Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Analysis
2.7. Publication Bias
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Effectiveness and Safety of Acupuncture in Included RCTs
3.4.1. Acupuncture as a Monotherapy
3.4.2. Acupuncture as an Adjunctive Therapy
3.5. Results in Included Before-After Studies
4. Discussion
4.1. Summary of Evidence
4.2. Implications of the Results
4.3. Limitations
- (1)
- Because the number of studies included in this review was small, quantitative synthesis was limited, and the results for each outcome were dependent on either one or two RCTs. In this situation, each RCT was implemented on a small scale, which may cause small-study effects, and its methodological quality is poor. Therefore, the reliability of the results obtained in this review was limited, and the level of evidence could be evaluated as weak.
- (2)
- The subgroup analysis planned in this review protocol [21] was not performed because of the lack of included studies. However, the characteristics of the participants and interventions in the included studies were not sufficiently homogeneous, so differences in effect estimates according to the severity of dementia, the severity of BPSD, and the duration of treatment should be further investigated in future studies.
- (3)
- The included studies also lacked the homogeneity of the evaluation tools used for BPSD evaluation. In particular, some studies used evaluation tools for individual BPSD symptoms such as HAMD, STAI, and PSQI and did not use an evaluation tool specific to BPSD such as BEHAVE-AD or NPI. Therefore, future studies require the uniformity of these evaluation tools, and the use of evaluation tools specific to BPSD is recommended.
- (4)
- Most of the included studies were conducted in China. In particular, all the included RCTs were implemented in China. Although publication bias using funnel plots was not evaluated in this review, studies implemented only in certain countries could potentially contribute to publication bias. In addition, as China has been using acupuncture for a long time, participants of acupuncture studies usually exhibit a favorable attitude toward this treatment method. These factors can act as obstacles to generalizing the results of this review to other countries.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.B.; Gitlin, L.N.; Kairalla, J.A.; Mann, W.C. Relationship between the number of behavioral symptoms in dementia and caregiver distress: What is the tipping point? Int. Psychogeriatr. 2018, 30, 1099–1107. [Google Scholar] [CrossRef]
- Beeri, M.S.; Werner, P.; Davidson, M.; Noy, S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int. J. Geriatr. Psychiatry 2002, 17, 403–408. [Google Scholar] [CrossRef]
- Tible, O.P.; Riese, F.; Savaskan, E.; von Gunten, A. Best practice in the management of behavioural and psychological symptoms of dementia. Ther. Adv. Neurol. Disord. 2017, 10, 297–309. [Google Scholar] [CrossRef]
- Kales, H.C.; Lyketsos, C.G.; Miller, E.M.; Ballard, C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int. Psychogeriatr. 2019, 31, 83–90. [Google Scholar] [CrossRef]
- Masopust, J.; Protopopová, D.; Vališ, M.; Pavelek, Z.; Klímová, B. Treatment of behavioral and psychological symptoms of dementias with psychopharmaceuticals: A review. Neuropsychiatr. Dis. Treat. 2018, 14, 1211–1220. [Google Scholar] [CrossRef]
- White, N.; Leurent, B.; Lord, K.; Scott, S.; Jones, L.; Sampson, E.L. The management of behavioural and psychological symptoms of dementia in the acute general medical hospital: A longitudinal cohort study. Int. J. Geriatr. Psychiatry 2016, 32, 297–305. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, S.-H.; Hwang, H.-J. Behavioral and Psychological Symptoms of Dementia and Antipsychotic Drug Use in the Elderly with Dementia in Korean Long-Term Care Facilities. Drugs-Real World Outcomes 2015, 2, 363–368. [Google Scholar] [CrossRef][Green Version]
- Cohen-Mansfield, J.; Thein, K.; Marx, M.S.; Dakheel-Ali, M. What Are the Barriers to Performing Nonpharmacological Interventions for Behavioral Symptoms in the Nursing Home? J. Am. Med Dir. Assoc. 2012, 13, 400–405. [Google Scholar] [CrossRef]
- Rubin, R. Medicare Proposes Coverage of Acupuncture for Lower Back Pain. JAMA 2019, 322, 716. [Google Scholar] [CrossRef]
- Harris, M.L.; Titler, M.G.; Struble, L.M. Acupuncture and Acupressure for Dementia Behavioral and Psychological Symptoms: A Scoping Review. West. J. Nurs. Res. 2019, 42, 867–880. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, M.; Zhou, K.; Mita, C.; Liu, J.; Wayne, P.M. Publication Trends in Acupuncture Research: A 20-Year Bibliometric Analysis Based on PubMed. PLoS ONE 2016, 11, e0168123. [Google Scholar] [CrossRef]
- Birch, S. Treating the patient not the symptoms: Acupuncture to improve overall health–Evidence, acceptance and strategies. Integr. Med. Res. 2019, 8, 33–41. [Google Scholar] [CrossRef]
- Chan, M.W.C.; Wu, X.Y.; Wu, J.C.Y.; Wong, S.Y.-S.; Chung, V.C.H. Safety of Acupuncture: Overview of Systematic Reviews. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Casanova, M.F.; Starkstein, S.E.; Jellinger, K.A. Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropathol. 2011, 122, 117–135. [Google Scholar] [CrossRef]
- Guo, X.; Ma, T. Effects of Acupuncture on Neurological Disease in Clinical- and Animal-Based Research. Front. Integr. Neurosci. 2019, 13, 47. [Google Scholar] [CrossRef]
- Bensamoun, D.; Guignard, R.; Furst, A.J.; Derreumaux, A.; Manera, V.; Darcourt, J.; Benoit, M.; Robert, P.H.; David, R.; Initiative, F.T.A.D.N. Associations between Neuropsychiatric Symptoms and Cerebral Amyloid Deposition in Cognitively Impaired Elderly People. J. Alzheimers Dis. 2015, 49, 387–398. [Google Scholar] [CrossRef]
- Chen, Y.; Dang, M.; Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and –specific lesion patterns. Mol. Neurodegener. 2021, 16, 1–22. [Google Scholar] [CrossRef]
- Kwon, C.-Y.; Lee, B. Acupuncture for behavioral and psychological symptoms of dementia. Medicine 2021, 100, e24341. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sclan, S.G.; Saillon, A.; Franssen, E.; Hugonot-Diener, L.; Saillon, A.; Reisberg, B. The behavior pathology in Alzheimer’s disease rating scale (Behave-AD): Reliability and analysis of symptom category scores. Int. J. Geriatr. Psychiatry 1996, 11, 819–830. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The brief psychiatric rating scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Galasko, D.; Bennett, D.A.; Sano, M.; Marson, D.; Kaye, J.; Edland, S.D. ADCS Prevention Instrument Project: Assessment of Instrumental Activities of Daily Living for Community-dwelling Elderly Individuals in Dementia Prevention Clinical Trials. Alzheimer Dis. Assoc. Disord. 2006, 20, S152–S169. [Google Scholar] [CrossRef]
- Kasper, J.D.; Black, B.S.; Shore, A.D.; Rabins, P.V. Evaluation of the validity and reliability of the Alzheimer’s Disease-Related Quality of Life (ADRQL) assessment instrument. Alzheimer Dis. Assoc. Disord. 2009, 23, 275. [Google Scholar] [CrossRef]
- Novak, M.; Guest, C. Application of a multidimensional caregiver burden inventory. Gerontologist 1989, 29, 798–803. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D. Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 25 February 2021).
- Guyatt, G.; Rennie, D.; Meade, M.; Cook, D. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice; AMA Press: Chicago, IL, USA, 2002; Volume 706. [Google Scholar]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.L. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Ou, Y.-Q. Clinical observation of electric acupuncture combined with fentazin in treating mental symptom of alzheimer’s disease. Shanghai J. Acupunct. Moxibustion 2000, 19, 16. [Google Scholar]
- Huang, D.; Pang, S.; Lu, Y.; Liao, L.; Jiang, R.; Jiang, W. Study on the effect of scalp-point cluster needling method on depression caused by Alzheimer’s disease and its rehabilitation. Guangxi Med. J. 2014, 36, 1279–1280. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Yu, J.; Han, J.; Yu, T.; Shi, J.; Zhao, L.; Nie, K. Acupuncture for patients with mild to moderate Alzheimer’s disease: A randomized controlled trial. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Ma, L.; Cao, M. Clinical Observation of Electroacupuncture Combined with Midazolam Maleate in Improving Alzheimer’s Disease Sleep Disorder. Shaanxi J. Tradit. Chin. Med. 2017, 38, 1471–1472. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, L.; Zhao, L.; Zhang, J.; Yang, T.; Li, G. Effects of Acusector Combined with Music Therapy on Cognitive Function and Activities of Daily Living in Patients with Mild and Moderate Alzheimer’s Disease. World Chin. Med. 2020, 15, 1998–2001. [Google Scholar] [CrossRef]
- Lombardo, N.B.E.; Dresser, M.V.B.; Malivert, M.; McManus, C.A.; Vehvilainen, L.; Ooi, W.L.; Xu, G.; Rosowsky, E.; Drebing, C.; Sheridan, P.L.; et al. Acupuncture as treatment for anxiety and depression in persons with dementia: Results of a feasibility and effectiveness study. Alzheimers Care Q. 2001, 2, 28–41. [Google Scholar]
- Ying, J. Clinical Study on the Effect of Acupuncture on Mental and Behavioral Symptoms of Alzheimer’s Disease. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2006. [Google Scholar]
- Teare, M.D.; Dimairo, M.; Shephard, N.; Hayman, A.; Whitehead, A.; Walters, S.J. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: A simulation study. Trials 2014, 15, 264. [Google Scholar] [CrossRef]
- Pagones, R.; Lee, J.L.; Hurst, S. Long-Term Acupuncture Therapy for Low-Income Older Adults with Multimorbidity: A Qualitative Study of Patient Perceptions. J. Altern. Complement. Med. 2018, 24, 161–167. [Google Scholar] [CrossRef]
- Çevik, C.; Anil, A.; Işeri, S. Özlem Effective chronic low back pain and knee pain treatment with acupuncture in geriatric patients. J. Back Musculoskelet. Rehabil. 2015, 28, 517–520. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, C.-Z.; Lu, Y.-J.; Cai, X.-W.; Zhang, G.-F.; Shan, B.-C.; Cui, S.-Y.; Chen, J.-Q.; Qu, S.-S.; Zhong, Z.; et al. Long-term acupuncture treatment has a multi-targeting regulation on multiple brain regions in rats with Alzheimer’s disease: A positron emission tomography study. Neural Regen. Res. 2017, 12, 1159–1165. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, J.; Wang, Z.-Q.; Zhao, Z.-L.; Zhang, M.; Xu, J.-Y.; Han, Y.; Li, K.-C.; Lu, J. Neuronal Specificity of Acupuncture in Alzheimer’s Disease and Mild Cognitive Impairment Patients: A Functional MRI Study. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Pach, D.; Napadow, V.; Park, K.; Long, X.; Neumann, J.; Maeda, Y.; Nierhaus, T.; Liang, F.; Witt, C.M. Characterizing Acupuncture Stimuli Using Brain Imaging with fMRI-A Systematic Review and Meta-Analysis of the Literature. PLoS ONE 2012, 7, e32960. [Google Scholar] [CrossRef]
- Alves, G.; Carvalho, A.; Carvalho, L.; Sudo, F.; Siqueira-Neto, J.; Oertel-Knöchel, V.; Jurcoane, A.; Knöchel, C.; Boecker, H.; Laks, J.; et al. Neuroimaging Findings Related to Behavioral Disturbances in Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2016, 14, 61–75. [Google Scholar] [CrossRef]
- Massimo, L.; Powers, C.; Moore, P.; Vesely, L.; Avants, B.; Gee, J.; Libon, D.J.; Grossman, M. Neuroanatomy of Apathy and Disinhibition in Frontotemporal Lobar Degeneration. Dement. Geriatr. Cogn. Disord. 2009, 27, 96–104. [Google Scholar] [CrossRef]
- Van Dam, D.; Vermeiren, Y.; Dekker, A.; Naudé, P.J.; De Deyn, P.P. Neuropsychiatric Disturbances in Alzheimer’s Disease: What Have We Learned from Neuropathological Studies? Curr. Alzheimer Res. 2016, 13, 1145–1164. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef]
- Lee, N.Y.; Choo, I.H.; Kim, K.W.; Jhoo, J.H.; Youn, J.C.; Lee, U.Y.; Woo, J.I. White Matter Changes Associated With Psychotic Symptoms in Alzheimer’s Disease Patients. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 191–198. [Google Scholar] [CrossRef]
- Tu, C.-H.; Macdonald, I.; Chen, Y.-H. The Effects of Acupuncture on Glutamatergic Neurotransmission in Depression, Anxiety, Schizophrenia, and Alzheimer’s Disease: A Review of the Literature. Front. Psychiatry 2019, 10, 14. [Google Scholar] [CrossRef]
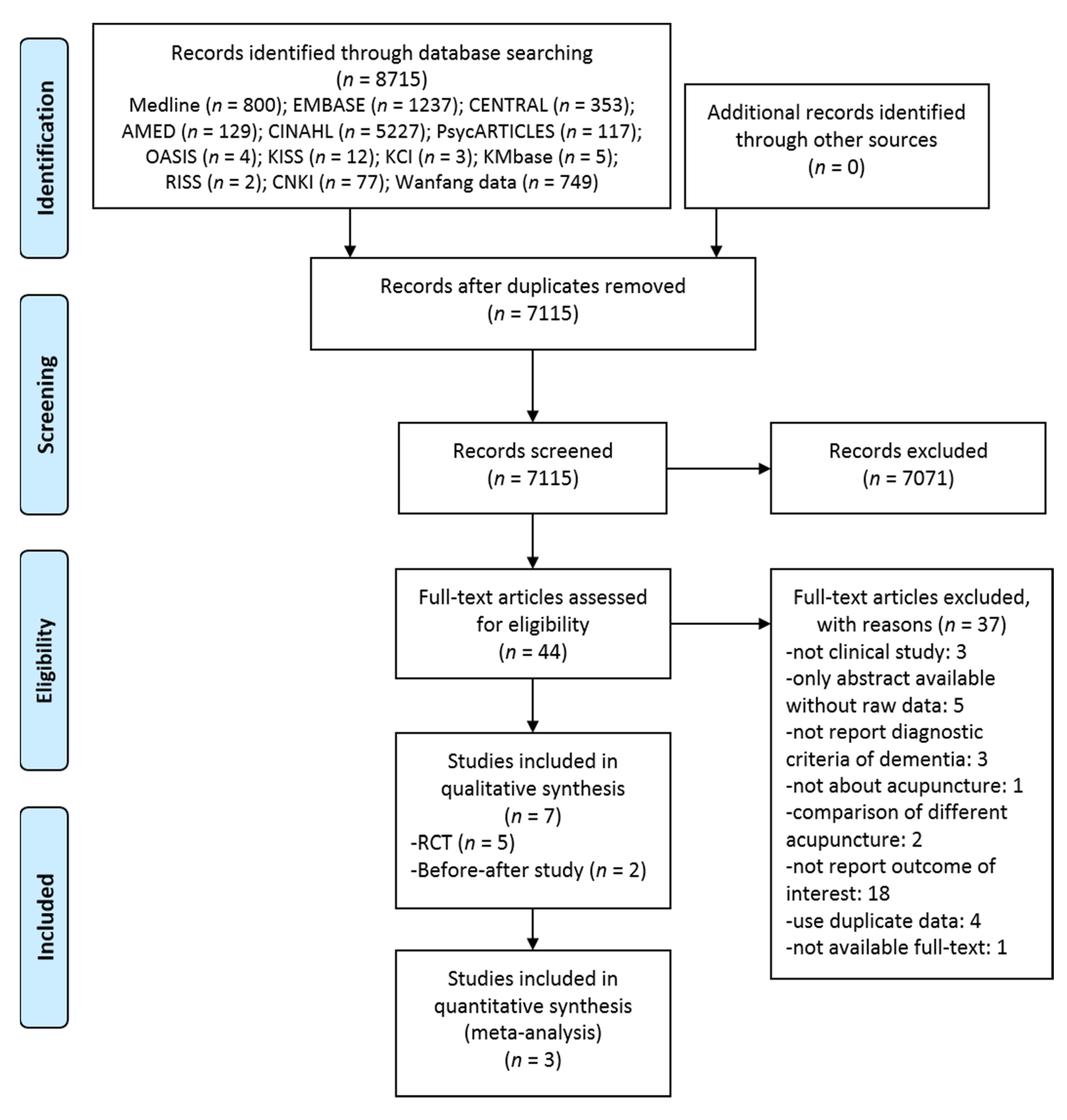
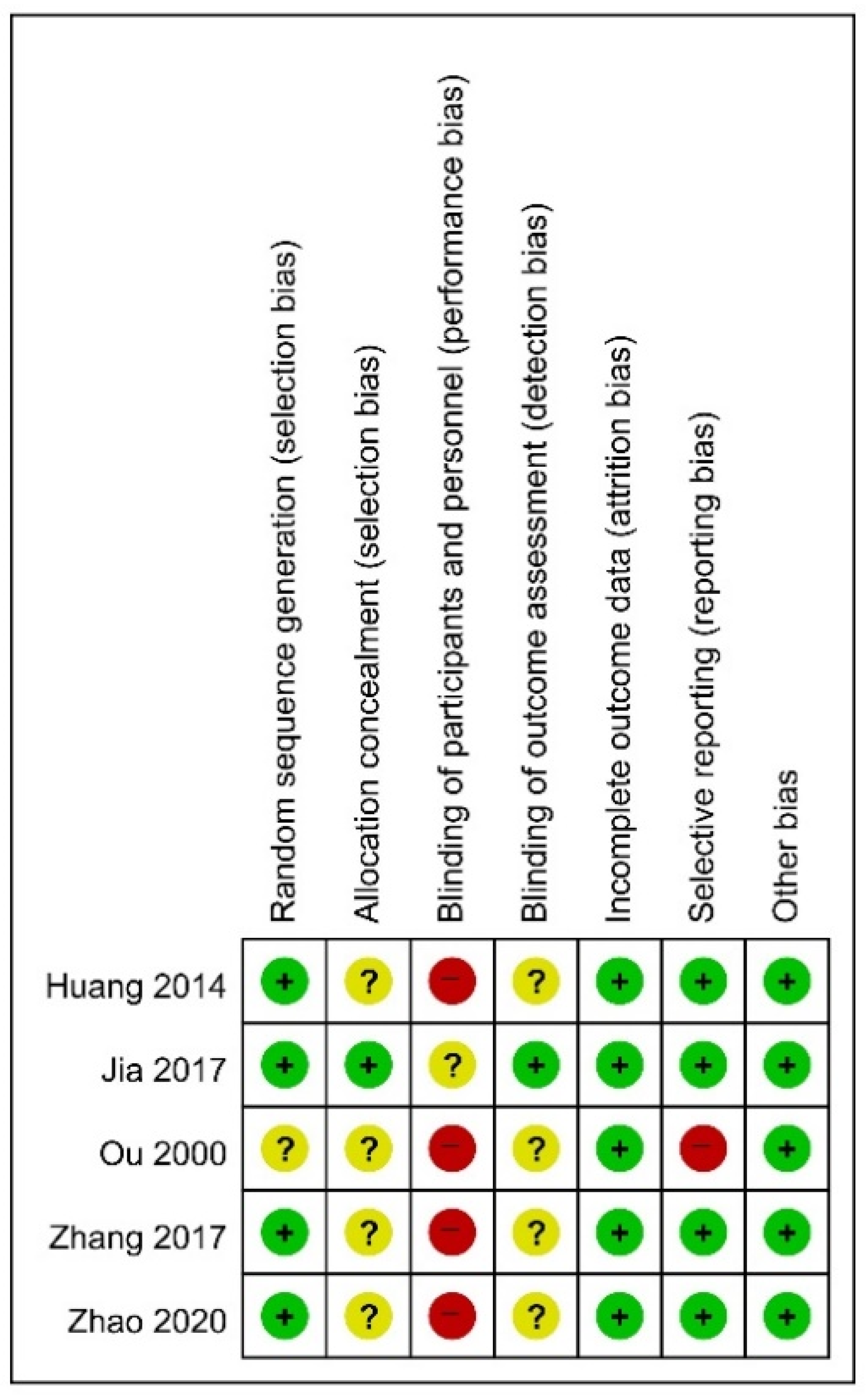
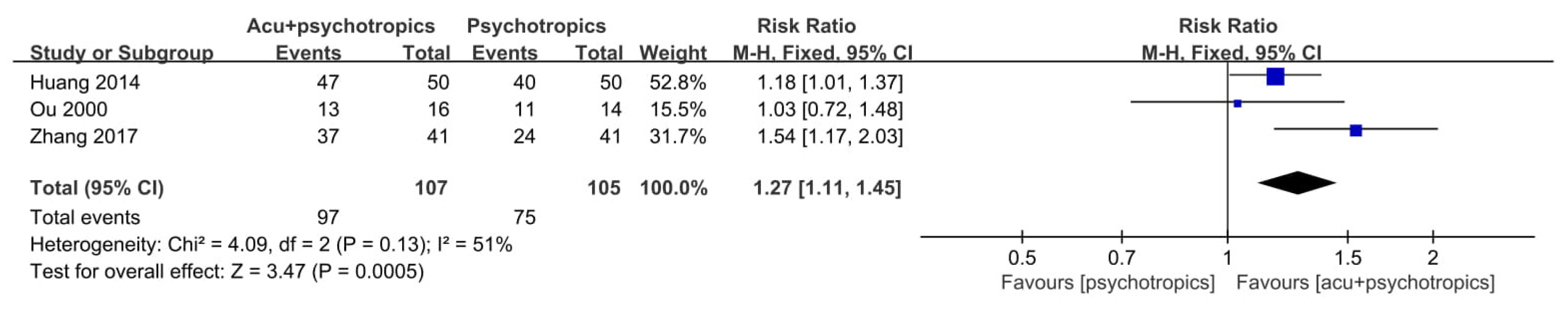
| Study, Year, [Reference] | Sample Size(Included→Analyzed) | Mean Age (yr) | Sex (M:F) | Population | Intervention | Treatment Duration/F/U | Outcome |
|---|---|---|---|---|---|---|---|
| Ou, 2000, [35] | 30(16:14) →30(16:14) | TG: 65.5 ± 6.8 CG: 64.7 ± 7.6 | TG: 16(10:6) CG: 14(9:4) | -AD (mild, moderate) -baseline BPRS TG: 42.85 ± 5.25 CG: 41.91 ± 4.88 | TG: EA + perphenazine (4–30 mg/d) CG: perphenazine (8–40 mg/d) | 8 wk/none | 1. TER (BPRS) 2. BPRS 3. CGI |
| Huang 2014, [36] | 100(50:50) →100(50:50) | TG: 70.9 ± 8.6 CG: 71.5 ± 7.9 | TG: 50(24:26) CG: 50(23:27) | -AD (mild, moderate, severe) -baseline HAMD TG: 22.30 ± 6.93 CG: 21.45 ± 7.01 | TG: SA + fluoxetine (20 mg/d) CG: fluoxetine (20 mg/d) | 8 wk/none | 1. TER (HAMD) 2. HAMD 3. ADL |
| Jia 2017, [37] | 87(43:44) →80(41:39) | TG: 75.11 ± 6.53 CG: 74.50 ± 6.83 | TG: 43(13:30) CG: 44(16:28) | -AD (mild, moderate) -baseline NPITG: 9.28 ± 2.49 CG: 8.97 ± 2.69 | TG: MA CG: donepezil (first 4 weeks: 5 mg/d; thereafter: 10 mg/d) | 12 wk/12 wk | 1. ADAS-cog 2. CIBIC + 3. ADCS-ADL23 4. NPI |
| Zhang 2017, [38] | 82(41:41) →82(41:41) | TG: 66.12 ± 11.33 CG: 65.25 ± 10.62 | TG: 41(23:18) CG: 41(22:19) | -AD -baseline PSQI TG: 14.12 ± 2.12 CG: 14.91 ± 3.32 | TG: EA + midazolam (7.5 mg/d) CG: midazolam (7.5 mg/d) | 30 d/none | 1. TER (PSQI) 2. PSQI |
| Zhao 2020, [39] | 120(60:60) →120(60:60) | TG: 72.0 ± 10.9 CG: 70.9 ± 11.2 | TG: 60(30:30) CG: 60(28:32) | -AD -baseline MMSE TG: 14.34 ± 2.87 CG: 14.62 ± 3.01 | TG: EA + passive music therapy + routine care (routine nursing, psychological comfort, daily cognitive training, diet modification) CG: routine care | 4 wk/none | 1. TER (MMSE, MoCA, SF-36) 2. MMSE 3. MoCA 4. ADL 5. BEHAVE-AD 6. SF-36 7. Levels of acetylcholine, choline acetylase, acetylcholinesterase, norepinephrine, 5-HT, and dopamine |
| Lombardo 2001, [40] | 11→11 | 76 | 11(3:8) | -AD or VD -baseline MMSE 21.9 ± 5.2 | MA | 9–12 wk/none | 1. POMS-anxiety 2. STAI-state anxiety 3. CSDD 4. GDS 5. MMSE 6. Boston naming test 7. Controlled oral word association test 8. Caregiver: SF-36-vitality, anxiety, depression 9. Patient: SF-36-vitality, anxiety, depression |
| Ying 2006, [41] | 33→30 | 74.96 ± 6.41 | 30(12:18) | -AD -baseline MMSE 12.47 ± 2.62 -baseline BEHAVE-AD 18.90 ± 6.67 | MA | 8 wk/none | 1. BEHAVE-AD 2. MMSE 3. ADL 4. TCM symptom score |
| Study, Year, [Reference] | Type of Acupuncture | Acupoints | Stimulation Method | Needle Retention Time | Treatment Frequency |
|---|---|---|---|---|---|
| Ou, 2000, [35] | EA | GV20, Yintang, GV14 | De qi GV20 to Yintang or GV20 to GV14 Wave: continuous wave; Frequency: 2–4 Hz; Intensity: visible twitching of the local muscles, but comfortable and tolerable. | 30 min | 6 session/wk |
| Huang 2014, [36] | SA | anterior vertex zone, frontal zone | Manual stimulation | NR | 6 session/wk |
| Jia 2017, [37] | MA | -Basic acupoints: CV17, CV12, CV6, ST36, TW5, SP10-Additional acupoints: LV3, GB39, ST40, BL17, ST44, ST25, CV4 | De qi | 30 min | 3 session/wk |
| Zhang 2017, [38] | EA | GV20, GV24, EX-HN1, Anmian, Taiyang, P6, HT7, SP6, KI1 | De qi Taiyang to EX-HN1 Wave: dilatational wave; Frequency: 2–100 Hz; Intensity: 2–4 V | 25 min | 1 session/d for 10d and rest for 3d |
| Zhao 2020, [39] | EA | GV20, BL23 | Not reporting on De qi Around GV20 to GV20, around BL23 to BL23 Wave: continuous wave; Frequency: 50 Hz; Intensity: 2 V, 1 mA | 20 min | 1 session/d for 7d and rest for 1d |
| Lombardo 2001, [40] | MA | -Basic acupoints: GB9, GV16, GV20, GV23, GV24, PC6, HT7, SP6, EX-HN1, Yintang -Additional acupoints: ST36, LI4, GB20, GV17, SP4, KI3, SI3, BL62, BL23, GV26, EX-B2 | NR | 30 min | 3 session/wk (1–2 wk), 2–3 session/wk (additional 7–10 wk) |
| Ying 2006, [41] | MA | -Basic acupoints: GV24, HT7, GV20, GV16, GV14, EX-B2 -Additional acupoints: BL23, GB39, LV3, SP6, HT5, HT7, ST36, SP10, BL17 | De qi Manual stimulation every 10 min during needle retention | 30 min | 1 session/d for 6d and rest for 1d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, C.-Y.; Lee, B. Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3087. https://doi.org/10.3390/jcm10143087
Kwon C-Y, Lee B. Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(14):3087. https://doi.org/10.3390/jcm10143087
Chicago/Turabian StyleKwon, Chan-Young, and Boram Lee. 2021. "Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 14: 3087. https://doi.org/10.3390/jcm10143087
APA StyleKwon, C.-Y., & Lee, B. (2021). Acupuncture for Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(14), 3087. https://doi.org/10.3390/jcm10143087







