5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
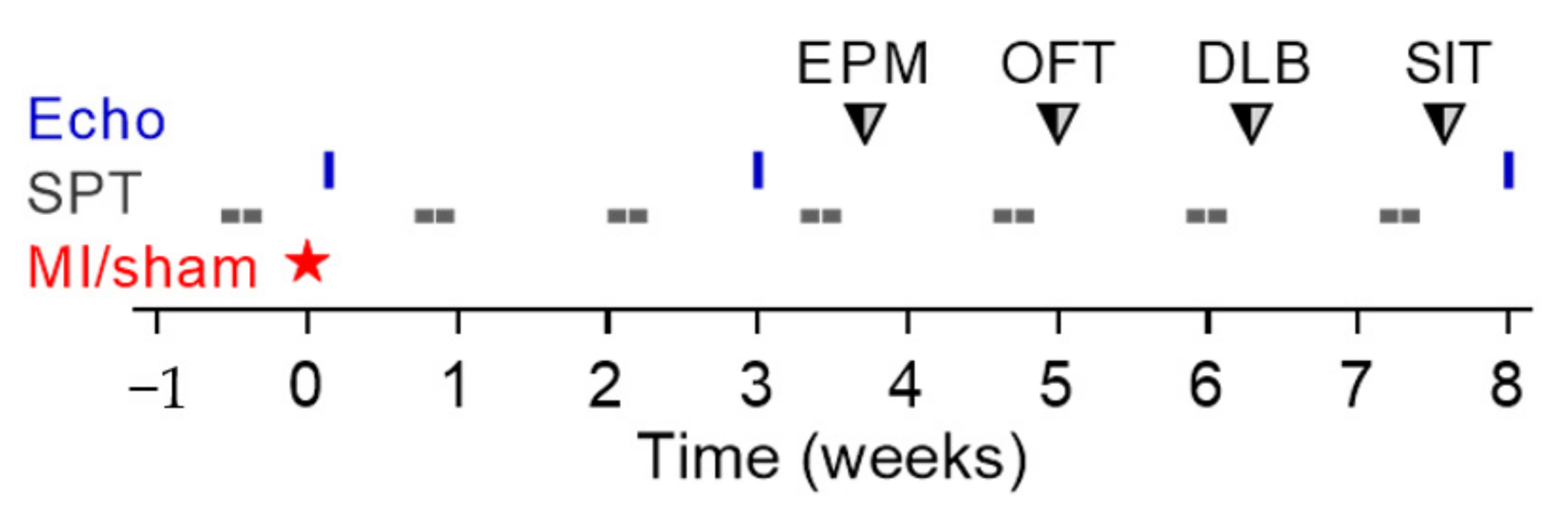
2.3. Experimental Design
2.3.1. Experiment 1: Baseline Cardiological Evaluation
2.3.2. Experiment 2: Long-Term Study
2.3.3. Experiment 3: Short-Term Study
2.4. Myocardial Infarction (MI) and Sham Operation
2.5. Echocardiographic Analysis
2.6. Hemodynamic Study
2.7. Electrocardiography
2.8. Sucrose Preference Test (SPT)
2.9. Exploration-Based Approach-Avoidance Conflict Tests
2.10. Sample Collection, Determination of Infarct Size, Ventricular Remodeling, and Quantification of Heart Failure
2.11. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.12. High-Performance Liquid Chromatography (HPLC)
2.13. Infarct Size Measurement in the Short-Term Study after Myocardial Infarction (Evans Blue/TTC-Staining)
2.14. Histological Evaluation of the Neutrophil Influx into the Scar
2.15. FACS Analysis of the Infarcted Tissue
2.16. Collagen Quantification
2.17. Statistical Analysis
3. Results
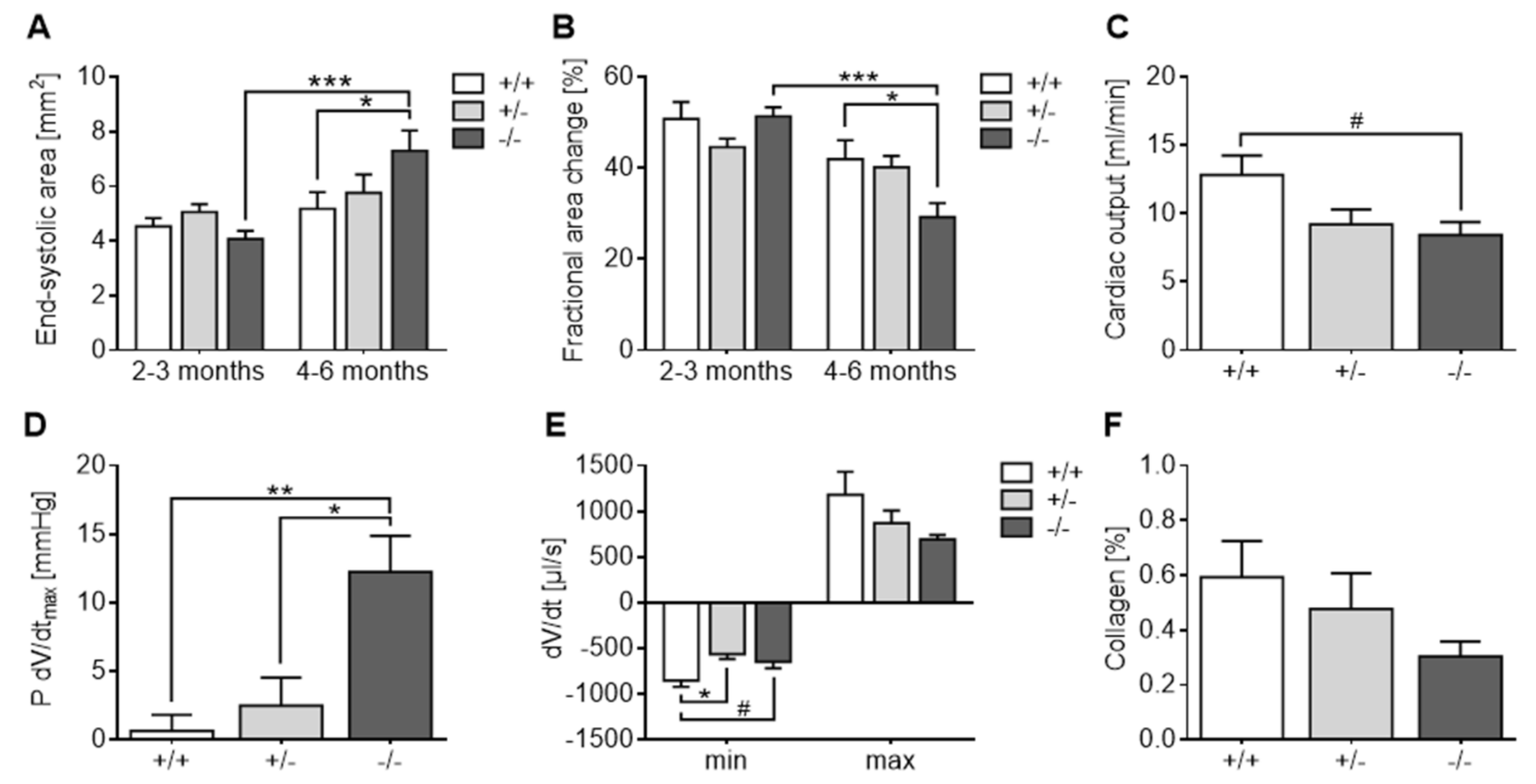
3.1. Experiment 1: Baseline Cardiological Evaluation
3.2. Experiment 2: Long-Term Study
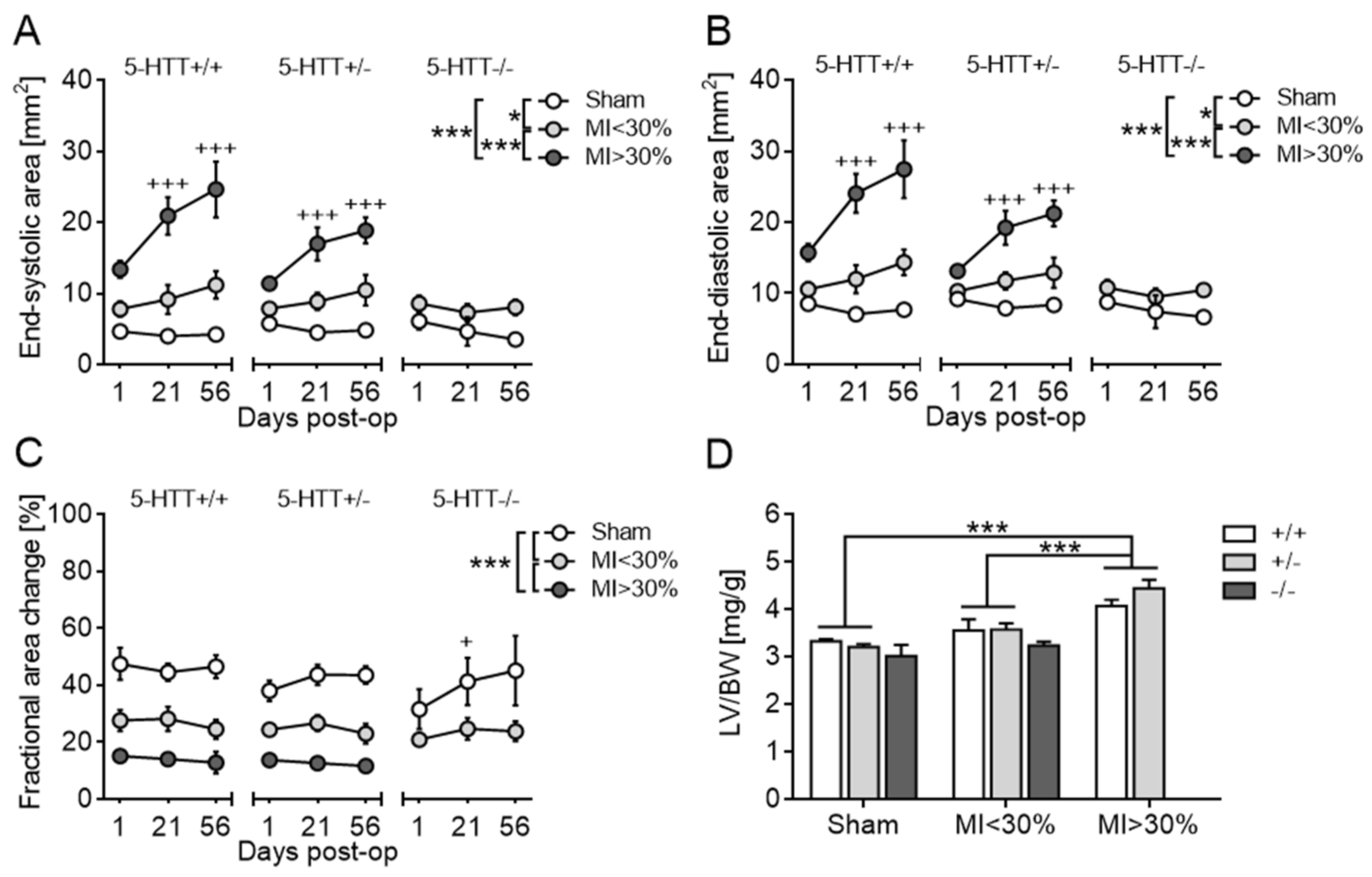
3.2.1. Impaired Survival of 5-HTT−/− Mice after MI
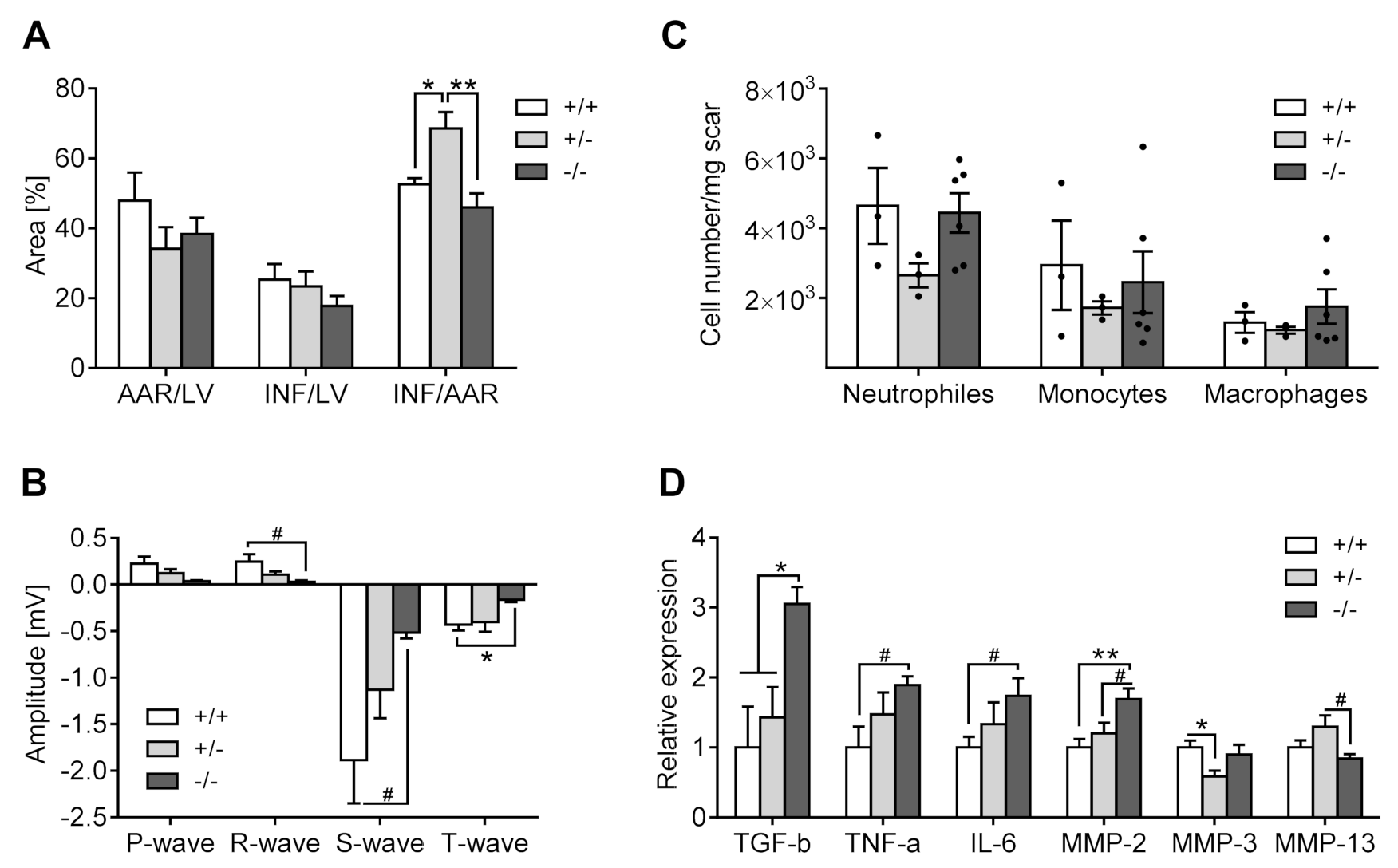
3.2.2. Infarct Size Quantification
3.2.3. Echocardiography and Terminal Organ Weights
3.2.4. Collagen Quantification
3.2.5. Body Weight, Liquid Intake and Sucrose Preference
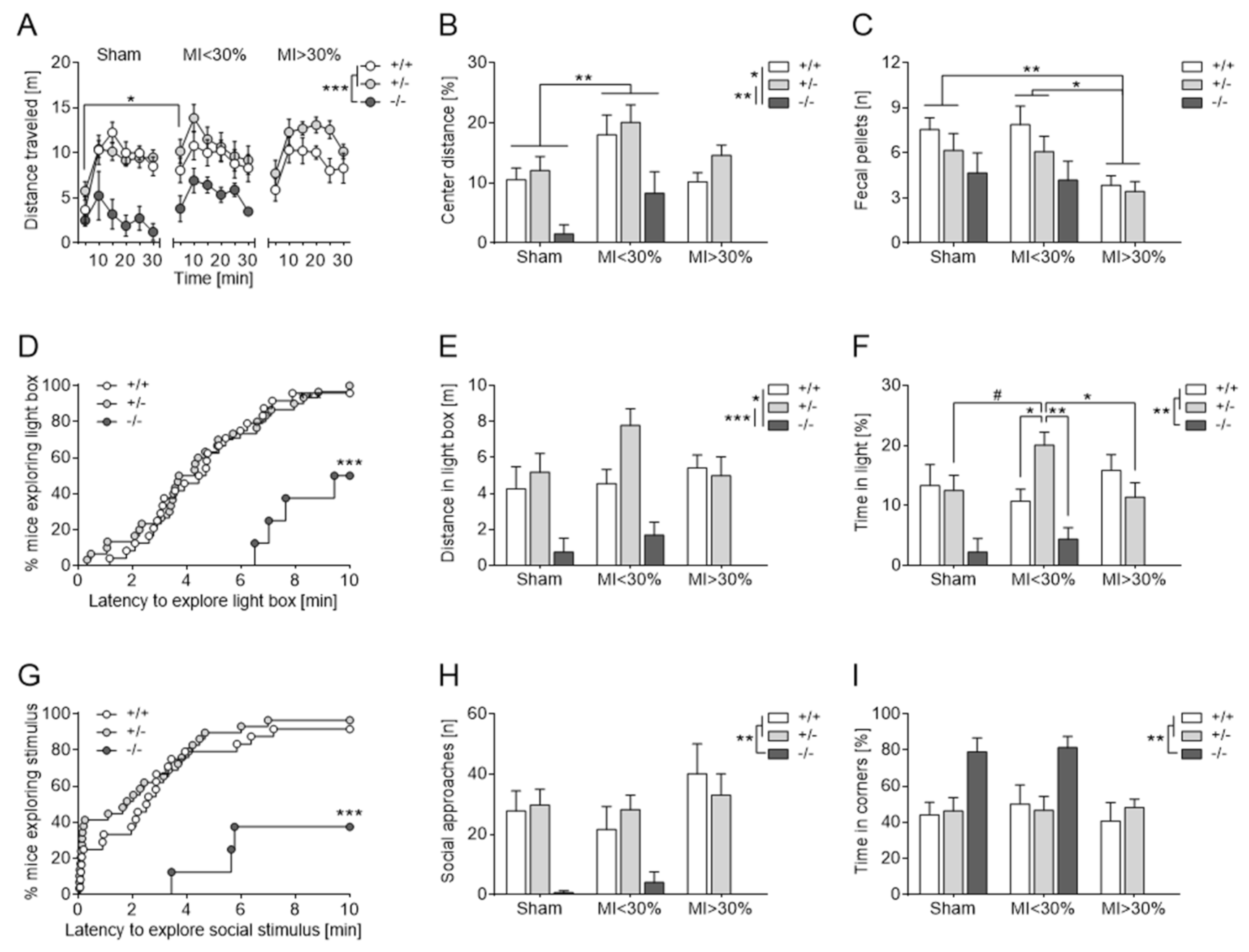
3.2.6. Approach-Avoidance Conflict Tests
Elevated Plus Maze
Open Field Test
Dark-Light Box
Social Interaction Test
3.3. Experiment 3: Short-Term Study
3.3.1. Infarct Size Measurement (Evans Blue/TTC Staining)
3.3.2. Electrocardiography
3.3.3. Inflammation
3.3.4. Gene Expression
Inflammation
Modifications of the Extracellular Matrix
Modification of Serotonin Metabolism
3.3.5. HPLC
4. Discussion
4.1. Baseline Characterization
4.2. Long-Term Study and Behavioral Changes after Myocardial Infarction
4.3. Mechanistic Considerations in the Short-Term Study
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano, C.V., Jr.; Tiemi Setani, K.; Sakamoto, E.; Andrei, A.M.; Fraguas, R. Association between depression and development of coronary artery disease: Patho-physiologic and diagnostic implications. Vasc. Health Risk Manag. 2011, 7, 159–164. [Google Scholar]
- McDermott, M.M.; Schmitt, B.; Wallner, E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Arch. Intern. Med. 1997, 157, 1921–1929. [Google Scholar] [CrossRef]
- Celano, C.M.; Millstein, R.A.; Bedoya, C.A.; Healy, B.C.; Roest, A.; Huffman, J.C. Association between anxiety and mortality in patients with coronary artery disease: A meta-analysis. Am. Heart J. 2015, 170, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angermann, C.E.; Gelbrich, G.; Störk, S.; Schowalter, M.; Deckert, J.; Ertl, G.; Faller, H. Somatic correlates of comorbid major depression in patients with systolic heart failure. Int. J. Cardiol. 2011, 147, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kuchibhatla, M.; Cuffe, M.S.; Christopher, E.J.; Alexander, J.D.; Clary, G.L.; Blazing, M.A.; Gaulden, L.H.; Califf, R.M.; Krishnan, R.R.; et al. Prognostic Value of Anxiety and Depression in Patients with Chronic Heart Failure. Circulation 2004, 110, 3452–3456. [Google Scholar] [CrossRef] [Green Version]
- Frey, A.; Popp, S.; Post, A.; Langer, S.; Lehmann, M.; Hofmann, U.; Sirén, A.-L.; Hommers, L.; Schmitt, A.; Strekalova, T.; et al. Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front. Behav. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Agrimi, J.; Spalletti, C.; Baroni, C.; Keceli, G.; Zhu, G.; Caragnano, A.; Matteucci, M.; Chelko, S.; Ramirez-Correa, G.A.; Bedja, D.; et al. Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion. EBioMedicine 2019, 47, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Lee, I.; Hammamieh, R.; Wang, K.; Baxter, D.; Scherler, K.; Etheridge, A.; Kulchenko, A.; Gautam, A.; Muhie, S.; et al. Molecular evidence of stress-induced acute heart injury in a mouse model simulating posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA 2014, 111, 3188–3193. [Google Scholar] [CrossRef] [Green Version]
- Razzoli, M.; Lindsay, A.; Law, M.L.; Chamberlain, C.M.; Southern, W.M.; Berg, M.; Osborn, J.; Engeland, W.C.; Metzger, J.M.; Ervasti, J.M.; et al. Social stress is lethal in the mdx model of Duchenne muscular dystrophy. EBioMedicine 2020, 55, 102700. [Google Scholar] [CrossRef] [Green Version]
- Agrimi, J.; Scalco, A.; Agafonova, J.; Iii, L.W.; Pansari, N.; Keceli, G.; Jun, S.; Wang, N.; Mastorci, F.; Tichnell, C.; et al. Psychosocial Stress Hastens Disease Progression and Sudden Death in Mice with Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2020, 9, 3804. [Google Scholar] [CrossRef]
- Carnevali, L.; Mastorci, F.; Audero, E.; Graiani, G.; Rossi, S.; Macchi, E.; Callegari, S.; Bartolomucci, A.; Nalivaiko, E.; Quaini, F.; et al. Stress-Induced Susceptibility to Sudden Cardiac Death in Mice with Altered Serotonin Homeostasis. PLoS ONE 2012, 7, e41184. [Google Scholar] [CrossRef]
- Dominguez-Lopez, S.; Howell, R.; Gobbi, G. Characterization of serotonin neurotransmission in knockout mice: Implications for major depression. Rev. Neurosci. 2012, 23, 429–443. [Google Scholar] [CrossRef]
- Mann, D.A.; Oakley, F. Serotonin paracrine signaling in tissue fibrosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Snider, J.C.; Riley, L.A.; Mallory, N.T.; Bersi, M.R.; Umbarkar, P.; Gautam, R.; Zhang, Q.; Mahadevan-Jansen, A.; Hatzopoulos, A.K.; Maroteaux, L.; et al. Targeting 5-HT 2B Receptor Signaling Prevents Border Zone Expansion and Improves Microstructural Remodeling After Myocardial Infarction. Circulation 2021, 143, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Jaffré, F.; Messaddeq, N.; Hickel, P.; Monassier, L.; Launay, J.-M.; Maroteaux, L. Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation 2003, 107, 3223–3229. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pedraza, J.Á.; Garcia, M.A.G.; Martín, M.; Rodriguezbarbero, A.; Morán, A. 5-HT2 receptor blockade exhibits 5-HT vasodilator effects via nitric oxide, prostacyclin and ATP-sensitive potassium channels in rat renal vasculature. Vasc. Pharmacol. 2016, 79, 51–59. [Google Scholar] [CrossRef]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and Blood Pressure Regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.L.; Lesch, K.-P. Targeting the murine serotonin transporter: Insights into human neurobiology. Nat. Rev. Neurosci. 2008, 9, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Maggiorani, D.; Manzella, N.; Edmondson, D.E.; Mattevi, A.; Parini, A.; Binda, C.; Mialet-Perez, J. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxid. Med. Cell Longev. 2017, 2017, 3017947. [Google Scholar] [CrossRef] [PubMed]
- Araragi, N.; Lesch, K.-P. Serotonin (5-HT) in the regulation of depression-related emotionality: Insight from 5-HT transporter and tryptophan hydroxylase-2 knockout mouse models. Curr. Drug Targets 2013, 14, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Tolliver, T.J.; Huang, S.-J.; Martin, B.J.; Andrews, A.M.; Wichems, C.; Holmes, A.; Lesch, K.-P.; Murphy, D.L. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 2005, 49, 798–810. [Google Scholar] [CrossRef]
- Mathews, T.A.; Fedele, D.E.; Coppelli, F.M.; Avila, A.M.; Murphy, D.L.; Andrews, A.M. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods 2004, 140, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Beaufour, C.; Evrard, A.; Rioux, A.; Hanoun, N.; Lesch, K.P.; Murphy, D.L.; Lanfumey, L.; Hamon, M.; Martres, M.-P. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur. J. Neurosci. 2000, 12, 2299–2310. [Google Scholar] [CrossRef]
- Li, Q.; Wichems, C.H.; Ma, L.; Van de Kar, L.D.; Garcia, F.; Murphy, D.L. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J. Neurochem. 2003, 84, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Yang, R.J.; Lesch, K.-P.; Crawley, J.N.; Murphy, D.L. Mice Lacking the Serotonin Transporter Exhibit 5-HT1A Receptor-Mediated Abnormalities in Tests for Anxiety-like Behavior. Neuropsychopharmacology 2003, 28, 2077–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.B.; Martin, V.S.; Trigo, J.M.; Chevarin, C.; Maldonado, R.; Fink, L.H.; Cunningham, K.A.; Hamon, M.; Lanfumey, L.; Mongeau, R. 5-HT2C Receptor Desensitization Moderates Anxiety in 5-HTT Deficient Mice: From Behavioral to Cellular Evidence. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.; Yang, R.J.; Murphy, D.L.; Crawley, J.N. Evaluation of Antidepressant-related Behavioral Responses in Mice Lacking the Serotonin Transporter. Neuropsychopharmacology 2002, 27, 914–923. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Fox, M.A.; Gallagher, P.S.; Murphy, D.L. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007, 6, 389–400. [Google Scholar] [CrossRef]
- Karabeg, M.M.; Grauthoff, S.; Kollert, S.Y.; Weidner, M.; Heiming, R.S.; Jansen, F.; Popp, S.; Kaiser, S.; Lesch, K.-P.; Sachser, N.; et al. 5-HTT Deficiency Affects Neuroplasticity and Increases Stress Sensitivity Resulting in Altered Spatial Learning Performance in the Morris Water Maze but Not in the Barnes Maze. PLoS ONE 2013, 8, e78238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krakenberg, V.; Von Kortzfleisch, V.T.; Kaiser, S.; Sachser, N.; Richter, S.H. Differential Effects of Serotonin Transporter Genotype on Anxiety-Like Behavior and Cognitive Judgment Bias in Mice. Front. Behav. Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef] [Green Version]
- Kolter, J.F.; Hildenbrand, M.F.; Popp, S.; Nauroth, S.; Bankmann, J.; Rother, L.; Waider, J.; Deckert, J.; Asan, E.; Jakob, P.M.; et al. Serotonin transporter genotype modulates resting state and predator stress-induced amygdala perfusion in mice in a sex-dependent manner. PLoS ONE 2021, 16, e0247311. [Google Scholar] [CrossRef]
- Houwing, D.J.; Buwalda, B.; Van Der Zee, E.A.; De Boer, S.F.; Olivier, J.D.A. The Serotonin Transporter and Early Life Stress: Translational Perspectives. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.; Li, Q.; Murphy, D.L.; Gold, E.; Crawley, J.N. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: The influence of genetic background. Genes Brain Behav. 2003, 2, 365–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Sato, A.; Kasai, S.; Hagino, Y.; Kotajima-Murakami, H.; Kashii, H.; Takamatsu, Y.; Nishito, Y.; Inagaki, M.; Mizuguchi, M.; et al. Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol. Autism 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Lira, A.; Zhou, M.; Castanon, N.; Ansorge, M.S.; A Gordon, J.; Francis, J.H.; Bradley-Moore, M.; Lira, J.; Underwood, M.; Arango, V.; et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry 2003, 54, 960–971. [Google Scholar] [CrossRef]
- Mekontso-Dessap, A.; Brouri, F.; Pascal, O.; Lechat, P.; Hanoun, N.; Lanfumey, L.; Seif, I.; Benhaiem-Sigaux, N.; Kirsch, M.; Hamon, M.; et al. Deficiency of the 5-Hydroxytryptamine Transporter Gene Leads to Cardiac Fibrosis and Valvulopathy in Mice. Circulation 2006, 113, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Eddahibi, S.; Hanoun, N.; Lanfumey, L.; Lesch, K.-P.; Raffestin, B.; Hamon, M.; Adnot, S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J. Clin. Investig. 2000, 105, 1555–1562. [Google Scholar] [CrossRef] [Green Version]
- Bengel, D.; Murphy, D.L.; Andrews, A.; Wichems, C.H.; Feltner, D.; Heils, A.; Mössner, R.; Westphal, H.; Lesch, K.-P. Altered Brain Serotonin Homeostasis and Locomotor Insensitivity to 3,4-Methylenedioxymethamphetamine (“Ecstasy”) in Serotonin Transporter-Deficient Mice. Mol. Pharmacol. 1998, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Schütt, M.; Palm, F.; Vogel, C.; Meier, M.; Schmitt, A.; Lesch, K.-P.; Mössner, R.; Sommer, C. Lack of the serotonin transporter in mice reduces locomotor activity and leads to gender-dependent late onset obesity. Int. J. Obes. 2010, 34, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Joeyen-Waldorf, J.; Edgar, N.; Sibille, E. The roles of sex and serotonin transporter levels in age- and stress-related emotionality in mice. Brain Res. 2009, 1286, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Margolis, K.J.; Gershon, M.D.; Schwartz, G.J.; Sze, J.Y. Reduced Serotonin Reuptake Transporter (SERT) Function Causes Insulin Resistance and Hepatic Steatosis Independent of Food Intake. PLoS ONE 2012, 7, e32511. [Google Scholar] [CrossRef] [Green Version]
- Du, X.-J. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc. Res. 2004, 63, 510–519. [Google Scholar] [CrossRef]
- Shioura, K.M.; Geenen, D.L.; Goldspink, P.H. Sex-related changes in cardiac function following myocardial infarction in mice. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R528–R534. [Google Scholar] [CrossRef] [Green Version]
- Najjar, F.; Ahmad, M.; Lagace, D.; Leenen, F.H.H. Sex differences in depression-like behavior and neuroinflammation in rats post-MI: Role of estrogens. Am. J. Physiol. Circ. Physiol. 2018, 315, H1159–H1173. [Google Scholar] [CrossRef] [PubMed]
- Gouweleeuw, L.; Hovens, I.B.; Liu, H.; Naude, P.J.W.; Schoemaker, R.G. Differences in the association between behavior and neutrophil gelatinase-associated lipocalin in male and female rats after coronary artery ligation. Physiol. Behav. 2016, 163, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Saxon, V.-M.; Popp, S.; Lehmann, M.; Mathes, D.; Pachel, C.; Hofmann, U.; Ertl, G.; Lesch, K.-P.; Frantz, S. Early citalopram treatment increases mortality due to left ventricular rupture in mice after myocardial infarction. J. Mol. Cell. Cardiol. 2016, 98, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bieber, M.; Werner, R.A.; Tanai, E.; Hofmann, U.; Higuchi, T.; Schuh, K.; Heuschmann, P.U.; Frantz, S.; Ritter, O.; Kraft, P.; et al. Stroke-induced chronic systolic dysfunction driven by sympathetic overactivity. Ann. Neurol. 2017, 82, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Aßmus, B.; Augustin, A.M.; Häbich, H.; Abeßer, M.; Machado, J.M.; Werner, F.; Erkens, R.; Arias-Loza, A.-P.; Umbenhauer, S.; et al. SPRED2 deficiency elicits cardiac arrhythmias and premature death via impaired autophagy. J. Mol. Cell. Cardiol. 2019, 129, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Jeron, A.; Koren, G. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. Circ. Physiol. 1998, 274, H747–H751. [Google Scholar] [CrossRef]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Riederer, P.; Burger, R. Grundlagen der Neuro-Psychopharmakologie; Springer: Berlin/Heidelberg, Germany, 2009; pp. 26–31. [Google Scholar]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.-J.; Frangogiannis, N.; Frantz, S.; Gourdie, R.G.; Holmes, J.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, C.W.; Ververs, F.F.T.; Nikkels, P.G.J.; Van Echteld, C.J.A.; Visser, G.H.A.; Smidt, M.P. Modulation of Serotonin Transporter Function during Fetal Development Causes Dilated Heart Cardiomyopathy and Lifelong Behavioral Abnormalities. PLoS ONE 2008, 3, e2782. [Google Scholar] [CrossRef]
- Matsuo, N.; Takao, K.; Nakanishi, K.; Yamasaki, N.; Tanda, K.; Miyakawa, T. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 2010, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, C.; Zhang, N.N.; Sokoloff, G.; Fanselow, M.S.; Ennes, H.S.; Palmer, A.A.; McRoberts, J.A. Behavioral Differences among C57BL/6 Substrains: Implications for Transgenic and Knockout Studies. J. Neurogenet. 2008, 22, 315–331. [Google Scholar] [CrossRef]
- Bruns, B.; Schmitz, T.; Diemert, N.; Schwale, C.; Werhahn, S.M.; Weyrauther, F.; Gass, P.; Vogt, M.A.; Katus, H.; Herzog, W.; et al. Learned helplessness reveals a population at risk for depressive-like behaviour after myocardial infarction in mice. ESC Heart Fail. 2019, 6, 711–722. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; A Bartsch, D.; A Henn, F.; Gass, P. Stress-Induced Anhedonia in Mice is Associated with Deficits in Forced Swimming and Exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef]
- Weeke, P.; Jensen, A.; Folke, F.; Gislason, G.; Olesen, J.B.; Andersson, C.; Fosbøl, E.; Larsen, J.K.; Lippert, F.; Nielsen, S.L.; et al. Antidepressant Use and Risk of Out-of-Hospital Cardiac Arrest: A Nationwide Case–Time–Control Study. Clin. Pharmacol. Ther. 2012, 92, 72–79. [Google Scholar] [CrossRef]
- Leonard, C.E.; Bilker, W.B.; Newcomb, C.; Kimmel, S.E.; Hennessy, S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol. Drug Saf. 2011, 20, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.R. 3 Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [PubMed] [Green Version]
- Matsumura, S.; Iwanaga, S.; Mochizuki, S.; Okamoto, H.; Ogawa, S.; Okada, Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J. Clin. Investig. 2005, 115, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, N.; Wada, H.; Kanda, T.; Niwa, T.; Yamada, Y.; Saito, K.; Fujiwara, H.; Sekikawa, K.; Seishima, M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-α. J. Am. Coll. Cardiol. 2002, 39, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Hilfiker-Kleiner, D.; Shukla, P.; Klein, G.; Schaefer, A.; Stapel, B.; Hoch, M.; Muller, W.; Scherr, M.; Theilmeier, G.; Ernst, M.; et al. Continuous Glycoprotein-130–Mediated Signal Transducer and Activator of Transcription-3 Activation Promotes Inflammation, Left Ventricular Rupture, and Adverse Outcome in Subacute Myocardial Infarction. Circulation 2010, 122, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Tsutsui, H.; Shiomi, T.; Matsusaka, H.; Matsushima, S.; Wen, J.; Kubota, T.; Takeshita, A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 2004, 64, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainer, P.P.; Hao, S.; Vanhoutte, D.; Lee, D.I.; Koitabashi, N.; Molkentin, J.D.; Kass, D.A. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ. Res. 2014, 114, 1246–1257. [Google Scholar] [CrossRef] [Green Version]
- Piepenburg, S.M.; Faller, H.; Störk, S.; Ertl, G.; Angermann, C.E. Symptom patterns and clinical outcomes in women versus men with systolic heart failure and depression. Clin. Res. Cardiol. 2018, 108, 244–253. [Google Scholar] [CrossRef]
- Postigo, A.; Martínez-Sellés, M. Sex Influence on Heart Failure Prognosis. Front. Cardiovasc. Med. 2020, 7, 616273. [Google Scholar] [CrossRef] [PubMed]
- Ayme-Dietrich, E.; Aubertin-Kirch, G.; Maroteaux, L.; Monassier, L. Cardiovascular remodeling and the peripheral serotonergic system. Arch. Cardiovasc. Dis. 2017, 110, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef]
- Veniaminova, E.; Cespuglio, R.; Chernukha, I.; Schmitt-Boehrer, A.G.; Morozov, S.; Kalueff, A.V.; Kuznetsova, O.; Anthony, D.; Lesch, K.-P.; Strekalova, T. Metabolic, Molecular, and Behavioral Effects of Western Diet in Serotonin Transporter-Deficient Mice: Rescue by Heterozygosity? Front. Neurosci. 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popp, S.; Schmitt-Böhrer, A.; Langer, S.; Hofmann, U.; Hommers, L.; Schuh, K.; Frantz, S.; Lesch, K.-P.; Frey, A. 5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction. J. Clin. Med. 2021, 10, 3104. https://doi.org/10.3390/jcm10143104
Popp S, Schmitt-Böhrer A, Langer S, Hofmann U, Hommers L, Schuh K, Frantz S, Lesch K-P, Frey A. 5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction. Journal of Clinical Medicine. 2021; 10(14):3104. https://doi.org/10.3390/jcm10143104
Chicago/Turabian StylePopp, Sandy, Angelika Schmitt-Böhrer, Simon Langer, Ulrich Hofmann, Leif Hommers, Kai Schuh, Stefan Frantz, Klaus-Peter Lesch, and Anna Frey. 2021. "5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction" Journal of Clinical Medicine 10, no. 14: 3104. https://doi.org/10.3390/jcm10143104
APA StylePopp, S., Schmitt-Böhrer, A., Langer, S., Hofmann, U., Hommers, L., Schuh, K., Frantz, S., Lesch, K.-P., & Frey, A. (2021). 5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction. Journal of Clinical Medicine, 10(14), 3104. https://doi.org/10.3390/jcm10143104





