, M.; Louro, J.; Sánchez, M.; Román, M.; Vidal, C.; Sala, M.; Baré, M.; Castells, X.; BELE Study Group. Mammographic Breast Density: How It Affects Performance Indicators in Screening Programmes? Eur. J. Radiol. 2019, 110, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Pfeiffer, R.M. Breast Cancer Risk Model Requirements for Counseling, Prevention, and Screening. J. Natl. Cancer Inst. 2018, 110, 994–1002. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A Comprehensive Breast Cancer Risk Prediction Model Incorporating Genetic and Nongenetic Risk Factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A Breast Cancer Prediction Model Incorporating Familial and Personal Risk Factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Brentnall, A.R.; Harkness, E.F.; Astley, S.M.; Donnelly, L.S.; Stavrinos, P.; Sampson, S.; Fox, L.; Sergeant, J.C.; Harvie, M.N.; Wilson, M.; et al. Mammographic Density Adds Accuracy to Both the Tyrer-Cuzick and Gail Breast Cancer Risk Models in a Prospective UK Screening Cohort. Breast Cancer Res. 2015, 17, 147. [Google Scholar] [CrossRef]
- Lee, C.I.; Chen, L.E.; Elmore, J.G. Risk-Based Breast Cancer Screening: Implications of Breast Density. Med. Clin. N. Am. 2017, 101, 725–741. [Google Scholar] [CrossRef]
- Destounis, S.V.; Santacroce, A.; Arieno, A. Update on Breast Density, Risk Estimation, and Supplemental Screening. AJR Am. J. Roentgenol. 2020, 214, 296–305. [Google Scholar] [CrossRef]
- Lian, J.; Li, K. A Review of Breast Density Implications and Breast Cancer Screening. Clin. Breast Cancer 2020, 20, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS Fifth Edition: A Summary of Changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Ciatto, S.; Houssami, N.; Apruzzese, A.; Bassetti, E.; Brancato, B.; Carozzi, F.; Catarzi, S.; Lamberini, M.P.; Marcelli, G.; Pellizzoni, R.; et al. Categorizing Breast Mammographic Density: Intra- and Interobserver Reproducibility of BI-RADS Density Categories. Breast 2005, 14, 269–275. [Google Scholar] [CrossRef]
- Sacchetto, D.; Morra, L.; Agliozzo, S.; Bernardi, D.; Björklund, T.; Brancato, B.; Bravetti, P.; Carbonaro, L.A.; Correale, L.; Fantò, C.; et al. Mammographic Density: Comparison of Visual Assessment with Fully Automatic Calculation on a Multivendor Dataset. Eur. Radiol. 2016, 26, 175–183. [Google Scholar] [CrossRef]
- Yaffe, M.J. Mammographic Density. Measurement of Mammographic Density. Breast Cancer Res. 2008, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Highnam, R.; Jeffreys, M.; McCormack, V.; Warren, R.; Davey Smith, G.; Brady, M. Comparing Measurements of Breast Density. Phys. Med. Biol. 2007, 52, 5881–5895. [Google Scholar] [CrossRef]
- Keller, B.M.; Chen, J.; Daye, D.; Conant, E.F.; Kontos, D. Preliminary Evaluation of the Publicly Available Laboratory for Breast Radiodensity Assessment (LIBRA) Software Tool: Comparison of Fully Automated Area and Volumetric Density Measures in a Case-Control Study with Digital Mammography. Breast Cancer Res. 2015, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Gastounioti, A.; Kasi, C.D.; Scott, C.G.; Brandt, K.R.; Jensen, M.R.; Hruska, C.B.; Wu, F.F.; Norman, A.D.; Conant, E.F.; Winham, S.J.; et al. Evaluation of LIBRA Software for Fully Automated Mammographic Density Assessment in Breast Cancer Risk Prediction. Radiology 2020, 296, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, E.U.; McEntee, M.F. Measurement of Breast Density with Digital Breast Tomosynthesis—A Systematic Review. Br. J. Radiol. 2014, 87, 20140460. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.M.; Fogelman, I. Technical Principles of Dual Energy X-Ray Absorptiometry. Semin. Nucl. Med. 1997, 27, 210–228. [Google Scholar] [CrossRef]
- Manji, F.; Wang, J.; Norman, G.; Wang, Z.; Koff, D. Comparison of Dual Energy Subtraction Chest Radiography and Traditional Chest X-Rays in the Detection of Pulmonary Nodules. Quant. Imaging Med. Surg. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Skarpathiotakis, M.; Yaffe, M.J.; Bloomquist, A.K.; Rico, D.; Muller, S.; Rick, A.; Jeunehomme, F. Development of Contrast Digital Mammography. Med. Phys. 2002, 29, 2419–2426. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Smidt, M.L.; Houwers, J.; Tjan-Heijnen, V.C.; Wildberger, J.E. Contrast Enhanced Mammography: Techniques, Current Results, and Potential Indications. Clin. Radiol. 2013, 68, 935–944. [Google Scholar] [CrossRef]
- Patel, B.K.; Lobbes, M.B.I.; Lewin, J. Contrast Enhanced Spectral Mammography: A Review. Semin. Ultrasound CT MR 2018, 39, 70–79. [Google Scholar] [CrossRef]
- Lalji, U.; Lobbes, M. Contrast-Enhanced Dual-Energy Mammography: A Promising New Imaging Tool in Breast Cancer Detection. Women’s Health 2014, 10, 289–298. [Google Scholar] [CrossRef]
- Gilbert, F.J.; Pinker-Domenig, K. Diagnosis and Staging of Breast Cancer: When and How to Use Mammography, Tomosynthesis, Ultrasound, Contrast-Enhanced Mammography, and Magnetic Resonance Imaging. In Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2019; ISBN 9783030111489. [Google Scholar]
- Lobbes, M.B.I.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-Enhanced Spectral Mammography in Patients Referred from the Breast Cancer Screening Programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef]
- Houben, I.P.L.; Van de Voorde, P.; Jeukens, C.R.L.P.N.; Wildberger, J.E.; Kooreman, L.F.; Smidt, M.L.; Lobbes, M.B.I. Contrast-Enhanced Spectral Mammography as Work-up Tool in Patients Recalled from Breast Cancer Screening Has Low Risks and Might Hold Clinical Benefits. Eur. J. Radiol. 2017, 94, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic Accuracy of Contrast-Enhanced Spectral Mammography in Comparison to Conventional Full-Field Digital Mammography in a Population of Women with Dense Breasts. Breast Cancer 2017, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef]
- Sung, J.S.; Lebron, L.; Keating, D.; D’Alessio, D.; Comstock, C.E.; Lee, C.H.; Pike, M.C.; Ayhan, M.; Moskowitz, C.S.; Morris, E.A.; et al. Performance of Dual-Energy Contrast-Enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019, 293, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Himes, D.O.; Root, A.E.; Gammon, A.; Luthy, K.E. Breast Cancer Risk Assessment: Calculating Lifetime Risk Using the Tyrer-Cuzick Model. J. Nurse Pract. 2016, 12, 581–592. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer; National Institute for Health and Care Excellence: London, UK, 2020; ISBN 9781473101609. [Google Scholar]
- Branderhorst, W.; de Groot, J.E.; van Lier, M.G.J.T.B.; Highnam, R.P.; den Heeten, G.J.; Grimbergen, C.A. Technical Note: Validation of Two Methods to Determine Contact Area between Breast and Compression Paddle in Mammography. Med. Phys. 2017, 44, 4040–4044. [Google Scholar] [CrossRef] [PubMed]
- Waade, G.G.; Danielsen, A.S.; Holen, Å.S.; Larsen, M.; Hanestad, B.; Hopland, N.-M.; Kalcheva, V.; Hofvind, S. Assessment of Breast Positioning Criteria in Mammographic Screening: Agreement between Artificial Intelligence Software and Radiographers. J. Med. Screen. 2021, 969141321998718. [Google Scholar] [CrossRef]
- Dance, D.R.; Skinner, C.L.; Young, K.C.; Beckett, J.R.; Kotre, C.J. Additional Factors for the Estimation of Mean Glandular Breast Dose Using the UK Mammography Dosimetry Protocol. Phys. Med. Biol. 2000, 45, 3225–3240. [Google Scholar] [CrossRef]
- Dance, D.R.; Young, K.C.; van Engen, R.E. Further Factors for the Estimation of Mean Glandular Dose Using the United Kingdom, European and IAEA Breast Dosimetry Protocols. Phys. Med. Biol. 2009, 54, 4361–4372. [Google Scholar] [CrossRef] [PubMed]
- Dance, D.R.; Young, K.C. Estimation of Mean Glandular Dose for Contrast Enhanced Digital Mammography: Factors for Use with the UK, European and IAEA Breast Dosimetry Protocols. Phys. Med. Biol. 2014, 59, 2127–2137. [Google Scholar] [CrossRef]
- Sogani, J.; Morris, E.A.; Kaplan, J.B.; D’Alessio, D.; Goldman, D.; Moskowitz, C.S.; Jochelson, M.S. Comparison of Background Parenchymal Enhancement at Contrast-Enhanced Spectral Mammography and Breast MR Imaging. Radiology 2017, 282, 63–73. [Google Scholar] [CrossRef]
- Lalji, U.C.; Jeukens, C.R.L.P.N.; Houben, I.; Nelemans, P.J.; van Engen, R.E.; van Wylick, E.; Beets-Tan, R.G.H.; Wildberger, J.E.; Paulis, L.E.; Lobbes, M.B.I. Evaluation of Low-Energy Contrast-Enhanced Spectral Mammography Images by Comparing Them to Full-Field Digital Mammography Using EUREF Image Quality Criteria. Eur. Radiol. 2015, 25, 2813–2820. [Google Scholar] [CrossRef]
- Alonzo-Proulx, O.; Mawdsley, G.E.; Patrie, J.T.; Yaffe, M.J.; Harvey, J.A. Reliability of Automated Breast Density Measurements. Radiology 2015, 275, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Engmann, N.J.; Scott, C.G.; Jensen, M.R.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A.P.; Malkov, S.; Whaley, D.H.; Hruska, C.B.; Wu, F.F.; et al. Longitudinal Changes in Volumetric Breast Density with Tamoxifen and Aromatase Inhibitors. Cancer Epidemiol. Biomark. Prev. 2017, 26, 930–937. [Google Scholar] [CrossRef]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast-Enhanced Spectral Mammography in Neoadjuvant Chemotherapy Monitoring: A Comparison with Breast Magnetic Resonance Imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef]
- Brentnall, A.R.; Warren, R.; Harkness, E.F.; Astley, S.M.; Wiseman, J.; Fox, J.; Fox, L.; Eriksson, M.; Hall, P.; Cuzick, J.; et al. Mammographic Density Change in a Cohort of Premenopausal Women Receiving Tamoxifen for Breast Cancer Prevention over 5 Years. Breast Cancer Res. 2020, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Nye, L.; Powers, K.R.; Nydegger, J.L.; Kreutzjans, A.L.; Phillips, T.A.; Metheny, T.; Winblad, O.; Zalles, C.M.; Hagan, C.R.; et al. Effect of Bazedoxifene and Conjugated Estrogen (Duavee) on Breast Cancer Risk Biomarkers in High-Risk Women: A Pilot Study. Cancer Prev. Res. 2019, 12, 711–720. [Google Scholar] [CrossRef]
- Manni, A.; Richie, J.P.; Schetter, S.E.; Calcagnotto, A.; Trushin, N.; Aliaga, C.; El-Bayoumy, K. Stearoyl-CoA Desaturase-1, a Novel Target of Omega-3 Fatty Acids for Reducing Breast Cancer Risk in Obese Postmenopausal Women. Eur. J. Clin. Nutr. 2017, 71, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Lecler, A.; Dunant, A.; Delaloge, S.; Wehrer, D.; Moussa, T.; Caron, O.; Balleyguier, C. Breast Tissue Density Change after Oophorectomy in BRCA Mutation Carrier Patients Using Visual and Volumetric Analysis. Br. J. Radiol. 2018, 91, 20170163. [Google Scholar] [CrossRef] [PubMed]
- Masala, G.; Assedi, M.; Sera, F.; Ermini, I.; Occhini, D.; Castaldo, M.; Pierpaoli, E.; Caini, S.; Bendinelli, B.; Ambrogetti, D.; et al. Can Dietary and Physical Activity Modifications Reduce Breast Density in Postmenopausal Women? The DAMA Study, a Randomized Intervention Trial in Italy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 41–50. [Google Scholar] [CrossRef]
- Vohra, N.A.; Kachare, S.D.; Vos, P.; Schroeder, B.F.; Schuth, O.; Suttle, D.; Fitzgerald, T.L.; Wong, J.H.; Verbanac, K.M. The Short-Term Effect of Weight Loss Surgery on Volumetric Breast Density and Fibroglandular Volume. Obes. Surg. 2017, 27, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Shia, W.-C.; Wu, H.-K.; Huang, Y.-L.; Lin, L.-S.; Chen, D.-R. Mammographic Density Distribution of Healthy Taiwanese Women and Its Naturally Decreasing Trend with Age. Sci. Rep. 2018, 8, 14937. [Google Scholar] [CrossRef] [PubMed]
- Broeders, M.J.M.; Ten Voorde, M.; Veldkamp, W.J.H.; van Engen, R.E.; van Landsveld-Verhoeven, C.; ’t Jong-Gunneman, M.N.L.; de Win, J.; Greve, K.D.; Paap, E.; den Heeten, G.J. Comparison of a Flexible versus a Rigid Breast Compression Paddle: Pain Experience, Projected Breast Area, Radiation Dose and Technical Image Quality. Eur. Radiol. 2015, 25, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Maki, A.K.; Mainprize, J.G.; Pisano, E.D.; Mawdsley, G.E.; Skarpathiotakis, M.; Yaffe, M.J. Technical Note: Volumetric Coverage in Breast Tomosynthesis Images—Phantom QC Results from the TMIST Study. Med. Phys. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Ng, K.H.; Abdul Aziz, Y.F. Volumetric Breast Density Measurement: Sensitivity Analysis of a Relative Physics Approach. Br. J. Radiol. 2016, 89, 20160258. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Subgroup | Values |
|---|---|---|
| Number of women | / | 150 |
| Age (years) | mean ± SD median range | 51.0 ± 8.8 51 (35–76) |
| Menopausal status | Premenopausal Perimenopausal Postmenopausal | 68 (45.33%) 14 (9.33%) 68 (45.33%) |
| Risk category | BRCA1 1 BRCA2 2 HIGH 3 INTERMEDIATE 4 | 30 (20.00%) 36 (24.00%) 65 (41.33%) 22 (14.67%) |
| BI-RADS breast density 5 | Predominantly fatty (A) Scattered fibroglandular (B) Heterogeneously dense (C) Extremely dense (D) | 9 (6.00%) 27 (18.00%) 55 (36.67%) 59 (39.33%) |
| Background parenchymal enhancement (BPE) | Minimal Mild Moderate Marked | 65 (43.33%) 44 (29.33%) 38 (25.33%) 3 (2.00%) |
| Previous/subsequent exam | Mammography Tomosynthesis | 120 (80.00%) 30 (20.00%) |
| Variable | LE-CEM Median | DM/DBT Median | Hodges–Lehmann Median Difference | 95% CI | p-Value |
|---|---|---|---|---|---|
| VBD (%) | 12.73 | 12.39 | 0.075 | −0.19 to 0.34 | 0.5855 |
| Breast volume (cm2) | 508.15 | 534.57 | 25.58 | 18.94 to 32.68 | <0.0001 |
| Glandular volume (cm2) | 55.90 | 59.44 | 3.315 | 1.975 to 4.660 | <0.0001 |
| Breast thickness (mm) | 47.7 | 50.0 | 2.35 | 1.90 to 2.80 | <0.0001 |
| Compression force (N) | 102 | 85 | −15.0 | −17.5 to −12.0 | <0.0001 |
| Compression pressure (kPa) | 11.83 | 10.46 | 1.655 | −1.995 to 1.310 | <0.0001 |
| Contact area (mm2) | 8529.46 | 8311.63 | −144.14 | −222.61 to 68.77 | 0.0003 |
| Nipple distance from posterior edge (mm) | 88.40 | 88.80 | 0.60 | 0.10 to 1.15 | 0.00180 |
| Mean glandular dose (mGy) | 1.544 | 1.527 | −0.043 | −0.074 to −0.012 | 0.0083 |
| Independent Variable Difference | Regression Coefficient | p-Value |
|---|---|---|
| (Constant) | 0.1741 | |
| Breast volume (cm3) | 0.005017 | 0.1311 |
| Glandular volume (cm3) | 0.1174 | <0.0001 |
| Breast thickness (mm) | −0.2748 | <0.0001 |
| Compression force (N) | 0.05008 | 0.0036 |
| Compression pressure (kPa) | −0.5031 | 0.0001 |
| Contact area (mm2) | −0.0009143 | <0.0001 |
| Nipple-to-posterior-edge distance (mm) | −0.1453 | 0.0011 |
| Mean glandular dose (mGy) | −0.1373 | 0.6684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gennaro, G.; Hill, M.L.; Bezzon, E.; Caumo, F. Quantitative Breast Density in Contrast-Enhanced Mammography. J. Clin. Med. 2021, 10, 3309. https://doi.org/10.3390/jcm10153309
Gennaro G, Hill ML, Bezzon E, Caumo F. Quantitative Breast Density in Contrast-Enhanced Mammography. Journal of Clinical Medicine. 2021; 10(15):3309. https://doi.org/10.3390/jcm10153309
Chicago/Turabian StyleGennaro, Gisella, Melissa L. Hill, Elisabetta Bezzon, and Francesca Caumo. 2021. "Quantitative Breast Density in Contrast-Enhanced Mammography" Journal of Clinical Medicine 10, no. 15: 3309. https://doi.org/10.3390/jcm10153309
APA StyleGennaro, G., Hill, M. L., Bezzon, E., & Caumo, F. (2021). Quantitative Breast Density in Contrast-Enhanced Mammography. Journal of Clinical Medicine, 10(15), 3309. https://doi.org/10.3390/jcm10153309






