Effect of Deep versus Moderate Neuromuscular Blockade on Quantitatively Assessed Postoperative Atelectasis Using Computed Tomography in Thoracic Surgery; a Randomized Double-Blind Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Allocation, Randomization and Blindness
2.4. Anesthesia Protocols
2.5. Monitoring of Neuromuscular Blockade
2.6. Outcome Measurement
2.7. Statistical Analysis
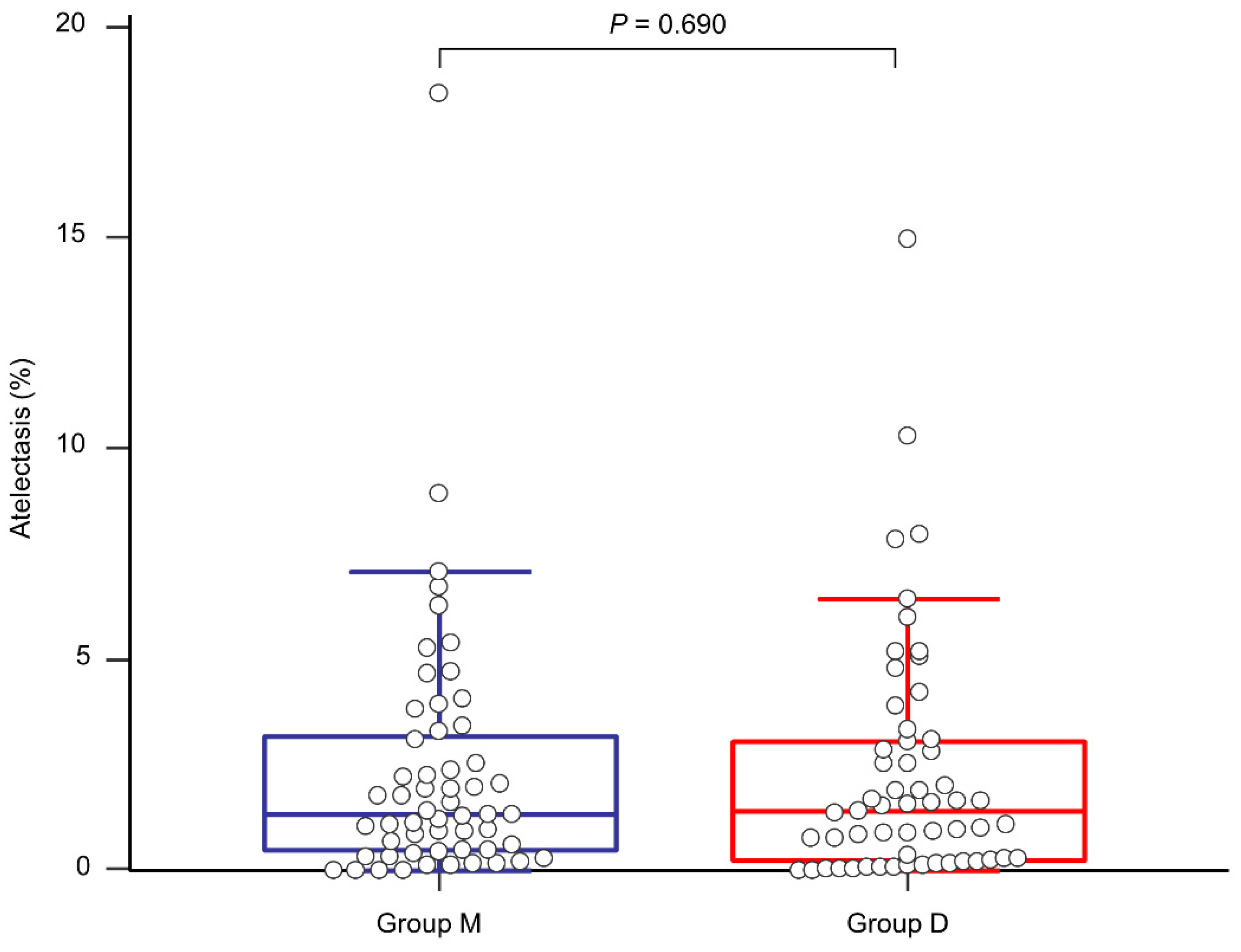
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez Ruiz, R.; Lee, J.W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.S.; Colquhoun, D.A.; Durieux, M.E.; Kozower, B.D.; McMurry, T.L.; Bender, S.P.; Naik, B.I. Management of One-lung Ventilation: Impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology 2016, 124, 1286–1295. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef] [Green Version]
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019, 123, 898–913. [Google Scholar] [CrossRef] [Green Version]
- Cammu, G. Residual Neuromuscular Blockade and Postoperative Pulmonary Complications: What Does the Recent Evidence Demonstrate? Curr. Anesthesiol. Rep. 2020, 10, 131–136. [Google Scholar] [CrossRef]
- Baete, S.; Vercruysse, G.; Vander Laenen, M.; De Vooght, P.; Van Melkebeek, J.; Dylst, D.; Beran, M.; Van Zundert, J.; Heylen, R.; Boer, W.; et al. The Effect of Deep Versus Moderate Neuromuscular Block on Surgical Conditions and Postoperative Respiratory Function in Bariatric Laparoscopic Surgery: A Randomized, Double Blind Clinical Trial. Anesth. Analg. 2017, 124, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.K.; Caldwell, J.E.; Brull, S.J.; Soto, R.G. Reversal of profound rocuronium-induced blockade with sugammadex: A randomi‘zed comparison with neostigmine. Anesthesiology 2008, 109, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Freundlich, R.E.; Gupta, R.K.; Hayhurst, C.J.; Le, C.H.; Martin, B.J.; Shotwell, M.S.; Wanderer, J.P. Postoperative Pulmonary Complications’ Association with Sugammadex versus Neostigmine. Anesthesiology 2021, 134, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Terekhov, M.A.; Martin, B.J.; Dmochowski, R.R.; Hayes, R.M.; Ehrenfeld, J.M. Nondepolarizing Neuromuscular Blocking Agents, Reversal, and Risk of Postoperative Pneumonia. Anesthesiology 2016, 125, 647–655. [Google Scholar] [CrossRef]
- Meira, M.N.; Carvalho, C.R.; Galizia, M.S.; Borges, J.B.; Kondo, M.M.; Zugaib, M.; Vieira, J.E. Atelectasis observed by computerized tomography after Caesarean section. Br. J. Anaesth. 2010, 104, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.C.; Lee, J.H.; Lee, S.C.; Park, S.Y.; Rim, J.C.; Choi, S.R. Use of sugammadex in lung cancer patients undergoing video-assisted thoracoscopic lobectomy. Korean J. Anesthesiol. 2017, 70, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Mulier, J.P.; Dillemans, B. Anaesthetic Factors Affecting Outcome After Bariatric Surgery, a Retrospective Levelled Regression Analysis. Obes. Surg. 2019, 29, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Duggan, M.; Kavanagh, B.P. Pulmonary atelectasis: A pathogenic perioperative entity. Anesthesiology 2005, 102, 838–854. [Google Scholar] [CrossRef]
- Lumb, A.B. Just a little oxygen to breathe as you go off to sleep...Is it always a good idea? Br. J. Anaesth. 2007, 99, 769–771. [Google Scholar] [CrossRef] [Green Version]
- Duggan, M.; Kavanagh, B.P. Atelectasis in the perioperative patient. Curr. Opin. Anaesthesiol. 2007, 20, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Jeong, S.Y.; Jeong, J.H.; Kim, J.H.; Choi, S.R. Comparison of postoperative pulmonary complications between sugammadex and neostigmine in lung cancer patients undergoing video-assisted thoracoscopic lobectomy: A prospective double-blinded randomized trial. Anesth. Pain Med. 2021, 16, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Carron, M. Respiratory benefits of deep neuromuscular block during laparoscopic surgery in a patient with end-stage lung disease. Br. J. Anaesth. 2015, 114, 158–159. [Google Scholar] [CrossRef] [Green Version]
- Agostini, P.; Lugg, S.T.; Adams, K.; Vartsaba, N.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B.; Rushton, A.; Bishay, E. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: A propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomydagger. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 931–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledowski, T.; O’Dea, B.; Meyerkort, L.; Hegarty, M.; von Ungern-Sternberg, B.S. Postoperative Residual Neuromuscular Paralysis at an Australian Tertiary Children’s Hospital. Anesthesiol. Res. Pract. 2015, 2015, 410248. [Google Scholar] [CrossRef]
- Light, R.W.; George, R.B. Incidence and significance of pleural effusion after abdominal surgery. Chest 1976, 69, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst. Rev. 2017, 8, CD012763. [Google Scholar] [CrossRef] [PubMed]
- Tenenbein, P.K.; Debrouwere, R.; Maguire, D.; Duke, P.C.; Muirhead, B.; Enns, J.; Meyers, M.; Wolfe, K.; Kowalski, S.E. Thoracic epidural analgesia improves pulmonary function in patients undergoing cardiac surgery. Can. J. Anaesth. 2008, 55, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Shear, T.D.; Deshur, M.; Benson, J.; Newmark, R.L.; Maher, C.E. Comparison of the TOFscan and the TOF-Watch SX during Recovery of Neuromuscular Function. Anesthesiology 2018, 129, 880–888. [Google Scholar] [CrossRef] [PubMed]
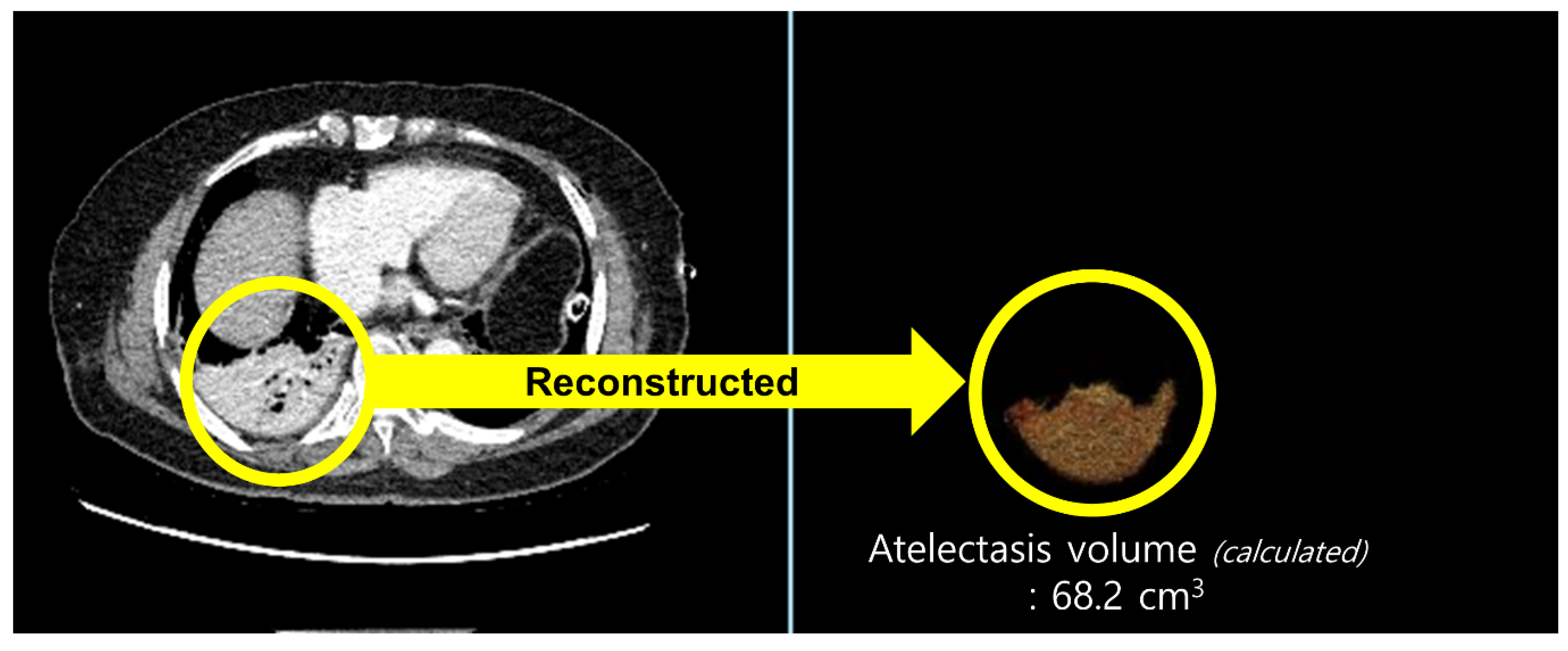


| Variables | Group M (n = 59) | Group D (n = 58) | p |
|---|---|---|---|
| Age | 64 (59–71) | 64 (58–73) | 0.611 |
| Sex, male/female | 29 (49.2)/30 (50.8) | 43 (74.1)/15 (25.4) | 0.005 |
| ASA physical status 1 | 8 (13.6) | 7 (11.9) | 0.910 |
| 2 | 44 (74.6) | 46 (78.0) | |
| 3 | 7 (11.9) | 6 (10.2) | |
| Height (cm) | 161 (152.0–165.7) | 165 9 (158.7–169.4) | 0.006 |
| Weight (kg) | 62 (56.5–71.6) | 62.8 (53.9–69.3) | 0.844 |
| Body mass index (kg/m2) | 24.3 (22.2–26.3) | 23.6 (20.2–25.8) | 0.064 |
| Preoperative abnormal chest radiography | 2 (3.4%) | 16 (27.1) | 0.023 |
| Pleural effusion | 0 (0.0) | 8 (13.6) | |
| Pneumothorax | 2 (3.4) | 4 (6.8) | |
| Others (Pulmonary edema or infiltration, atelectasis, hemothorax) | 0 (0.0) | 4 (6.8) | |
| Type of surgery | 0.070 | ||
| Wedge resection | 6 (10.2) | 9 (15.3) | |
| Segmentectomy | 12 (20.3) | 7 (11.9) | |
| Lobectomy | 33 (55.9) | 29 (49.2) | |
| Lobectomy + Wedge resection | 2 (3.4) | 0 (0.0) | |
| Lobectomy + Segmentectomy | 0 (0.0) | 1 (1.7) | |
| Pneumonectomy | 0 (0.0) | 1 (1.7) | |
| Decortication | 1 (1.7) | 9 (15.3) | |
| Etc. (including Pleura, Mediastinum, Esophagus) | 5 (8.5) | 3 (5.1) | |
| Incidence of open conversion | 7 (11.9) | 11(19.0) | 0.308 |
| Variables | Group M (n = 59) | Group D (n = 58) | p |
|---|---|---|---|
| Total anesthesia time (min) | 260 (220–316) | 250 (205–331) | 0.799 |
| Total operation time (min) | 200 (136–243) | 183 (132–241) | 0.524 |
| One-lung ventilation time (min) | 172 (125–220) | 165 (125–217) | 0.787 |
| Parameters during one-lung ventilation * | |||
| Mean tidal volume (mL) | 292 (196–386) | 302 (214–428) | 0.122 |
| Mean respiratory rate (/min) | 15 (14–16) | 15 (15–16) | 0.723 |
| Mean positive end-expiratory pressure (cmH2O) | 5 (5–5) | 5 (5–5) | 0.950 |
| Mean lung compliance (mL/cmH2O) | 24.1 (21.8–27.2) | 26.6 (23.5–30.6) | 0.026 |
| PaO2, lowest (mmHg) | 85.7 (72.7–105.8) | 81.9 (71.8–97.4) | 0.347 |
| SaO2, lowest (mmHg) | 94.6 (93.0–96.5) | 94.1 (92.8–96.1) | 0.663 |
| Incidence of FiO2 increase (>0.5) | 21 (35.6) | 12 (20.3) | 0.167 |
| Incidence of SaO2 < 95% | 34 (58.6) | 36 (61.0) | 0.792 |
| Incidence of additional NMBA administration | 23 (39.0) | 22 (37.3) | 0.850 |
| Incidence of conversion to open surgery | 7 (11.9) | 11 (18.6) | 0.308 |
| Total amount of administered propofol (mg) | 1359 (1090–1756) | 1413 [1056–1681) | 0.771 |
| Total amount of administered remifentanil (µg) | 1591 (1184–2132) | 1508 [1100–2030) | 0.333 |
| Total amount of administered crystalloid (mL) | 1200 (880–1495) | 1300 [900–1760) | 0.314 |
| Total amount of transfused RBCs (unit) | 0 (0–0) | 0 [0–0) | 0.172 |
| Urine output (mL) | 390 (250–508) | 320 [230–500) | 0.302 |
| Variables | Group M (n = 58) | Group D (n = 56) | p |
|---|---|---|---|
| Postoperative pulmonary complications | |||
| Overall | 43 (74.1) | 42 (73.7) | 0.831 |
| Pleural effusion | 0.779 | ||
| Ipsilateral | 19 (32.8) | 21 (36.8) | |
| Contralateral | 2 (3.4) | 4 (7.0) | |
| Bilateral | 15 (25.8) | 12 (21.1) | |
| Pneumothorax | 4 (6.9) | 5 (8.8) | 0.730 |
| Pulmonary edema | 0 (0) | 1 (1.8) | 0.317 |
| Pneumonia | 1 (1.7) | 2 (3.5) | 0.560 |
| Postoperative arterial blood gas analysis | |||
| pH | 7.352 (7.305–7.377) | 7.361 (7.326–7.399) | 0.135 |
| PaO2 (mmHg) | 133.0 (97.8–172.0) | 156.5 (103.4–198.5) | 0.085 |
| PaCO2 (mmHg) | 44.0 [40.7–47.7) | 42.1 (38.7–46.5) | 0.087 |
| HCO3−(mEq/L) | 23.5 (22.4–25.1) | 23.3 (21.4–25.2) | 0.520 |
| SaO2 (%) | 97.7 (97.5–98.4) | 98.1 (97.1–98.5) | 0.149 |
| Intensive care unit stay (days) | 1 (1–1) | 1 (1–1) | 0.905 |
| Hospital stay (days) | 11 (9–16) | 12 (9–16) | 0.668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-J.; Lee, H.N.; Chung, J.-Y.; Kim, D.; Kim, J.I.; Seo, H. Effect of Deep versus Moderate Neuromuscular Blockade on Quantitatively Assessed Postoperative Atelectasis Using Computed Tomography in Thoracic Surgery; a Randomized Double-Blind Controlled Trial. J. Clin. Med. 2021, 10, 3228. https://doi.org/10.3390/jcm10153228
Lee B-J, Lee HN, Chung J-Y, Kim D, Kim JI, Seo H. Effect of Deep versus Moderate Neuromuscular Blockade on Quantitatively Assessed Postoperative Atelectasis Using Computed Tomography in Thoracic Surgery; a Randomized Double-Blind Controlled Trial. Journal of Clinical Medicine. 2021; 10(15):3228. https://doi.org/10.3390/jcm10153228
Chicago/Turabian StyleLee, Bong-Jae, Han Na Lee, Jun-Young Chung, Daehyun Kim, Jung Im Kim, and Hyungseok Seo. 2021. "Effect of Deep versus Moderate Neuromuscular Blockade on Quantitatively Assessed Postoperative Atelectasis Using Computed Tomography in Thoracic Surgery; a Randomized Double-Blind Controlled Trial" Journal of Clinical Medicine 10, no. 15: 3228. https://doi.org/10.3390/jcm10153228
APA StyleLee, B.-J., Lee, H. N., Chung, J.-Y., Kim, D., Kim, J. I., & Seo, H. (2021). Effect of Deep versus Moderate Neuromuscular Blockade on Quantitatively Assessed Postoperative Atelectasis Using Computed Tomography in Thoracic Surgery; a Randomized Double-Blind Controlled Trial. Journal of Clinical Medicine, 10(15), 3228. https://doi.org/10.3390/jcm10153228






