Association of Low Alanine Aminotransferase Values with Extubation Failure in Adult Critically Ill Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Participants
2.3. Outcome Measures and Variables
2.4. Statistical Analysis
3. Results
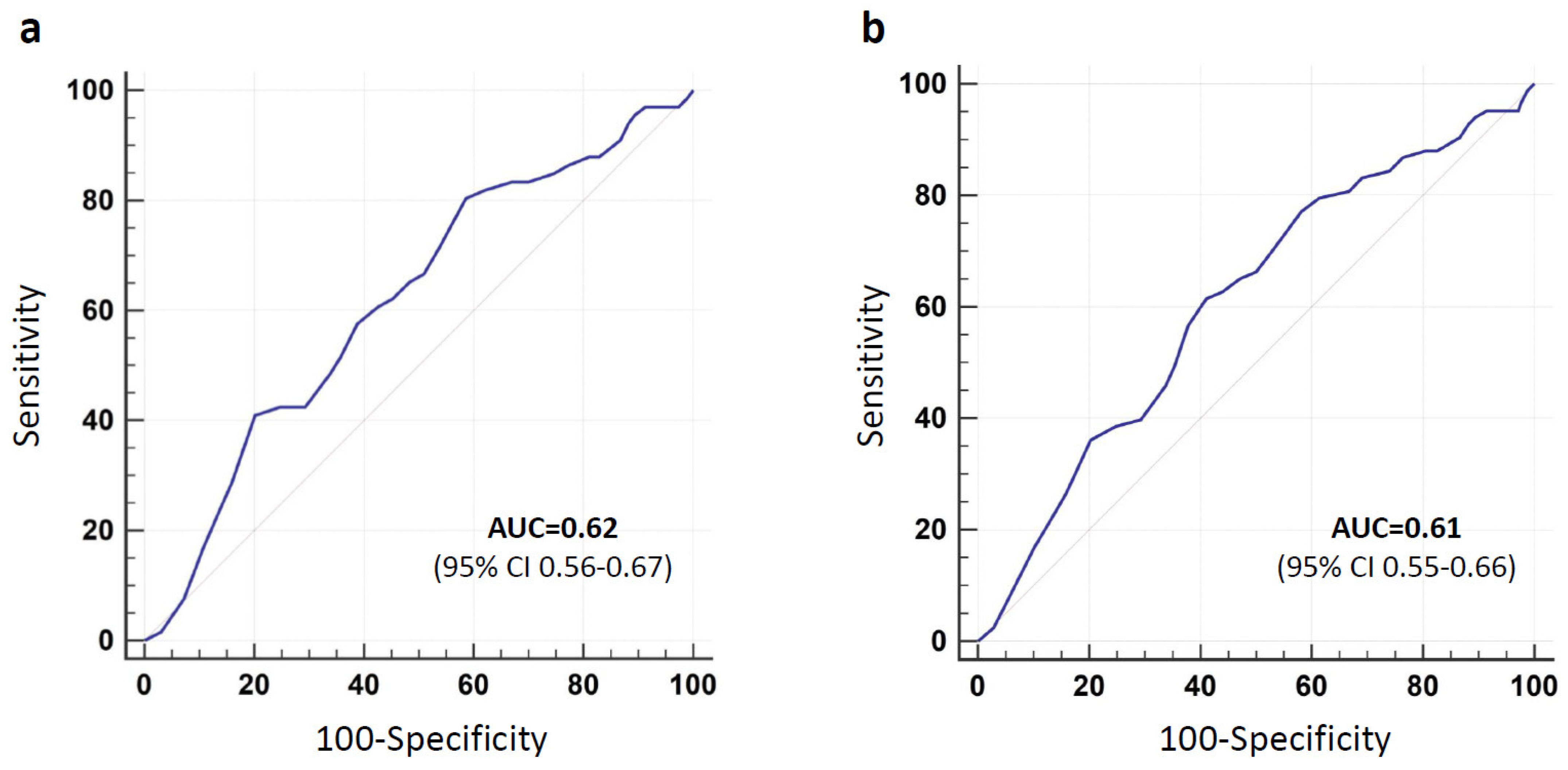
3.1. Short-Term (48 h) Extubation Failure
3.2. Long-Term (7 Days) Extubation Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.C.; Tseng, K.L.; Ho, C.H.; Chiang, S.R.; Chen, C.M.; Chan, K.S.; Chao, C.M.; Hsing, S.C.; Cheng, K.C. Prognosis of patients with acute respiratory failure and prolonged intensive care unit stay. J. Thorac. Dis. 2019, 11, 2051–2057. [Google Scholar] [CrossRef]
- Slutsky, A.S. History of Mechanical Ventilation. From Vesalius to Ventilator-induced Lung Injury. Am. J. Respir. Crit. Care Med. 2015, 191, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Cortés-Puch, I.; Esteban, A. Weaning from the ventilator and extubation in ICU. Curr. Opin. Crit. Care 2013, 19, 57–64. [Google Scholar] [CrossRef]
- Jeganathan, N.; Kaplan, C.A.; Balk, R.A. Ventilator liberation for high-risk-for-failure patients: Improving value of the spontaneous breathing trial. Respir. Care 2015, 60, 290–296. [Google Scholar] [CrossRef]
- Thille, A.W.; Richard, J.C.M.; Brochard, L. The decision to extubate in the intensive care unit. Am. J. Respir. Crit. Care Med. 2013, 187, 1294–1302. [Google Scholar] [CrossRef]
- Thille, A.W.; Muller, G.; Gacouin, A.; Coudroy, R.; Decavèle, M.; Sonneville, R.; Beloncle, F.; Girault, C.; Dangers, L.; Lautrette, A.; et al. Effect of Postextubation High-Flow Nasal Oxygen with Noninvasive Ventilation vs High-Flow Nasal Oxygen Alone on Reintubation among Patients at High Risk of Extubation Failure: A Randomized Clinical Trial. JAMA 2019, 322, 1465–1475. [Google Scholar] [CrossRef]
- Yeung, J.; Couper, K.; Ryan, E.G.; Gates, S.; Hart, N.; Perkins, G.D. Non-invasive ventilation as a strategy for weaning from invasive mechanical ventilation: A systematic review and Bayesian meta-analysis. Intensive Care Med. 2018, 44, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.W.; Yeh, C.H.; Tsai, H.; Hsu, C.C.; Hsieh, Y.C.; Chen, W.T.; Cheng, H.T.; Yu, M.C.; Lee, C.W. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS ONE 2019, 14, e0220699. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.Y.; Oh, S.Y.; Lee, H.; Ryu, H.G. Evaluation of the association between decreased skeletal muscle mass and extubation failure after long-term mechanical ventilation. Clin. Nutr. 2020, 39, 2764–2770. [Google Scholar] [CrossRef]
- Kizilarslanoglu, M.C.; Kuyumcu, M.E.; Yesil, Y.; Halil, M. Sarcopenia in critically ill patients. J. Anesth. 2016, 30, 884–890. [Google Scholar] [CrossRef]
- Looijaard, W.G.P.M.; Stapel, S.N.; Dekker, I.M.; Rusticus, H.; Remmelzwaal, S.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Identifying critically ill patients with low muscle mass: Agreement between bioelectrical impedance analysis and computed tomography. Clin. Nutr. 2019, 39. [Google Scholar] [CrossRef]
- Lasman, N.; Shalom, M.; Turpashvili, N.; Goldhaber, G.; Lifshitz, Y.; Leibowitz, E.; Berger, G.; Saltzman-Shenhav, G.; Brom, A.; Cohen, D.; et al. Baseline low ALT activity is associated with increased long-term mortality after COPD exacerbations. BMC Pulm. Med. 2020, 20, 133. [Google Scholar] [CrossRef]
- Portal, D.; Hofstetter, L.; Eshed, I.; Dan Lantsman, C.; Sella, T.; Urban, D.; Onn, A.; Bar, J.; Segal, G. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag. Res. 2019, 11, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Vespasiani-Gentilucci, U.; de Vincentis, A.; Ferrucci, L.; Bandinelli, S.; Antonelli Incalzi, R.; Picardi, A. Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.; Klempfner, R.; Lotan, D.; Wasserstrum, Y.; Goldenberg, I.; Segal, G. Low ALT blood levels are associated with lower baseline fitness amongst participants of a cardiac rehabilitation program. J. Exerc. Sci. Fit. 2018, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Itelman, E.; Segev, A.; Ahmead, L.; Leibowitz, E.; Agbaria, M.; Avaky, C.; Negro, L.; Shenhav-Saltzman, G.; Wasserstrum, Y.; Segal, G. Low ALT Values amongst Hospitalized Patients are Associated with Increased Risk of Hypoglycemia and Overall Mortality. A Retrospective, Big-data Analysis of 51,831 Patients. QJM 2020. [Google Scholar] [CrossRef]
- Reiner Benaim, A.; Almog, R.; Gorelik, Y.; Hochberg, I.; Nassar, L.; Mashiach, T.; Khamaisi, M.; Lurie, Y.; Azzam, Z.S.; Khoury, J.; et al. Analyzing Medical Research Results Based on Synthetic Data and Their Relation to Real Data Results: Systematic Comparison From Five Observational Studies. JMIR Med. Inform. 2020, 8, e16492. [Google Scholar] [CrossRef]
- Hochberg, I. Insulin Detemir Use Is Associated with Higher Occurrence of Hypoglycemia in Hospitalized Patients with Hypoalbuminemia. Diabetes Care 2018, 41, e44–e46. [Google Scholar] [CrossRef]
- Gorelik, Y.; Yaseen, H.; Heyman, S.N.; Khamaisi, M. Negligible Risk of Acute Renal Failure among Hospitalized Patients after Contrast-Enhanced Imaging with Iodinated versus Gadolinium-Based Agents. Investig. Radiol. 2019, 54, 312–318. [Google Scholar] [CrossRef]
- Even Dar, R.; Kurnik, D.; Bishop, B.; Bogner, I.; Azzam, Z.; Paul, M.; Neuberger, A. Are corticosteroids or end-stage renal failure associated with afebrile presentation of Gram-negative bacteremia? Int. J. Antimicrob. Agents 2020, 56, 106070. [Google Scholar] [CrossRef]
- Dagan, A.; Sella, T.; Urban, D.; Bar, Y.; Onn, A.; Segal, G. Low alanine transaminase is not associated with increased rate of mortality in patients with advanced lung cancer. JCSM Clin. Rep. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Epstein, S.K.; Ciubotaru, R.L.; Wong, J.B. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997, 112, 186–192. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Agarwal, V. Extubation failure in intensive care unit: Predictors and management. Indian J. Crit. Care Med. 2008, 12, 1. [Google Scholar] [CrossRef]
- Thille, A.; Harrois, A.; Schortgen, F.; Brun-Buisson, C.; Brochard, L. Outcomes of extubation failure in medical intensive care unit patients. Crit. Care Med. 2011, 39, 2612–2618. [Google Scholar] [CrossRef]
- Thille, A.W.; Boissier, F.; Ben-Ghezala, H.; Razazi, K.; Mekontso-Dessap, A.; Brun-Buisson, C.; Brochard, L. Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: A prospective before-after study. Crit. Care 2016, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- de Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis acquired in the intensive care unit: A prospective multicenter study. J. Am. Med. Assoc. 2002, 288, 2859–2867. [Google Scholar] [CrossRef]
- Khalil, Y.; Mustafa, E.E.D.; Youssef, A.; Imam, M.H.; Behiry, A.F. El Neuromuscular dysfunction associated with delayed weaning from mechanical ventilation in patients with respiratory failure. Alex. J. Med. 2012, 48, 223–232. [Google Scholar] [CrossRef][Green Version]
- Hafner, S.; Radermacher, P.; Frick, M.; Dietl, P.; Calzia, E. Hyperglycemia, oxidative stress, and the diaphragm: A link between chronic co-morbidity and acute stress? Crit. Care 2014, 18, 149. [Google Scholar] [CrossRef]
- Parsons, E.C.; Kross, E.K.; Ali, N.A.; Vandevusse, L.K.; Caldwell, E.S.; Watkins, T.R.; Heckbert, S.R.; Hough, C.L. Red blood cell transfusion is associated with decreased in-hospital muscle strength among critically ill patients requiring mechanical ventilation. J. Crit. Care 2013, 28, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Janssen, I. The epidemiology of sarcopenia. Clin. Geriatr. Med. 2011, 27, 355–363. [Google Scholar] [CrossRef]
- van Mook, W.N.K.A.; Hulsewé-Evers, R.P.M.G. Critical illness polyneuropathy. Curr. Opin. Crit. Care 2002, 8, 302–310. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. Low alanine aminotransferase levels predict low muscle strength in older patients with diabetes: A nationwide cross-sectional study in Korea. Geriatr. Gerontol. Int. 2020, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]


| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Age, years (IQR) | 62.4 (48.1–71.2) | |
| Elderly (≥75 years), n (%) | 56 (17%) | |
| Male gender, n (%) | 205 (62.3%) | |
| Body mass index, kg/m2 (IQR) | 26.85 (23.5–31) | |
| Comorbidities | ||
| Diabetes Mellitus, n (%) | 113 (34.4%) | |
| Chronic Kidney Disease, n (%) | 54 (16.4%) | |
| Congestive Heart Failure, n (%) | 89 (27.1%) | |
| Cerebrovascular disease, n (%) | 52 (15.8%) | |
| COPD, n (%) | 75 (22.8%) | |
| Hypertension, n (%) | 171 (52%) | |
| Ischemic Heart Disease, n (%) | 74 (22.5%) | |
| Indication for mechanical ventilation | ||
| Neurologic disorders, n (%) | 88 (26.7%) | |
| Sepsis, n (%) | 60 (18.2%) | |
| Pneumonia, n (%) | 43 (13.1%) | |
| Acute exacerbation of COPD, n (%) | 35 (10.6%) | |
| Cardiac disorders, n (%) | 29 (8.8%) | |
| Shock (non-septic), n (%) | 12 (3.6%) | |
| Airway disorder, n (%) | 8 (2.4%) | |
| Asthma, n (%) | 5 (1.5%) | |
| Other indication, n (%) | 49 (14.9%) | |
| Admission APACHE-II score (SD) * | 27.8 (6.5) | |
| Medications | ||
| Corticosteroids (during 7 days before extubation), n (%) | 113 (34.4%) | |
| Muscle relaxants (during index admission), n (%) | 60 (18.2%) | |
| Vasopressors (during 24 h before extubation), n (%) | 59 (17.9%) | |
| Length of ICU stay before first extubation trial, days (IQR) | 3.81 (1.64–7.47) | |
| Duration of mechanical ventilation, days (IQR) | 2.86 (1.19–5.87) | |
| High risk of extubation failure, n (%) | 210 (63.8%) | |
| Very high risk of extubation failure, n (%) | 144 (43.8%) | |
| Laboratory values (last value before extubation) | ||
| Albumin, g/dL (IQR) | 2.8 (2.48–3.2) | |
| Creatinine, mg/dL (IQR) | 0.78 (0.62–1.27) | |
| Blood urea nitrogen, mg/dL (IQR) | 20 (13–32.6) | |
| Hemoglobin, g/dL (IQR) | 10.2 (8.7–11.9) | |
| Sodium, mEq/L (IQR) | 140 (138–143) | |
| Potassium, mmol/L (IQR) | 3.8 (3.5–4.1) | |
| Phosphorus, mg/dL (IQR) | 3.25 (2.49–4.1) | |
| Magnesium, mg/dL (IQR) | 1.99 (1.76–2.1) | |
| Brain natriuretic peptide, pg/mL (IQR) ** | 1296 (396–3711) | |
| pH | 7.41 (7.36–7.47) | |
| pO2, mmHg (IQR) | 103 (82–135) | |
| pCO2, mmHg (IQR) | 46 (39–53) | |
| Bicarbonate, mmol/L (IQR) | 29.3 (24.9–35.1) | |
| Lactate, mmol/L (IQR) | 1.2 (0.9–1.7) | |
| Ionized calcium, mmol/L (IQR) | 1.1 (1.04–1.14) | |
| Hyperglycemia (>180 mg/dL) during 48 h preceding extubation trial, n (%) | 198 (60.2%) | |
| Vital signs (last value before extubation) | ||
| Heart rate, bpm (IQR) | 91 (80–107) | |
| Systolic blood pressure, mmHg (IQR) | 139 (121–157) | |
| Diastolic blood pressure, mmHg (IQR) | 66 (57–75) | |
| Core Temperature, °C (IQR) | 36.8 (36.3–37.4) | |
| Oxygen saturation, % (IQR) | 98 (96–100) | |
| Fraction of inspired oxygen, % (IQR) | 40 (35–40) | |
| Muscle related laboratory values (lowest value over the preceding 10 days) | ||
| Alanine transaminase, IU/L (IQR) | 18 (11–25) | |
| Creatine phosphokinase, U/L (IQR) *** | 132 (51–422) | |
| 30-day mortality (after extubation attempt), n (%) | 28 (8.5%) | |
| Short-Term (48 h) Extubation Outcome | Long-Term (7 Days) Extubation Outcome | ||||
|---|---|---|---|---|---|
| Failure (n = 66) | Success (n = 263) | Failure (n = 83) | Success (n = 246) | ||
| Age, years (IQR) | 63.6 (54.3–75.4) | 62.3 (46.3–71) | 65.7 (55–76.2) | 62.1 (45.7–70) | |
| Male gender, n (%) | 34 (51.5%) | 171 (65%) | 45 (54.2%) | 160 (65%) | |
| Body mass index, kg/m2 (IQR) | 27.3 (24.2–29.3) | 26.7 (23.4–31.2) | 27.5 (24.2–30.9) | 26.2 (23.4–31.03) | |
| Comorbidities | |||||
| Diabetes Mellitus, n (%) | 26 (39.4%) | 87 (33.1%) | 35 (42.2%) | 78 (31.7%) | |
| Chronic Kidney Disease, n (%) | 10 (15.2%) | 44 (16.7%) | 14 (16.9%) | 40 (16.3%) | |
| Congestive Heart Failure, n (%) | 20 (30.3%) | 69 (26.2%) | 26 (31.3%) | 63 (25.6%) | |
| Cerebrovascular disease, n (%) | 12 (18.2%) | 40 (15.2%) | 15 (18.1%) | 37 (15%) | |
| COPD, n (%) | 10 (15.2%) | 65 (24.7%) | 14 (16.9%) | 61 (24.1%) | |
| Hypertension, n (%) | 34 (51.5%) | 137 (52.1%) | 48 (57.3%) | 123 (50%) | |
| Ischemic Heart Disease, n (%) | 16 (24.2%) | 58 (22.1%) | 26 (31.3%) | 48 (19.5%) | |
| Admission APACHE-II score (SD) * | 28 (23–33) | 28 (23–32) | 29.29 (6.66) | 27.24 (6.31) | |
| Medications | |||||
| Corticosteroids (during 7 days before extubation), n (%) | 26 (39.4%) | 87 (33.1%) | 32 (38.6%) | 81 (32.9%) | |
| Muscle relaxants (during index admission), n (%) | 12 (18.2%) | 48 (18.3%) | 17 (20.5%) | 43 (17.5%) | |
| Vasopressors (during 24 h before extubation), n (%) | 10 (15.2%) | 49 (18.6%) | 13 (15.7%) | 46 (18.7%) | |
| Length of ICU stay before first extubation trial, days (IQR) | 4.68 (2.23–7.35) | 3.53 (1.5–7.54) | 4.87 (2.63–7.72) | 3.34 (1.46–6.77) | |
| Duration of mechanical ventilation, days (IQR) | 3.7 (1.73–6.35) | 2.78 (1.08–5.7) | 3.71 (1.74–6.27) | 2.63 (1.01–5.66) | |
| High-risk of extubation failure, n (%) | 42 (63.6%) | 168 (63.9%) | 58 (69.9%) | 152 (61.8%) | |
| Very high-risk of extubation failure, n (%) | 30 (45.5%) | 114 (43.3%) | 41 (49.4%) | 103 (41.9%) | |
| Laboratory values (last value before extubation) | |||||
| Albumin, g/dL (IQR) | 2.65 (2.3–3) | 2.9 (2.5–3.2) | 2.7 (2.3–3) | 2.9 (2.5–3.2) | |
| Creatinine, mg/dL (IQR) | 0.86 (0.6–1.47) | 0.77 (0.63–1.24) | 0.94 (0.61–1.6) | 0.75 (0.62–1.18) | |
| Blood urea nitrogen, mg/dL (IQR) | 25 (14–43.2) | 19.7 (12.7–32) | 27.9 (16.4–45.3) | 19 (12.3–29) | |
| Hemoglobin, g/dL (IQR) | 9.65 (8.5–11.9) | 10.3 (8.8–11.9) | 9.3 (8.4–11.48) | 10.4 (8.9–11.9) | |
| Sodium, mEq/L (IQR) | 140 (137–145) | 140 (138–143) | 141 (138–145) | 140 (138–143) | |
| Potassium, mmol/L (IQR) | 3.7 (3.4–4) | 3.8 (3.5–4.2) | 3.7 (3.4–4) | 3.8 (3.5–4.2) | |
| Phosphorus, mg/dL (IQR) | 3.07 (2.4–3.9) | 3.35 (2.52–4.11) | 3.14 (2.53–4.02) | 3.29 (2.48–4.11) | |
| Magnesium, mg/dL (IQR) | 1.93 (1.71–2.14) | 1.99 (1.77–2.24) | 1.96 (1.72–2.19) | 1.99 (1.76–2.23) | |
| Brain natriuretic peptide, pg/mL (IQR) ** | 1868 (301–3347) | 1230 (432–3875) | 2089 (316–3823) | 1182 (425–3711) | |
| pH | 7.41 (7.35–7.46) | 7.41 (7.36–7.47) | 7.42 (7.34–7.47) | 7.41 (7.36–7.47) | |
| pO2, mmHg (IQR) | 93 (78–123) | 106 (84–138) | 96 (80.5–124.5) | 106 (83–138) | |
| pCO2, mmHg (IQR) | 48 (42–56) | 45 (39–53) | 47 (41–54) | 45 (39–53) | |
| Bicarbonate, mmol/L (IQR) | 30.6 (25.8–36.4) | 29.1 (24.8–34.8) | 30.6 (24.8–36.4) | 29 (24.9–34.8) | |
| Lactate, mmol/L (IQR) | 1.2 (0.95–1.65) | 1.1 (0.85–1.7) | 1.2 (0.9–1.7) | 1.1 (0.8–1.7) | |
| Ionized calcium, mmol/L (IQR) | 1.1 (1.04–1.16) | 1.09 (1.04–1.14) | 1.1 (1.03–1.16) | 1.09 (1.04–1.14) | |
| Hyperglycemia (>180 mg/dL) during 48 h preceding extubation, n (%) | 37 (56.1%) | 161 (61.2%) | 52 (62.7%) | 146 (59.4%) | |
| Vital signs (last value before extubation) | |||||
| Heart rate, bpm (IQR) | 91 (80–110) | 92 (81–107) | 91 (80–109) | 92 (81–107) | |
| Systolic blood pressure, mmHg (IQR) | 141 (123–156) | 139 (121–158) | 142 (123–156) | 138 (121–158) | |
| Diastolic blood pressure, mmHg (IQR) | 67 (57–75) | 66 (57–75) | 64 (56–74) | 66 (57–75) | |
| Core Temperature, °C (IQR) | 36.7 (36.2–37.4) | 36.8 (36.3–37.4) | 36.7 (36.2–37.4) | 36.9 (36.3–37.4) | |
| Oxygen saturation, % (IQR) | 98 (95–100) | 98 (96–100) | 98 (96–100) | 98 (96–100) | |
| Fraction of inspired oxygen, % (IQR) | 40 (35–40) | 40 (35–40) | 40 (35–40) | 40 (35–40) | |
| Muscle related laboratory values (lowest value over the preceding 10 days) | |||||
| Alanine transaminase, IU/L (IQR) | 14 (9–21) | 19 (12–26) | 15 (9–21) | 20 (12–26) | |
| Creatine phosphokinase, U/L (IQR) *** | 105 (38–234) | 136 (52–475) | 98 (38–257) | 142 (56–478) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, Y.; Epstein, D.; Miller, A.; Segal, G.; Berger, G. Association of Low Alanine Aminotransferase Values with Extubation Failure in Adult Critically Ill Patients: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 3282. https://doi.org/10.3390/jcm10153282
Weber Y, Epstein D, Miller A, Segal G, Berger G. Association of Low Alanine Aminotransferase Values with Extubation Failure in Adult Critically Ill Patients: A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(15):3282. https://doi.org/10.3390/jcm10153282
Chicago/Turabian StyleWeber, Yoav, Danny Epstein, Asaf Miller, Gad Segal, and Gidon Berger. 2021. "Association of Low Alanine Aminotransferase Values with Extubation Failure in Adult Critically Ill Patients: A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 15: 3282. https://doi.org/10.3390/jcm10153282
APA StyleWeber, Y., Epstein, D., Miller, A., Segal, G., & Berger, G. (2021). Association of Low Alanine Aminotransferase Values with Extubation Failure in Adult Critically Ill Patients: A Retrospective Cohort Study. Journal of Clinical Medicine, 10(15), 3282. https://doi.org/10.3390/jcm10153282






