An Early Predictive Scoring Model for In-Hospital Cardiac Arrest of Emergent Hemodialysis Patients
Abstract
:1. Introduction
2. Methods
2.1. Data Source and Study Participants
2.2. Primary Outcome Measure
2.3. Predictor Variables
2.4. Prediction Model
2.5. Validation Model
2.6. Statistical Analyses
2.7. Ethical Consideration
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charytan, D.M.; Foley, R.; McCullough, P.A.; Rogers, J.D.; Zimetbaum, P.; Herzog, C.A.; Tumlin, J.A. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Makar, M.S.; Pun, P.H. Sudden Cardiac Death among Hemodialysis Patients. Am. J. Kidney Dis. 2017, 69, 684–695. [Google Scholar] [CrossRef] [Green Version]
- Karnik, J.A.; Young, B.S.; Lew, N.L.; Herget, M.; Dubinsky, C.; Lazarus, J.M.; Chertow, G.M. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001, 60, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Pun, P.H.; Lehrich, R.W.; Honeycutt, E.F.; Herzog, C.A.; Middleton, J.P. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011, 79, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starks, M.A.; Wu, J.; Peterson, E.D.; Stafford, J.A.; Matsouaka, R.A.; Boulware, E.; Svetkey, L.P.; Chan, P.S.; Pun, P.H. American Heart Association’s Get with the Guidelines-Resuscitation Investigators. In-Hospital Cardiac Arrest Resuscitation Practices and Outcomes in Maintenance Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2020, 15, 219–227. [Google Scholar] [CrossRef]
- Pun, P.H.; Lehrich, R.W.; Smith, S.R.; Middleton, J.P. Predictors of Survival after Cardiac Arrest in Outpatient Hemodialysis Clinics. Clin. J. Am. Soc. Nephrol. 2007, 2, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, O.K.; Vazquez, M.A.; Charles, L.; Berger, J.R.; Quiñones, H.; Fuquay, R.; Sanders, J.M.; Kapinos, K.A.; Halm, E.A.; Makam, A.N. Association of Scheduled vs. Emergency-Only Dialysis with Health Outcomes and Costs in Undocumented Immigrants With End-stage Renal Disease. JAMA Intern. Med. 2019, 179, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, R. When Access to Chronic Dialysis is limited: One Center’s Approach to Emergent Hemodialysis. Semin. Dial. 2012, 25, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Hsu, H.-K.; Lai, T.-S.; Chiang, W.-C.; Lin, S.-L.; Chen, Y.-M.; Chen, C.-C.; Chu, T.-S. Emergency department utilization and resuscitation rate among patients receiving maintenance hemodialysis. J. Formos. Med. Assoc. 2019, 118, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; Rivera, R.; Barbera, V.; Bellasi, A.; Cozzolino, M.; Russo, D.; De Pascalis, A.; Banerjee, D.; Floccari, F.; Ronco, C. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int. J. Cardiol. 2016, 217, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J. Am. Soc. Nephrol. 1996, 7, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Sherrard, D.J.; Gillen, D.L.; Wong, C.; Kestenbaum, B.; Seliger, S.; Ball, A.; Stehman-Breen, C. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am. J. Kidney Dis. 2002, 40, 307–314. [Google Scholar] [CrossRef]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS ONE 2018, 13, e0190410. [Google Scholar] [CrossRef]
- Smith, G.B.; Prytherch, D.; Meredith, P.; Schmidt, P.E.; Featherstone, P.I. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013, 84, 465–470. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Fang, C.-C.; Chen, S.-C.; Tsai, S.-H.; Kao, W.-F. Periarrest Modified Early Warning Score (MEWS) predicts the outcome of in-hospital cardiac arrest. J. Formos. Med. Assoc. 2016, 115, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.; Lander, H.; Snyder, A.; Hudson, P.; Churpek, M.; Edelson, D. Comparison of the Between the Flags calling criteria to the MEWS, NEWS and the electronic Cardiac Arrest Risk Triage (eCART) score for the identification of deteriorating ward patients. Resuscitation 2018, 123, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Tu, Y.-F.; Chiang, W.-C.; Wu, S.-Y.; Chang, Y.-H.; Chi, C.-H. Electrolyte abnormalities and laboratory findings in patients with out-of-hospital cardiac arrest who have kidney disease. Am. J. Emerg. Med. 2013, 31, 487–493. [Google Scholar] [CrossRef]
- Wu, C.; Hannan, E.L.; Walford, G.; Ambrose, J.A.; Holmes, D.R.; King, S.B.; Clark, L.T.; Katz, S.; Sharma, S.K.; Jones, R.H. A Risk Score to Predict In-Hospital Mortality for Percutaneous Coronary Interventions. J. Am. Coll. Cardiol. 2006, 47, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-H.; Tsai, J.-L.; Wang, J.-Y. Feasibility of using Microsoft Excel to draw forest plots. Taiwan J. Public Health 2019, 38, 102–110. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Ma, S.-Q.; Pan, C.; He, H.-L.; Cai, S.-X.; Hu, S.-L.; Liu, A.-R.; Liu, L.; Huang, Y.-Z.; Guo, F.-M.; et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: A prospective open label study. Crit. Care 2015, 19, 130. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Yun, D.; Kwon, S.; Jin, K.; Han, S.; Kim, D.K.; Oh, K.-H.; Joo, K.W.; Kim, Y.S.; Kim, S.; et al. Target value of mean arterial pressure in patients undergoing continuous renal replacement therapy due to acute kidney injury. BMC Nephrol. 2021, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.; Mendu, M.L.; Gibbons, F.K.; Christopher, K.B. Association between hyperkalemia at critical care initiation and mortality. Intensiv. Care Med. 2012, 38, 1834–1842. [Google Scholar] [CrossRef]
- Montford, J.R.; Linas, S. How Dangerous Is Hyperkalemia? J. Am. Soc. Nephrol. 2017, 28, 3155–3165. [Google Scholar] [CrossRef] [Green Version]
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Udy, A.; Licari, E.; Romero, L.; Bellomo, R. Sodium bicarbonate therapy for critically ill patients with metabolic acidosis: A scoping and a systematic review. J. Crit. Care 2019, 51, 184–191. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, S.; Zhang, M.; Zheng, Z.; Ma, Y. Albumin versus Other Fluids for Fluid Resuscitation in Patients with Sepsis: A Meta-Analysis. PLoS ONE 2014, 9, e114666. [Google Scholar] [CrossRef]
- Kim, W.Y.; Shin, Y.J.; Lee, J.M.; Huh, J.W.; Koh, Y.; Lim, C.-M.; Hong, S.B. Modified Early Warning Score Changes Prior to Cardiac Arrest in General Wards. PLoS ONE 2015, 10, e0130523. [Google Scholar] [CrossRef] [PubMed]
- Perera, Y.S.; Ranasinghe, P.; Adikari, A.M.; Welivita, W.D.; Perera, W.M.; Wijesundara, W.M.; Karunanayake, S.A.; Constantine, G.R. The value of the Modified Early Warning Score and biochemical parameters as predictors of patient outcome in acute medical admissions a prospective study. Acute. Med. 2011, 10, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef] [Green Version]


| IHCA within 3 Days | No (n = 146) | Yes (n = 44) | p Value | Incidence | Coefficient β | Univariate HR (95% CI) | Full Model HR (95% CI) | Final Model HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | −0.066 | ||||||||
| Female | 58 (39.7%) | 18 (40.9%) | 0.888 | 23.7% | 1.0 | ||||
| Male | 88 (60.3%) | 26 (59.1%) | 22.8% | 0.937 (0.513–1.708) | |||||
| Age (mean ± SD; year) | 65.82 ± 13.68 | 67.14 ± 15.54 | 0.587 | 0.007 | 1.007 (0.985–1.029) | ||||
| Hypertension | No | 43 (29.5%) | 20 (45.5%) | 0.048 * | 31.7% | 1.0 | 1.0 | 1.0 | |
| Yes | 103 (70.5%) | 24 (54.5%) | 18.9% | −0.639 | 0.528 (0.291–0.955) * | 0.563 (0.310–1.022) | 0.528 (0.291–0.955) * | ||
| DM | No | 59 (40.4%) | 16 (36.4%) | 0.630 | 21.3% | 1.0 | |||
| Yes | 87 (59.6%) | 28 (63.6%) | 24.3% | 0.114 | 1.121 (0.606–2.071) | ||||
| CAD | No | 91 (62.3%) | 28 (63.6%) | 0.875 | 23.5% | 1.0 | |||
| Yes | 55 (37.7%) | 16 (36.4%) | 22.5% | −0.084 | 0.919 (0.497–1.699) | ||||
| CVA | No | 119 (81.5%) | 37 (84.1%) | 0.695 | 23.7% | 1.0 | |||
| Yes | 27 (18.5%) | 7 (15.9%) | 20.6% | −0.199 | 0.820 (0.365–1.838) | ||||
| CKD | Stage 1 or 2 | 26 (17.8%) | 9 (20.5%) | 0.791 | 25.7% | 1.0 | |||
| Stage 3 or 4 | 10 (6.8%) | 4 (9.1%) | 28.6% | 0.044 | 1.045 (0.322–3.392) | ||||
| Stage 5 | 110 (75.3%) | 31 (70.5%) | 22.0% | −0.274 | 0.760 (0.362–1.597) | ||||
| Malignancy | No | 126 (86.3%) | 40 (90.9%) | 0.420 | 24.1% | 1.0 | |||
| Yes | 20 (13.7%) | 4 (9.1%) | 16.7% | −0.341 | 0.711 (0.254–1.987) | ||||
| Heart failure | No | 99 (67.8%) | 36 (81.8%) | 0.072 | 26.7% | 1.0 | 1.0 | ||
| Yes | 47 (32.2%) | 8 (18.2%) | 14.5% | −0.709 | 0.492 (0.229–1.059) | 0.530 (0.245–1.147) | |||
| IHCA within 3 Days | No (n = 146) | Yes (n = 44) | p Value | Coefficient β | Univariate HR (95% CI) | Full Model HR (95% CI) | Final Model HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Body temperature (°C) | 36.56 ± 1.10 | 36.77 ± 1.37 | 0.364 | 0.110 | 1.117 (0.860–1.450) | ||
| Pulse rate (beat/min) | 91.85 ± 26.04 | 85.30 ± 31.77 | 0.172 | −0.007 | 0.993 (0.982–1.004) | ||
| Respiratory rate (cycle/min) | 24.26 ± 7.65 | 24.00 ± 11.20 | 0.861 | −0.005 | 0.995 (0.958–1.033) | ||
| Systolic blood pressure (mmHg) | 144.44 ± 43.82 | 120.33 ± 42.86 | 0.002 ** | −0.011 | 0.989 (0.983–0.996) ** | 0.993 (0.980–1.007) | 0.991 (0.984–0.999) * |
| Diastolic blood pressure (mmHg) | 79.52 ± 28.02 | 66.90 ± 26.95 | 0.011 * | −0.015 | 0.985 (0.974–0.996) ** | 0.999 (0.977–1.021) | |
| Mean arterial pressure (mmHg) | 101.16 ± 31.86 | 84.71 ± 30.96 | 0.004 ** | −0.014 | 0.986 (0.977–0.995) ** | ||
| Oxygen saturation (%) | 90.47 ± 12.28 | 85.83 ± 12.57 | 0.039 * | −0.017 | 0.983 (0.967–1.000) * | 0.993 (0.973–1.012) | |
| Finger sugar (mg/dL) | 184.27 ± 132.57 | 173.35 ± 83.50 | 0.735 | −0.001 | 0.999 (0.995–1.003) | ||
| Glasgow Coma Scale (GCS) | 12.74 ± 3.85 | 11.91 ± 4.01 | 0.216 | −0.049 | 0.952 (0.889–1.021) | ||
| Eye opening (E) | 3.58 ± 0.95 | 3.43 ± 1.04 | 0.370 | −0.173 | 0.841 (0.639–1.107) | ||
| Verbal response (V) | 4.12 ± 1.55 | 3.60 ± 1.72 | 0.067 | −0.147 | 0.863 (0.730–1.022) | 0.927 (0.754–1.139) | |
| Motor response (M) | 5.24 ± 1.54 | 4.95 ± 1.77 | 0.300 | −0.095 | 0.909 (0.769–1.074) |
| IHCA within 3 Days | No (n = 146) | Yes (n = 44) | p Value | Coefficient β | Univariate HR (95% CI) | Full Model HR (95% CI) | Final Model HR (95% CI) |
|---|---|---|---|---|---|---|---|
| WBC (103/μL) | 11.92 ± 5.65 | 14.08 ± 6.33 | 0.033 * | 0.055 | 1.056 (1.009–1.105) * | 1.044 (0.983–1.108) | 1.064 (1.009–1.122) * |
| Hb (g/dL) | 9.80 ± 2.50 | 11.04 ± 3.36 | 0.028 * | 0.157 | 1.170 (1.050–1.304) ** | 1.064 (0.958–1.183) | |
| Plt (109/L) | 213.87 ± 90.48 | 185.74 ± 80.06 | 0.068 | −0.003 | 0.997 (0.993–1.000) | 0.995 (0.992–0.999) * | 0.995 (0.991–0.999) ** |
| PT-INR | 1.19 ± 0.63 | 1.36 ± 0.84 | 0.190 | 0.165 | 1.179 (0.874–1.592) | ||
| aPTT (sec) | 39.36 ± 27.21 | 42.58 ± 33.56 | 0.563 | 0.004 | 1.004 (0.994–1.013) | ||
| BUN (mg/dL) | 101.75 ± 47.60 | 117.72 ± 77.70 | 0.416 | 0.005 | 1.005 (0.996–1.013) | ||
| Cr (mg/dL) | 8.33 ± 4.61 | 8.26 ± 5.47 | 0.947 | −0.010 | 0.990 (0.916–1.069) | ||
| eGFR (ml/min/1.73 m2) | 9.01 ± 9.49 | 10.47 ± 15.23 | 0.520 | 0.018 | 1.019 (0.988–1.050) | ||
| Lactate (mmol/L) | 4.70 ± 5.21 | 5.93 ± 4.03 | 0.244 | 0.038 | 1.038 (0.980–1.100) | ||
| Arterial gas | |||||||
| pH | 7.32 ± 0.16 | 7.23 ± 0.17 | 0.001 ** | −2.527 | 0.080 (0.016–0.388) ** | 0.346(0.049–2.432) | |
| Base deficit (mmol/L) | −6.46 ± 7.54 | −10.67 ± 7.21 | 0.002 ** | −0.057 | 0.945 (0.912–0.979) ** | ||
| PCO2 (mmHg) | 37.73 ± 17.51 | 39.45 ± 20.00 | 0.587 | 0.003 | 1.003 (0.988–1.019) | ||
| PO2 (mmHg) | 115.69 ± 95.49 | 93.00 ± 93.91 | 0.171 | −0.003 | 0.997 (0.993–1.001) | ||
| Na (mmol/L) | 135.77 ± 6.54 | 139.09 ± 7.78 | 0.005 ** | 0.076 | 1.079 (1.035–1.126) *** | 1.061 (1.014–1.110) ** | 1.069 (1.024–1.115) ** |
| K (mmol/L) | 4.83 ± 1.34 | 5.87 ± 1.83 | 0.001 ** | 0.280 | 1.323 (1.127–1.552) ** | 1.235 (1.037–1.470) * | 1.296 (1.110–1.526) ** |
| Ca (mg/dL) | 8.78 ± 1.34 | 8.51 ± 1.96 | 0.521 | −0.076 | 0.926 (0.706–1.215) | ||
| Alb (g/dL) | 3.85 ± 0.82 | 3.02 ± 1.09 | 0.044 * | −0.672 | 0.511 (0.246–1.059) | ||
| Mg (mg/dL) | 2.66 ± 0.82 | 2.82 ± 1.12 | 0.651 | 0.173 | 1.189 (0.637–2.217) | ||
| TnT (ng/L) | 0.40 ± 1.08 | 0.57 ± 1.59 | 0.482 | 0.093 | 1.097 (0.876–1.374) | ||
| ALT (U/L) | 51.49 ± 201.90 | 52.88 ± 102.98 | 0.966 | 0.000 | 1.000 (0.999–1.002) |
| IHCA within 3 Days | No | Yes | p Value | Incidence | Univariate HR (95% CI) | Final Model HR (95% CI) | Coefficient β | Score | |
|---|---|---|---|---|---|---|---|---|---|
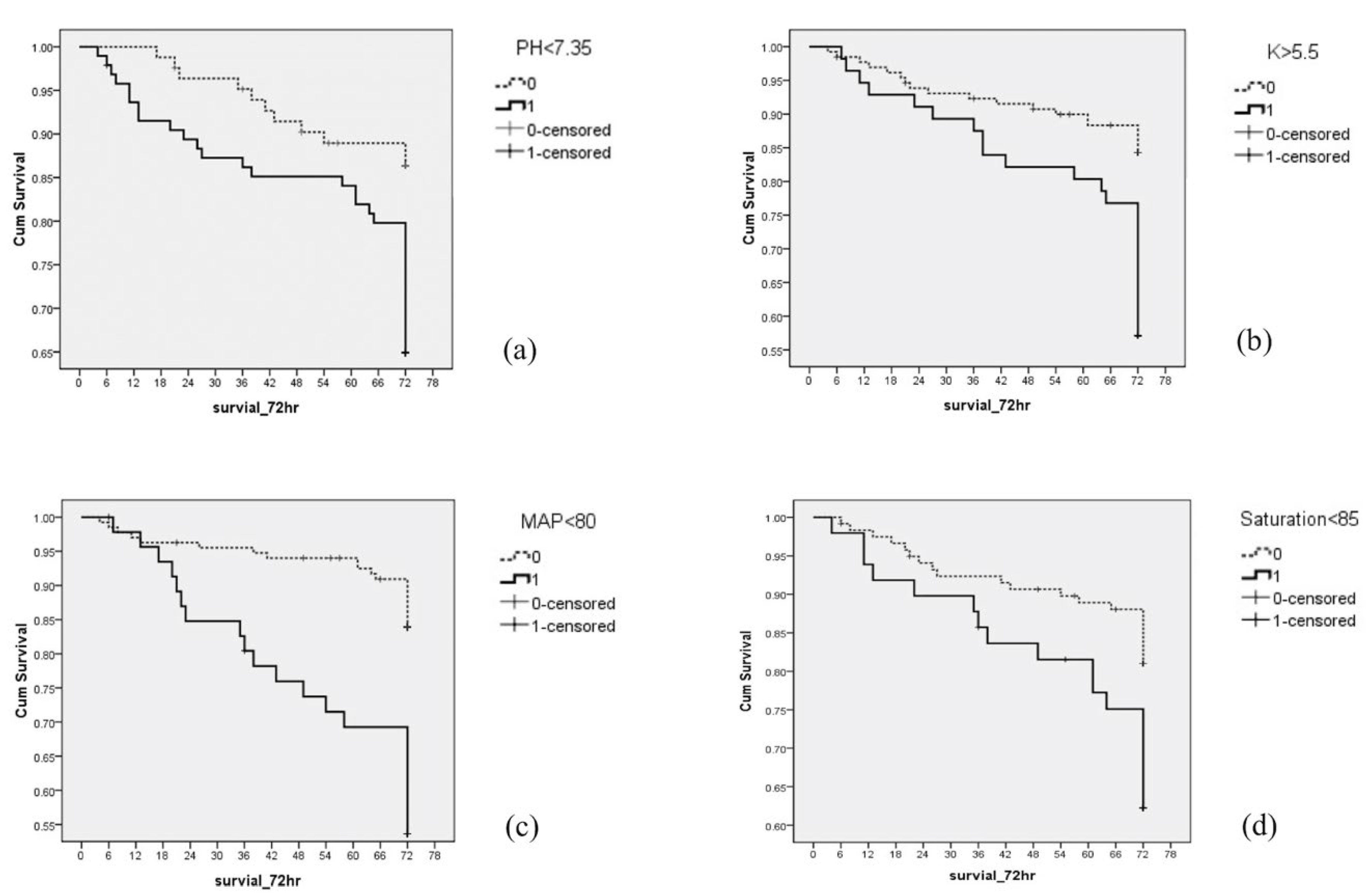
| pH < 7.35 | No | 72 (53.7%) | 11 (25%) | 0.001 ** | 13.3% | 1.0 | 1.0 | 0.686 | 1 |
| Yes | 62 (46.3%) | 33 (75%) | 34.7% | 2.768 (1.399–5.478) ** | 1.985 (0.968–4.073) | ||||
| K > 5.5 mmol/L | No | 111 (77.6%) | 20 (45.5%) | <0.001 *** | 15.3% | 1.0 | 1.0 | 0.521 | 1 |
| Yes | 32 (22.4%) | 24 (54.5%) | 42.9% | 2.943 (1.626–5.328) *** | 1.683 (0.860–3.293) | ||||
| MAP < 80 mmHg | No | 113 (81.3%) | 21 (50.0%) | <0.001 *** | 15.7% | 1.0 | 1.0 | 0.872 | 2 |
| Yes | 26 (18.7%) | 21 (50.0%) | 44.7% | 3.475 (1.896–6.369) *** | 2.392 (1.240–4.617) ** | ||||
| Oxygen saturation < 85% | No | 97 (75.8%) | 22 (55.0%) | 0.012 | 18.5% | 1.0 | 1.0 | 0.515 | 1 |
| Yes | 31 (24.2%) | 18 (45.0%) | 36.7% | 2.182 (1.170–4.070) * | 1.674 (0.866–3.238) |
| IHCA within 3 Days | No | Yes | p Value | Incidence |
|---|---|---|---|---|
| Original model | <0.001 | |||
| Score = 0 | 36 (31.9%) | 1 (2.6%) | 2.7% | |
| Score = 1 | 35 (31.0%) | 5 (13.2%) | 12.5% | |
| Score = 2 | 23 (20.4%) | 15 (39.5%) | 39.5% | |
| Score = 3 | 13 (11.5%) | 9 (23.7%) | 40.9% | |
| Score = 4 | 4 (3.5%) | 4 (10.5%) | 50.0% | |
| Score = 5 | 2 (1.8%) | 4 (10.5%) | 66.7% | |
| Low-risk: Score < 3 | 94 (83.2%) | 21 (55.3%) | <0.001 | 18.3% |
| High-risk: Score ≥ 3 | 19 (16.8%) | 17 (44.7%) | 47.2% | |
| Validation model | 0.042 | |||
| Score = 0 | 8 (17.0%) | 0 (0.0%) | 0.0% | |
| Score = 1 | 20 (42.6%) | 2 (25.0%) | 9.1% | |
| Score = 2 | 12 (25.5%) | 1 (12.5%) | 7.7% | |
| Score = 3 | 4 (8.5%) | 4 (50.0%) | 50.0% | |
| Score = 4 | 2 (4.3%) | 1 (12.5%) | 33.3% | |
| Score = 5 | 1 (2.1%) | 0 (0.0%) | 0.0% | |
| Low-risk: Score < 3 | 40 (85.1%) | 3 (37.5%) | 0.003 | 7.0% |
| High-risk: Score ≥ 3 | 7 (14.9%) | 5 (62.5%) | 41.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Cheng, Y.-Y.; Lin, C.-H. An Early Predictive Scoring Model for In-Hospital Cardiac Arrest of Emergent Hemodialysis Patients. J. Clin. Med. 2021, 10, 3241. https://doi.org/10.3390/jcm10153241
Chen S-H, Cheng Y-Y, Lin C-H. An Early Predictive Scoring Model for In-Hospital Cardiac Arrest of Emergent Hemodialysis Patients. Journal of Clinical Medicine. 2021; 10(15):3241. https://doi.org/10.3390/jcm10153241
Chicago/Turabian StyleChen, Shih-Hao, Ya-Yun Cheng, and Chih-Hao Lin. 2021. "An Early Predictive Scoring Model for In-Hospital Cardiac Arrest of Emergent Hemodialysis Patients" Journal of Clinical Medicine 10, no. 15: 3241. https://doi.org/10.3390/jcm10153241
APA StyleChen, S.-H., Cheng, Y.-Y., & Lin, C.-H. (2021). An Early Predictive Scoring Model for In-Hospital Cardiac Arrest of Emergent Hemodialysis Patients. Journal of Clinical Medicine, 10(15), 3241. https://doi.org/10.3390/jcm10153241








