Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting
Abstract
:1. Introduction
2. Approved Treatments for HCC before the “Era” of Immune Checkpoint Inhibitors
3. The Advent of Immune Checkpoint Inhibitors
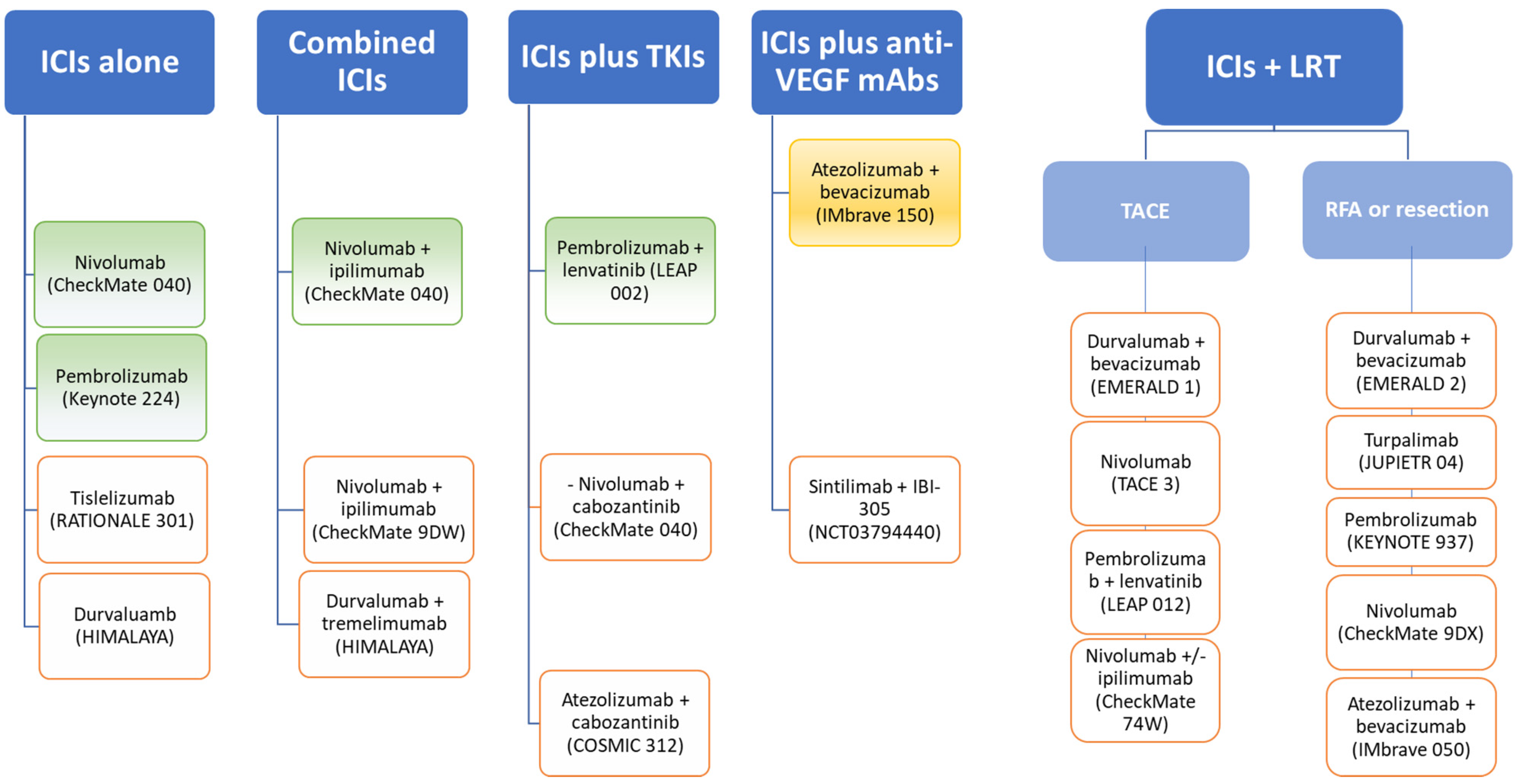
4. Immune Checkpoint Inhibitors in HCC
4.1. Immune Checkpoint Inhibitors in Monotherapy
4.2. Dual Immune Chechpoint Blockade
4.3. Immune Checkpoint Inhibitors Combined with Tyrosine Kinase Inhibitors
4.4. Immune Checkpoint Inhibitors Combined with Anti-VEGFR Agents
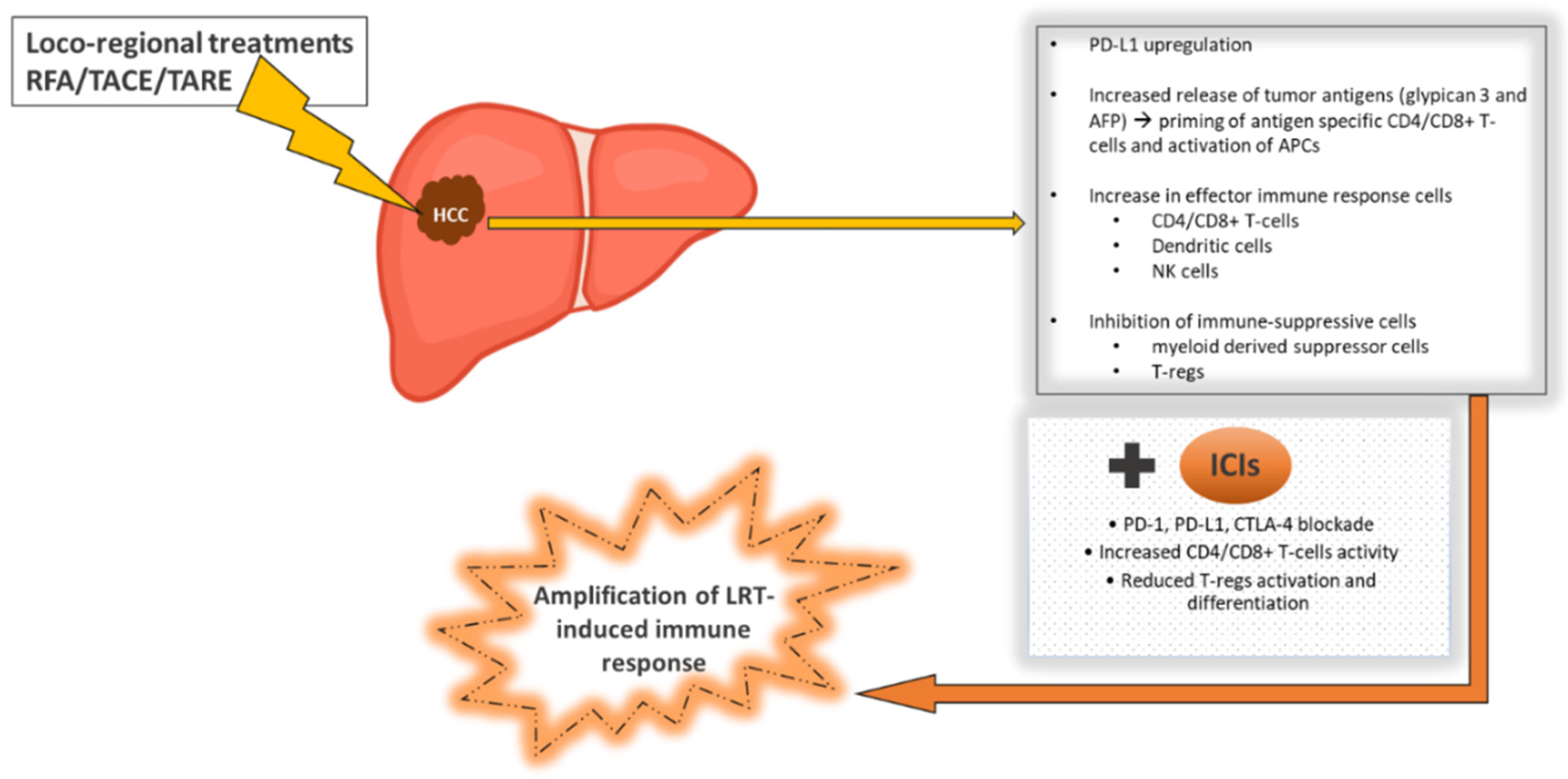
4.5. Immune Checkpoint Inhibitors Combined with Locoregional Treatments for HCC
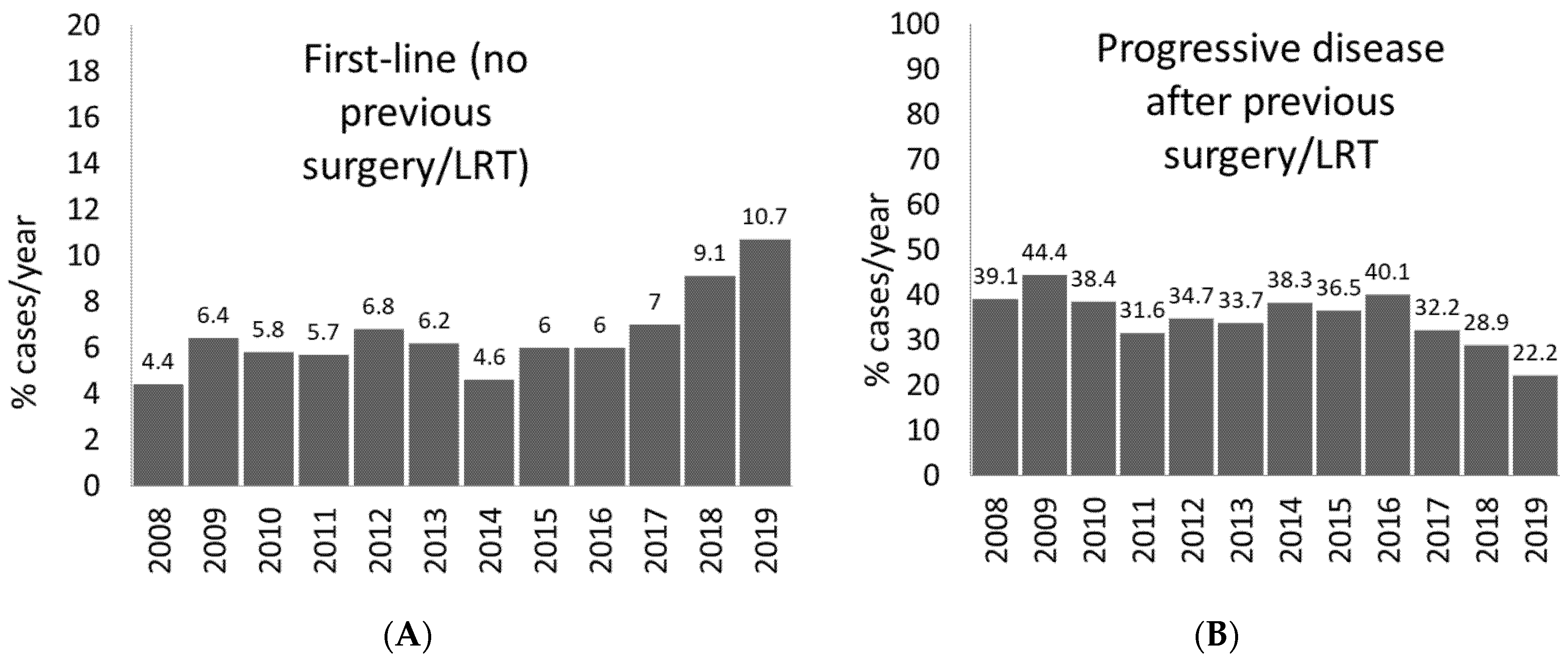
5. Amenability to Atezolizumab Plus Bevacizumab in Real-Life Setting
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.C.P.; Bodini, G.; Furnari, M.; Marabotto, E.; Zentilin, P.; Strazzabosco, M.; Giannini, E.G. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers 2020, 12, 1422. [Google Scholar] [CrossRef]
- Torres, M.C.P.; Aghemo, A.; Lleo, A.; Bodini, G.; Furnari, M.; Marabotto, E.; Miele, L.; Giannini, E.G. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients 2019, 11, 2971. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.D.; Park, J.-W.; Han, G.; Jassem, J.; et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC). J. Clin. Oncol. 2017, 35, 4001. [Google Scholar] [CrossRef]
- Kirstein, M.M.; Scheiner, B.; Marwede, T.; Wolf, C.; Voigtländer, T.; Semmler, G.; Wacker, F.; Manns, M.P.; Hinrichs, J.B.; Pinter, M.; et al. Sequential systemic treatment in patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 52, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.-I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.C.C.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Camma’, C.; Colombo, M.; on behalf of the SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Ganten, T.M.; Stauber, R.E.; Schott, E.; Malfertheiner, P.; Buder, R.; Galle, P.R.; Göhler, T.; Walther, M.; Koschny, R.; Gerken, G. Sorafenib in Patients with Hepatocellular Carcinoma—Results of the Observational INSIGHT Study. Clin. Cancer Res. 2017, 23, 5720–5728. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Lim, H.Y.; Pracht, M.; et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J. Clin. Oncol. 2018, 36, 4003. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.-Y.; Yen, C.-J.; Poon, R.; Pastorelli, D.; Blanc, J.-F.; Chung, H.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Giannini, E.G.; Trevisani, F. Ramucirumab as a second-line treatment for hepatocellular carcinoma: Reaching out further to patients with elevated alpha-fetoprotein. Hepatobiliary Surg. Nutr. 2019, 8, 515–518. [Google Scholar] [CrossRef]
- Lai, E.; Astara, G.; Ziranu, P.; Pretta, A.; Migliari, M.; Dubois, M.; Donisi, C.; Mariani, S.; Liscia, N.; Impera, V.; et al. Introducing immunotherapy for advanced hepatocellular carcinoma patients: Too early or too fast? Crit. Rev. Oncol. 2021, 157, 103167. [Google Scholar] [CrossRef]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2021, 41, 585–597. [Google Scholar] [CrossRef]
- Hoshida, Y.; Villanueva, A.; Kobayashi, M.; Peix, J.; Chiang, D.Y.; Camargo, A.; Gupta, S.; Moore, J.; Wrobel, M.J.; Lerner, J.; et al. Gene Expression in Fixed Tissues and Outcome in Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Shi, M.; Zeng, Z.; Qi, R.-Z.; Liu, Z.-W.; Zhang, J.-Y.; Yang, Y.-P.; Tien, P.; Wang, F.-S. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 2010, 128, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; de Moura, M.C.; Putra, J.; Campreciós, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khoueiry, A.B.; Melero, I.; Yau, T.C.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Choo, S.; Trojan, J.; Welling, T.; Meyer, T.; et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): Subanalyses of CheckMate-040. J. Clin. Oncol. 2018, 36, 475. [Google Scholar] [CrossRef]
- Crocenzi, T.S.; El-Khoueiry, A.B.; Yau, T.C.; Melero, I.; Sangro, B.; Kudo, M.; Hsu, C.; Trojan, J.; Kim, T.-Y.; Choo, S.-P.; et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J. Clin. Oncol. 2017, 35, 4013. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.; Furuse, J.; Galle, P.R.; Kelley, R.K.; Qin, S.; Armstrong, J.; Darilay, A.; Vlahovic, G.; Negro, A.; et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J. Clin. Oncol. 2018, 36, TPS4144. [Google Scholar] [CrossRef]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracian, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.F.; et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J. Clin. Oncol. 2019, 37, 327. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Aglitti, A.; Borzio, M.; Gambato, M.; Guarino, M.; Iavarone, M.; Lai, Q.; Sandri, G.B.L.; Melandro, F.; Morisco, F.; et al. Overview of Immune Checkpoint Inhibitors Therapy for Hepatocellular Carcinoma, and The ITA.LI.CA Cohort Derived Estimate of Amenability Rate to Immune Checkpoint Inhibitors in Clinical Practice. Cancers 2019, 11, 1689. [Google Scholar] [CrossRef] [Green Version]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, B.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Schulze, K.; Von Felden, J.; Koch, S.; Schwabl, P.; Hinrichs, J.B.; Waneck, F.; et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: Efficacy and safety data from an international multicentre real-world cohort. Aliment. Pharmacol. Ther. 2019, 49, 1323–1333. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate. J. Clin. Oncol. 2019, 37 (Suppl. S15), 4012. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Sangro, B.; Harris, W.P.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, W.M.D.; Lim, H.Y.; Yau, T.; et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J. Clin. Oncol. 2020, 38, 4508. [Google Scholar] [CrossRef]
- Tai, D.; Choo, S.P.; Chew, V. Rationale of Immunotherapy in Hepatocellular Carcinoma and Its Potential Biomarkers. Cancers 2019, 11, 1926. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qin, S.; Zhang, Y. The Evolving Landscape of Checkpoint Inhibitor Combination Therapy in the Treatment of Advanced Hepatocellular Carcinoma. Target. Oncol. 2021, 16, 153–163. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Gracián, A.C.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2020, 38, 478. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Ulbrich, G.; Sieghart, W.; Kölblinger, C.; Reiberger, T.; Li, S.; Ferlitsch, A.; Müller, C.; Lammer, J.; Peck-Radosavljevic, M. Hepatocellular Carcinoma: A Phase II Randomized Controlled Double-Blind Trial of Transarterial Chemoembolization in Combination with Biweekly Intravenous Administration of Bevacizumab or a Placebo. Radiology 2015, 277, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Knox, J.J.; Chen, H.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; et al. Phase II Trial Evaluating the Clinical and Biologic Effects of Bevacizumab in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998. [Google Scholar] [CrossRef] [Green Version]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Giannini, E.G.; Trevisani, F. Improving survival of cirrhosis patients with hepatocellular carcinoma through application of standard of care. Hepatology 2014, 60, 1446–1447. [Google Scholar] [CrossRef]
- Iwamoto, H.; Shimose, S.; Noda, Y.; Shirono, T.; Niizeki, T.; Nakano, M.; Okamura, S.; Kamachi, N.; Suzuki, H.; Sakai, M.; et al. Initial Experience of Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma in Real-World Clinical Practice. Cancers 2021, 13, 2786. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Duffy, A.G.; Korangy, F. Hepatocellular Carcinoma from an Immunologic Perspective. Clin. Cancer Res. 2013, 19, 6678–6685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Toom, S.; Avula, A.; Kumar, V.; E Rahma, O. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, A.G.; Ulahannan, S.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaru, L.; Pereira, S.; Alisa, A.; Pathan, A.A.; Williams, R.; Davidson, B.; Burroughs, A.K.; Meyer, T.; Behboudi, S. Unmasking of α-Fetoprotein-Specific CD4+ T Cell Responses in Hepatocellular Carcinoma Patients Undergoing Embolization. J. Immunol. 2007, 178, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Hänsler, J.; Hä, J.; Nsler, T.; Wissniowski, D.S.U.T. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J. Gastroenterol. 2006, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Hiroishi, K.; Eguchi, J.; Baba, T.; Shimazaki, T.; Ishii, S.; Hiraide, A.; Sakaki, M.; Doi, H.; Uozumi, S.; Omori, R.; et al. Strong CD8+ T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2009, 45, 451–458. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Nakamoto, Y.; Arai, K.; Yamashita, T.; Sakai, A.; Sakai, Y.; Kagaya, T.; Yamashita, T.; Honda, M.; Kaneko, S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011, 53, 1206–1216. [Google Scholar] [CrossRef]
- Zerbini, A.; Pilli, M.; Laccabue, D.; Pelosi, G.; Molinari, A.; Negri, E.; Cerioni, S.; Fagnoni, F.; Soliani, P.; Ferrari, C.; et al. Radiofrequency Thermal Ablation for Hepatocellular Carcinoma Stimulates Autologous NK-Cell Response. Gastroenterology 2010, 138, 1931–1942.e2. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Vence, L.; Blando, J.; Yadav, S.S.; Ikoma, N.; Pestana, R.C.; Vauthey, J.N.; Allison, J.P.; Sharma, P. Immunologic Correlates of Pathologic Complete Response to Preoperative Immunotherapy in Hepatocellular Carcinoma. Cancer Immunol. Res. 2019, 7, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Nobuoka, D.; Motomura, Y.; Shirakawa, H.; Yoshikawa, T.; Kuronuma, T.; Takahashi, M.; Nakachi, K.; Ishii, H.; Furuse, J.; et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2011, 40, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, W.M.D.; Loke, K.S.H.; Gogna, A.; Tan, S.H.; Ng, D.C.E.; Hennedige, T.P.; Irani, F.; Lee, J.J.X.; Too, C.W.; Ng, M.C.; et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209-678. J Clin. Oncol. 2020, 38, 4590. [Google Scholar] [CrossRef]
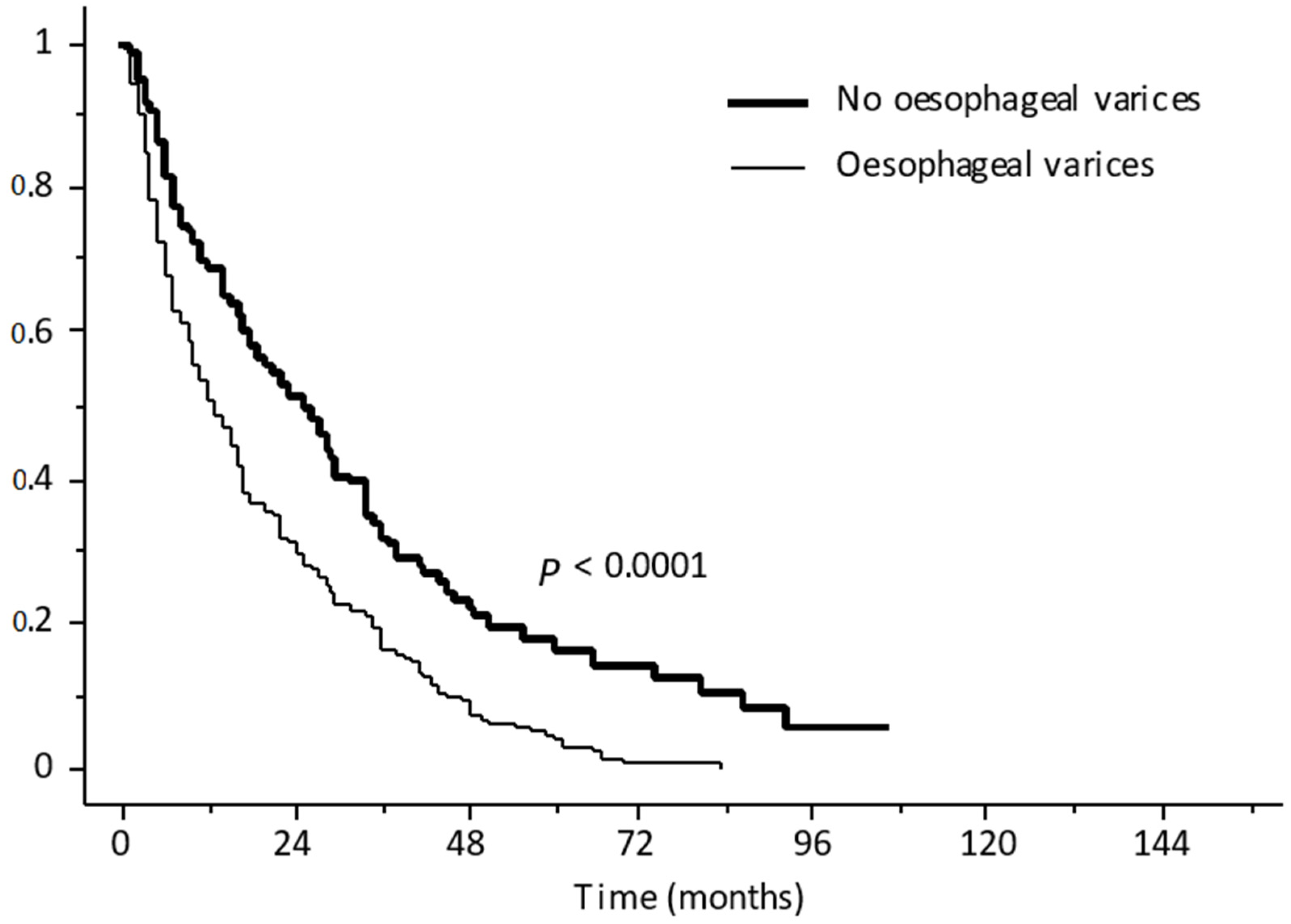
- Giannini, E.G.; Risso, D.; Testa, R.; Trevisani, F.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnù, L.; Rapaccini, G.L.; Farinati, F.; Zoli, M.; et al. Prevalence and Prognostic Significance of the Presence of Esophageal Varices in Patients with Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2006, 4, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, G.; Pasta, L.; Morabito, A.; Caltagirone, M.; Malizia, G.; Tinè, F.; Giannuoli, G.; Traina, M.; Vizzini, G.; Politi, F.; et al. Competing risks and prognostic stages of cirrhosis: A 25-year inception cohort study of 494 patients. Aliment. Pharmacol. Ther. 2014, 39, 1180–1193. [Google Scholar] [CrossRef] [Green Version]
- Russo, F.P.; Imondi, A.; Lynch, E.N.; Farinati, F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig. Liver Dis. 2018, 50, 640–646. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
| Trial Name | Phase | Line of Treatment | Design | Patients Enrolled | Endpoints | ClinicalTrial.gov | Company | Status |
|---|---|---|---|---|---|---|---|---|
| GO30140 | I | First-line | Atezolizumab + Bevacizumab (arm A) Atezolizumab + Bevacizumab (arm F1) Atezolizumab (arm F2) | 430 | Safety, efficacy, pharmacokinetics | NCT02715531 | Hoffmann-La Roche | Active, not recruiting |
| - | I | No restriction | Ramucirumab + MEDI4736 [HCC] (arm C) | 114 | DLTs | NCT02572687 | Eli Lilly & Co/Astra Zeneca | Active, not recruiting |
| NUANCE | I | Second-line | Nivolumab + bevacizumab | 1 | Safety and tolerability | NCT03382886 | University of Utah | Terminated |
| - | I | Neo-adjuvant | Nivolumab + cabozantinib | 15 | Safety and tolerability | NCT 03299946 | Sidney Kimmel Compehensive Cancer Center at John Hopkins | Active, not recruiting |
| - | Ib | First-line | Regorafenib + pembrolizumab | 57 | Safety and tolerability | NCT03347292 | Bayer | Recruiting |
| - | Ib | First-line | Pembrolizumab + lenvatinib | 104 | Safety and tolerability | NCT 03006926 | Eisai Co., Ltd. | Active, not recruiting |
| - | Ib | First-line | Nivolumab + lenvatinib | 30 | Safety and tolerability | NCT03418922 | Eisai Co., Ltd. | Active, not recruiting |
| - | Ib | Second-line | Sintilimab + IBI305 | 47 | AEs/ORR | NCT04401813 | Innovent Biologics (Suzhou) Co., Ltd. | Recruiting |
| - | I/IIa | First-line | Nivolumab + Pexastimogene devacirepvec | Safety and tolerability | NCT03071094 | Transgene | Active, not recruiting | |
| CheckMate 040 | I/II | Second-line | Cohort 4: Nivolumab + ipilimumab Cohort 6: Nivolumab + cabozantinib | 148 | Safety and tolerability | NCT01658878 | Bristol-Myers Squibb/Ono Pharmaceutical Co., Ltd. | Active, not recruiting |
| - | I/II | Second-line | SHR-1210 + apatinib | 60 | OS | NCT02942329 | The Affiliated Hospital of the Chinese Academy of Military Medical Sciences | Unknown |
| - | Ib/II | First-line | Pembrolizumab + talimogene laherarepvec | 244 | ORR/DLTs | NCT02509507 | Amgen | Recruiting |
| - | II | First-line and Second-line | Durvalumab + tremelimmumab [regimen 1] (arm A) Durvalumab (arm B) Tremelimumab (arm C) Durvalumab + tremelimumab [regimen 2] (arm D) Durvalumab + bevacizumab (arm E) | 545 | Safety and tolerability | NCT02519348 | MedImmune, LLC | Active, not recruiting |
| RESCUE | II | Second-line | SHR-1210 + apatinib | 190 | ORR | NCT03463876 | Jiangsu HengRui Medicine Co., Ltd. | Active, not recruiting |
| - | II | First-line/Second-line | SHR1210 + apatinib (arm A) SHR1210 + FOLFOX4 or GEMOX regimen (arm B) | 152 | Safety and tolerability | NCT03092895 | Jiangsu HengRui Medicine Co., Ltd. | Unknown |
| IMMUNIB | II | First-line | Nivolumab + lenvatinib | 50 | ORR/safety and tolerability | NCT03841201 | Institut fur Klinische Krebsforschung IKF GmbH | Recruiting |
| - | II | First-line/Second-line | Nivolumab + Ipilimumab vs. nivolumab | Safety and tolerability | NCT03222076 | MD Anderson Cancer Center | Active, not recruiting | |
| - | II/III | First-line | Sintilimab + IBI305 | 566 | OS/PFS | NCT03794440 | Innovent Biologics (Suzhou) Co., Ltd. | Recruiting |
| IMbrave150 | III | First-line | Atezolizumab + bevacizumab (arm A) Sorafenib (arm B) | 480 | OS/PFS | NCT03434379 | Hoffmann-La Roche | Active, not recruiting |
| COSMIC-312 | III | First-line | Cabozantinib + atezolizumab (arm A) Sorafenib (arm B) Cabozantinib (arm C) | 740 | PFS/OS | NCT03755791 | Exelixis | Recruiting |
| LEAP-002 | III | First-line | Pembrolizumab + Lenvatinib vs. placebo + lenvatinib | 750 | PFS/OS | NCT03713593 | Merck Sharp & Dohme Corp. | Active, not recruiting |
| - | III | First-line | SHR-1210 + FOLFOX4 vs. sorafenib or FOLFOX4 | 448 | OS | NCT03605706 | Jiangsu HengRui Medicine Co., Ltd. | Recruiting |
| HIMALAYA | III | First-line | Durvalumab (arm A) Durvalumab + tremelimumab [regimen 1] (arm B) Durvalumab + tremelimumab [regimen 2] (arm C) Sorafenib (arm D) | 1310 | OS | NCT03298451 | AstraZeneca | Active, not recruiting |
| - | III | First-line | CS1003 + lenvatinib vs. placebo + lenvatinib | 525 | PFS/OS | NCT04194775 | CStone Pharmaceuticals | Recruiting |
| IMBrave-150 Inclusion Criteria |
|---|
| Age ≥ 18 years |
| Locally advanced or metastatic and/or unresectable HCC |
| No prior systemic therapy for HCC |
| Disease that is not amenable to curative surgical and/or locoregional therapies, or progressive disease after surgical and/or locoregional therapies |
| At least one measurable (per RECIST 1.1) untreated lesion |
| Patients who received prior local therapy (e.g., radiofrequency ablation, percutaneous ethanol or acetic acid injection, cryoablation, high-intensity focused ultrasound, transarterial chemoembolization, transarterial embolization, etc.) are eligible provided the target lesion(s) have not been previously treated with local therapy or the target lesion(s) within the field of local therapy have subsequently progressed in accordance with RECIST version 1.1 |
| ECOG PS 0-1 |
| Child–Pugh class A |
| ANC ≥ 1.5 × 109/L (1500/mcL) without granulocyte colony-stimulating factor support |
| Lymphocyte count ≥ 0.5 × 109/L (500/µL) |
| Platelet count ≥ 75 × 109/L (75,000/µL) without transfusion |
| Hemoglobin ≥ 90 g/L (9 g/dL) |
| AST, ALT, and alkaline phosphatase (ALP) ≤ 5 × upper limit of normal (ULN) |
| Serum bilirubin ≤ 3 × ULN |
| Serum creatinine ≤ 1.5 × ULN or creatinine clearance ≥ 50 mL/min (Cockcroft–Gault formula) |
| Serum albumin ≥ 28 g/L |
| For patients not receiving therapeutic anticoagulation: INR or aPTT ≤ −2 × ULN |
| Urine dipstick for proteinuria < 2+ |
| Negative HIV test at screening |
| In case of active HBV, HBV DNA < 500 IU/mL and anti-HBV treatment for a minimum of 14 days prior to study entry |
| No history of leptomeningeal disease |
| No active or history of autoimmune disease or immune deficiency |
| No history of idiopathic pulmonary fibrosis, organizing pneumonia, drug-induced pneumonitis, or idiopathic pneumonitis, or evidence of active pneumonitis |
| No active tuberculosis |
| No significant cardiovascular disease (≥NYHA Class II) |
| No major surgical procedure, other than for diagnosis, within 4 weeks |
| No history of malignancy other than HCC within 5 years prior to screening |
| No severe infection within 4 weeks prior to initiation of study treatment |
| No treatment with therapeutic oral or IV antibiotics within 2 weeks prior to initiation of study treatment |
| No prior allogeneic stem cell or solid organ transplantation |
| No known fibrolamellar HCC, sarcomatoid HCC, or mixed cholangiocarcinoma and HCC |
| No untreated or incompletely treated varices with bleeding or high risk for bleeding |
| No moderate or severe ascites |
| No history of hepatic encephalopathy |
| No co-infection of HBV and HCV |
| No symptomatic, untreated, or actively progressing central nervous system (CNS) metastases |
| No uncontrolled pleural effusion, pericardial effusion, or ascites requiring recurrent drainage procedures |
| No uncontrolled or symptomatic hypercalcemia |
| No treatment with systemic immunosuppressive medication |
| No inadequately controlled arterial hypertension |
| No significant vascular disease |
| No history of intra-abdominal inflammatory process |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaz Torres, M.C.; Lai, Q.; Piscaglia, F.; Caturelli, E.; Cabibbo, G.; Biasini, E.; Pelizzaro, F.; Marra, F.; Trevisani, F.; Giannini, E.G. Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting. J. Clin. Med. 2021, 10, 3201. https://doi.org/10.3390/jcm10153201
Plaz Torres MC, Lai Q, Piscaglia F, Caturelli E, Cabibbo G, Biasini E, Pelizzaro F, Marra F, Trevisani F, Giannini EG. Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting. Journal of Clinical Medicine. 2021; 10(15):3201. https://doi.org/10.3390/jcm10153201
Chicago/Turabian StylePlaz Torres, Maria Corina, Quirino Lai, Fabio Piscaglia, Eugenio Caturelli, Giuseppe Cabibbo, Elisabetta Biasini, Filippo Pelizzaro, Fabio Marra, Franco Trevisani, and Edoardo G. Giannini. 2021. "Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting" Journal of Clinical Medicine 10, no. 15: 3201. https://doi.org/10.3390/jcm10153201
APA StylePlaz Torres, M. C., Lai, Q., Piscaglia, F., Caturelli, E., Cabibbo, G., Biasini, E., Pelizzaro, F., Marra, F., Trevisani, F., & Giannini, E. G. (2021). Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting. Journal of Clinical Medicine, 10(15), 3201. https://doi.org/10.3390/jcm10153201







