Effectiveness of Mind–Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Registration
2.2. Patients
2.3. Randomisation, Allocation Concealment and Blinding
2.4. Mind–Body Intervention
- Contemplative practices, including (a) exercises designed to activate a relaxation response in the body as well as body awareness (b) mindfulness meditation, and (c) zen-meditation.
- Psychoeducation, i.e., educational and informative lectures, materials and pen-and-paper exercises concerning (a) physical, psychological, and social health promotion, (b) stress prevention and resilience, and (c) disease-specific mechanisms (e.g., typical symptoms, possible developmental paths, possible risks and beneficial effects, and specific health-related advice).
- Dialogue, including therapist–group dialogue as well as participant–participant dialogue. Participants were paired in smaller units (2 participants per unit) and were encouraged to continue dialogues and discussion outside of the treatment sessions. Moreover, each participant was offered 5 individual consultations with the therapist.
2.5. Patient-Reported Outcome Measures
2.6. Statistical Methods
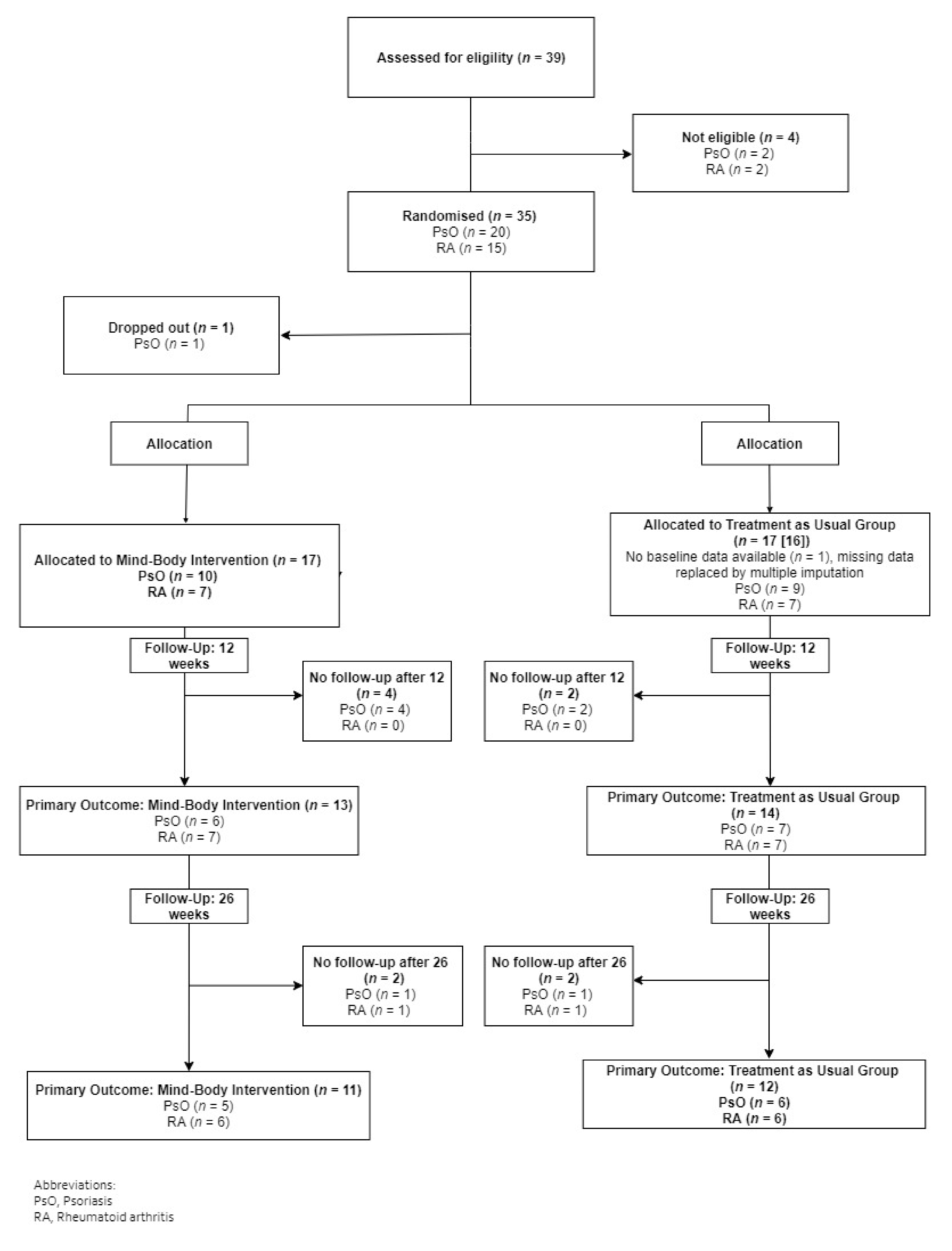
3. Results
3.1. Baseline Characteristics
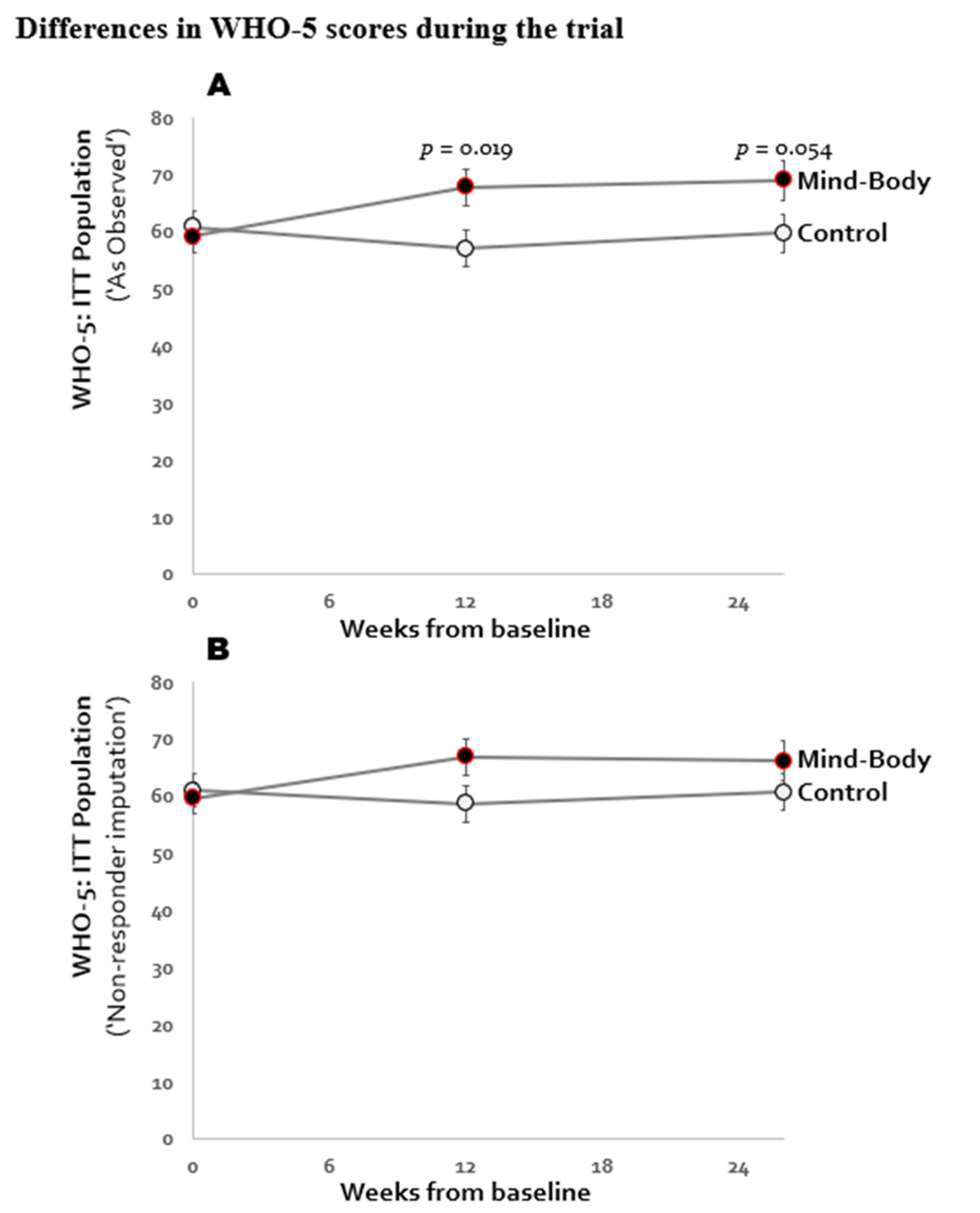
3.2. Primary Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO-5 | The World Health Organisation-Five Well-Being Index |
| SF-36 PCS | Medical Outcomes Study 36-Item Short-Form Health Survey, Physical Component Summary |
References
- Naylor, C.; Parsonage, M.; McDaid, D.; Knapp, M.; Fossey, M.; Galea, A. Long-Term Conditions and Mental Health: The Cost of Co-Morbidities. 2012. Available online: http://www.kingsfund.org.uk/publications/mental_health_ltcs.html (accessed on 8 December 2020).
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Rigas, H.M.; Bucur, S.; Ciurduc, D.M.; Nita, I.E.; Constantin, M.M. Psychological stress and depression in psoriasis patients—A dermatologist’s perspective. Maedica 2019, 14, 287. [Google Scholar]
- Sturgeon, J.A.; Finan, P.H.; Zautra, A.J. Affective disturbance in rheumatoid arthritis: Psychological and disease-related pathways. Nat. Rev. Rheumatol. 2016, 12, 532–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nast, A.; Gisondi, P.; Ormerod, A.D.; Saiag, P.; Smith, C.H.; Spuls, P.I.; Arenberger, P.; Bachelez, H.; Barker, J.; Dauden, E. European s3-guidelines on the systemic treatment of psoriasis vulgaris-update 2015-short version-edf in cooperation with eadv and ipc. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2277–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H. 2015 american college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Misery, L.; Shourick, J.; Sénéschal, J.; Paul, C.; de Pouvourville, G.; Jullien, D.; Mahé, E.; Bachelez, H.; Aubert, R.; Joly, P. Use of mind-body practices by patients with psoriasis: Results from a study on 2562 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 35, e305–e307. [Google Scholar]
- Timis, T.L.; Florian, I.A.; Mitrea, D.R.; Orasan, R. Mind-body interventions as alternative and complementary therapies for psoriasis: A systematic review of the english literature. Medicina 2021, 57, 410. [Google Scholar] [CrossRef] [PubMed]
- Leverone, D.; Epstein, B.J. Nonpharmacological interventions for the treatment of rheumatoid arthritis: A focus on mind-body medicine. J. Pharm. Pract. 2010, 23, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; DiRenzo, D.D. Complementary and alternative medicine use in rheumatoid arthritis. Curr. Rheumatol. Rep. 2020, 22, 61. [Google Scholar] [CrossRef]
- Gamret, A.C.; Price, A.; Fertig, R.M.; Lev-Tov, H.; Nichols, A.J. Complementary and alternative medicine therapies for psoriasis: A systematic review. JAMA Dermatol. 2018, 154, 1330–1337. [Google Scholar] [CrossRef]
- Tabish, S.A. Complementary and alternative healthcare: Is it evidence-based? Int. J. Health Sci. 2008, 2, 5–9. [Google Scholar]
- Beese, R.J.; Ringdahl, D. Enhancing spiritually based care through gratitude practices: A health-care improvement project. Creat. Nurs. 2018, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Cropley, M.; Smith, H.E.; Feigin, V.L.; McPherson, K. Mind and body therapy for fibromyalgia. Cochrane Database Syst. Rev. 2015, 4. [Google Scholar] [CrossRef]
- Niles, B.L.; Mori, D.L.; Polizzi, C.; Pless Kaiser, A.; Weinstein, E.S.; Gershkovich, M.; Wang, C. A systematic review of randomized trials of mind-body interventions for ptsd. J. Clin. Psychol. 2018, 74, 1485–1508. [Google Scholar] [CrossRef]
- Wahbeh, H.; Elsas, S.-M.; Oken, B.S. Mind–body interventions: Applications in neurology. Neurology 2008, 70, 2321–2328. [Google Scholar] [CrossRef] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.G.; Lansner, J.; Petersen, A.; Vangkilde, S.A.; Ringkøbing, S.P.; Frokjaer, V.G.; Adamsen, D.; Knudsen, G.M.; Denninger, J.W.; Hasselbalch, S.G. Open and calm—A randomized controlled trial evaluating a public stress reduction program in denmark. BMC Public Health 2015, 15, 1245. [Google Scholar] [CrossRef] [PubMed]
- Park, E.R.; Traeger, L.; Vranceanu, A.-M.; Scult, M.; Lerner, J.A.; Benson, H.; Denninger, J.; Fricchione, G.L. The development of a patient-centered program based on the relaxation response: The relaxation response resiliency program (3rp). Psychosomatics 2013, 54, 165–174. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life; Hyperion: New York, NY, USA, 1994. [Google Scholar]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The who-5 well-being index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Framework, I.C. The mos 36-item short-form health survey (sf-36). Med. Care 1992, 30, 473–483. [Google Scholar]
- Bech, P.; Lunde, M.; Bech-Andersen, G.; Lindberg, L.; Martiny, K. Psychiatric outcome studies (pos): Does treatment help the patients? A popperian approach to research in clinical psychiatry: 25th anniversary report from the psychiatric research unit, frederiksborg general hospital, denmark. Nord. J. Psychiatry 2007, 61, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Altman, D.G. Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detry, M.A.; Ma, Y. Analyzing repeated measurements using mixed models. JAMA 2016, 315, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Little, R.J.; D’Agostino, R.; Cohen, M.L.; Dickersin, K.; Emerson, S.S.; Farrar, J.T.; Frangakis, C.; Hogan, J.W.; Molenberghs, G.; Murphy, S.A. The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 2012, 367, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.H. Interpreting Incomplete Data in Studies of Diet and Weight Loss. N. Engl. J. Med. 2003, 348, 2136–2137. [Google Scholar] [CrossRef]
- Detry, M.A.; Lewis, R.J. The intention-to-treat principle: How to assess the true effect of choosing a medical treatment. JAMA 2014, 312, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Eberth, J.; Sedlmeier, P. The effects of mindfulness meditation: A meta-analysis. Mindfulness 2012, 3, 174–189. [Google Scholar] [CrossRef]
- Alsubaie, M.; Abbott, R.; Dunn, B.; Dickens, C.; Keil, T.F.; Henley, W.; Kuyken, W. Mechanisms of action in mindfulness-based cognitive therapy (mbct) and mindfulness-based stress reduction (mbsr) in people with physical and/or psychological conditions: A systematic review. Clin. Psychol. Rev. 2017, 55, 74–91. [Google Scholar] [CrossRef]
- Bohlmeijer, E.; Prenger, R.; Taal, E.; Cuijpers, P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: A meta-analysis. J. Psychosom. Res. 2010, 68, 539–544. [Google Scholar] [CrossRef]
- Gu, J.; Strauss, C.; Bond, R.; Cavanagh, K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin. Psychol. Rev. 2015, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khoury, B.; Sharma, M.; Rush, S.E.; Fournier, C. Mindfulness-based stress reduction for healthy individuals: A meta-analysis. J. Psychosom. Res. 2015, 78, 519–528. [Google Scholar] [CrossRef]
- Fordham, B.; Griffiths, C.; Bundy, C. A pilot study examining mindfulness-based cognitive therapy in psoriasis. Psychol. Health Med. 2015, 20, 121–127. [Google Scholar] [CrossRef]
- D’Alton, P.; Kinsella, L.; Walsh, O.; Sweeney, C.; Timoney, I.; Lynch, M.; O’Connor, M.; Kirby, B. Mindfulness-based interventions for psoriasis: A randomized controlled trial. Mindfulness 2019, 10, 288–300. [Google Scholar] [CrossRef]
- Zill, J.; Christalle, E.; Tillenburg, N.; Mrowietz, U.; Augustin, M.; Härter, M.; Dirmaier, J. Effects of psychosocial interventions on patient-reported outcomes in patients with psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 181, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Fordham, B.; Griffiths, C.E.; Bundy, C. Can stress reduction interventions improve psoriasis? A review. Psychol. Health Med. 2013, 18, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Tolahunase, M.; Kumar, U.; Dada, R. Impact of yoga based mind-body intervention on systemic inflammatory markers and co-morbid depression in active rheumatoid arthritis patients: A randomized controlled trial. Restor. Neurol. Neurosci. 2019, 37, 41–59. [Google Scholar] [CrossRef]
- Pukšić, S.; Mitrović, J.; Čulo, M.-I.; Živković, M.; Orehovec, B.; Bobek, D.; Morović-Vergles, J. Effects of Yoga in Daily Life program in rheumatoid arthritis: A randomized controlled trial. Complement. Ther. Med. 2021, 57, 102639. [Google Scholar] [CrossRef]
- Jensen, P.; Zachariae, C.; Christensen, R.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Effect of weight loss on the severity of psoriasis: A randomized clinical study. JAMA Dermatol. 2013, 149, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Stadtmüller, L.; Eckardt, M.; Zick, C.; Kupfer, J.; Schut, C. Investigation of predictors of interest in a brief mindfulness-based intervention and its effects in patients with psoriasis at a rehabilitation clinic (skinmind): An observational study and randomised controlled trial. BMJ Open 2020, 10, e033952. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.D.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Gniadecki, R.; Dam, T.N.; Iversen, L.; Skov, L. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: A danish nationwide study. Acta Derm. Venereol. 2019, 99, 1224–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Mind–Body Intervention Group (n = 17) | Treatment as Usual Group (n = 17) | Groups Combined (n = 34) | |||
|---|---|---|---|---|---|---|
| Males, no (%) | 5 | 29% | 5 | 29% | 10 | 29% |
| Mean | Std Dev | Mean | Std Dev | Mean | SD | |
| Age, years | 51.7 | 11.7 | 44.4 | 12.9 | 48.0 | 12.7 |
| Disease duration, years | 19.1 | 14.8 | 20.4 | 10.8 | 19.7 | 12.8 |
| Height, cm | 170.2 | 16.5 | 169.5 | 9.5 | 169.8 | 13.3 |
| Weight, kg | 82.2 | 20.5 | 77.7 | 17.0 | 80.0 | 18.7 |
| BMI, kg/m2 | 28.5 | 6.5 | 27.0 | 5.2 | 27.8 | 5.8 |
| WHO-5, score: 0–100 | 56.0 | 17.6 | 63.8 | 13.5 | 59.9 | 16.0 |
| SF-36 MCS, score: 0–100 | 49.7 | 11.7 | 50.8 | 6.5 | 50.2 | 9.4 |
| SF-36 PCS, score: 0–100 | 44.7 | 9.9 | 42.4 | 10.0 | 43.6 | 9.9 |
| SF-36 PF, score: 0–100 | 76.5 | 20.4 | 80.6 | 14.5 | 78.6 | 17.6 |
| SF-36 RP, score: 0–100 | 67.6 | 39.3 | 59.0 | 42.1 | 63.3 | 40.4 |
| SF-36 BP, score: 0–100 | 70.1 | 22.7 | 58.5 | 27.9 | 64.3 | 25.8 |
| SF-36 GH, score: 0–100 | 51.8 | 18.5 | 50.7 | 19.7 | 51.3 | 18.8 |
| SF-36 VT, score: 0–100 | 53.5 | 20.4 | 56.7 | 14.9 | 55.1 | 17.6 |
| SF-36 SF, score: 0–100 | 80.3 | 25.8 | 77.4 | 19.9 | 78.8 | 22.7 |
| SF-36 RE, score: 0–100 | 70.9 | 37.0 | 74.8 | 30.1 | 72.9 | 33.3 |
| SF-36 MH, score: 0–100 | 78.2 | 14.8 | 77.4 | 9.9 | 77.8 | 12.4 |
| MBI (n = 17) | TAU (n = 17) | MBI vs. TAU | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | LS Means | SE | LS Means | SE | Difference between Groups | LCL 95% to UCL 95% | p-Value | |
| WHO-5, score: 0–100 | 65.3 | 2.2 | 59.1 | 2.2 | −6.15 | (−12.56 to 0.26) | 0.060 | |
| SF-36 PCS, score: 0–100 | 45.1 | 1.1 | 42.1 | 1.1 | −3.05 | (−6.25 to 0.16) | 0.062 | |
| SF-36 MCS, score: 0–100 | 51.3 | 1.0 | 50.6 | 1.0 | −0.64 | (−3.60 to 2.32) | n.e. | |
| SF-36 PF, score: 0–100 | 81.6 | 2.5 | 74.3 | 2.5 | −7.26 | (−14.41 to −0.11) | n.e. | |
| SF-36 RP, score: 0–100 | 71.9 | 4.6 | 60.6 | 4.6 | −11.26 | (−24.58 to 2.05) | n.e. | |
| SF-36 BP, score: 0–100 | 69.4 | 2.9 | 62.3 | 2.9 | −7.07 | (−15.66 to 1.52) | n.e. | |
| SF-36 GH, score: 0–100 | 56.2 | 2.0 | 52.0 | 2.0 | −4.23 | (−10.01 to 1.55) | n.e. | |
| SF-36 VT, score: 0–100 | 58.6 | 2.8 | 49.5 | 2.7 | −9.16 | (−17.09 to −1.23) | n.e. | |
| SF-36 SF, score: 0–100 | 81.4 | 2.7 | 79.2 | 2.7 | −2.21 | (−10.10 to 5.68) | n.e. | |
| SF-36 RE, score: 0–100 | 80.01 | 3.7 | 76.9 | 3.7 | −3.16 | (−13.51 to 7.20) | n.e. | |
| SF-36 MH, score: 0–100 | 79.0 | 1.6 | 77.5 | 1.6 | −1.47 | (−5.99 to 3.05) | n.e. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Jensen, C.G.; Khoury, L.; Deleuran, B.; Blom, E.S.; Breinholt, T.; Christensen, R.; Skov, L. Effectiveness of Mind–Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial. J. Clin. Med. 2021, 10, 3107. https://doi.org/10.3390/jcm10143107
Nguyen TT, Jensen CG, Khoury L, Deleuran B, Blom ES, Breinholt T, Christensen R, Skov L. Effectiveness of Mind–Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial. Journal of Clinical Medicine. 2021; 10(14):3107. https://doi.org/10.3390/jcm10143107
Chicago/Turabian StyleNguyen, Thao Thi, Christian G. Jensen, Lina Khoury, Bent Deleuran, Esther S. Blom, Thomas Breinholt, Robin Christensen, and Lone Skov. 2021. "Effectiveness of Mind–Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial" Journal of Clinical Medicine 10, no. 14: 3107. https://doi.org/10.3390/jcm10143107
APA StyleNguyen, T. T., Jensen, C. G., Khoury, L., Deleuran, B., Blom, E. S., Breinholt, T., Christensen, R., & Skov, L. (2021). Effectiveness of Mind–Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial. Journal of Clinical Medicine, 10(14), 3107. https://doi.org/10.3390/jcm10143107






