Cutaneous Events Associated with Immunotherapy of Melanoma: A Review
Abstract
1. Introduction
2. Vitiligo
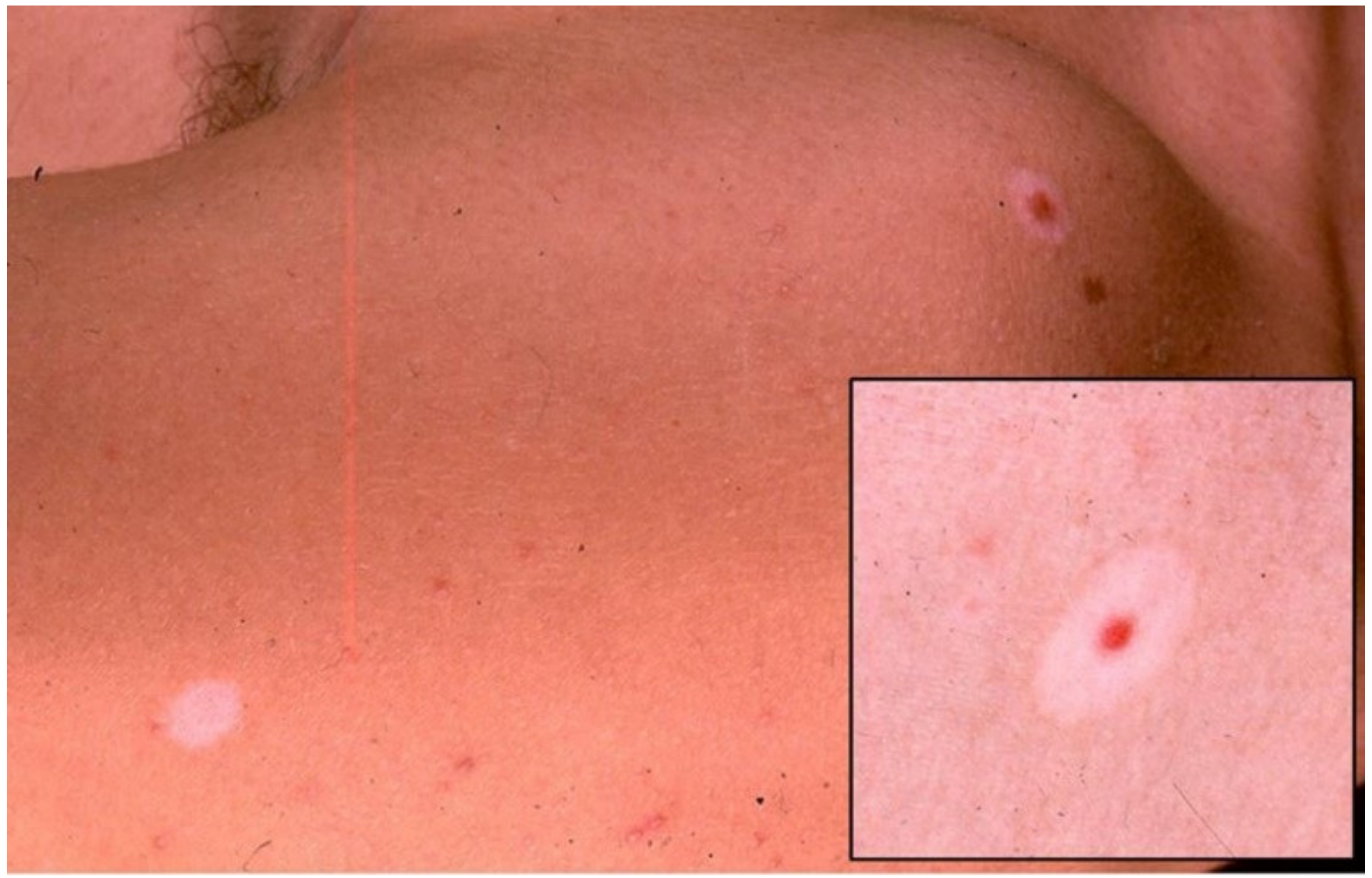
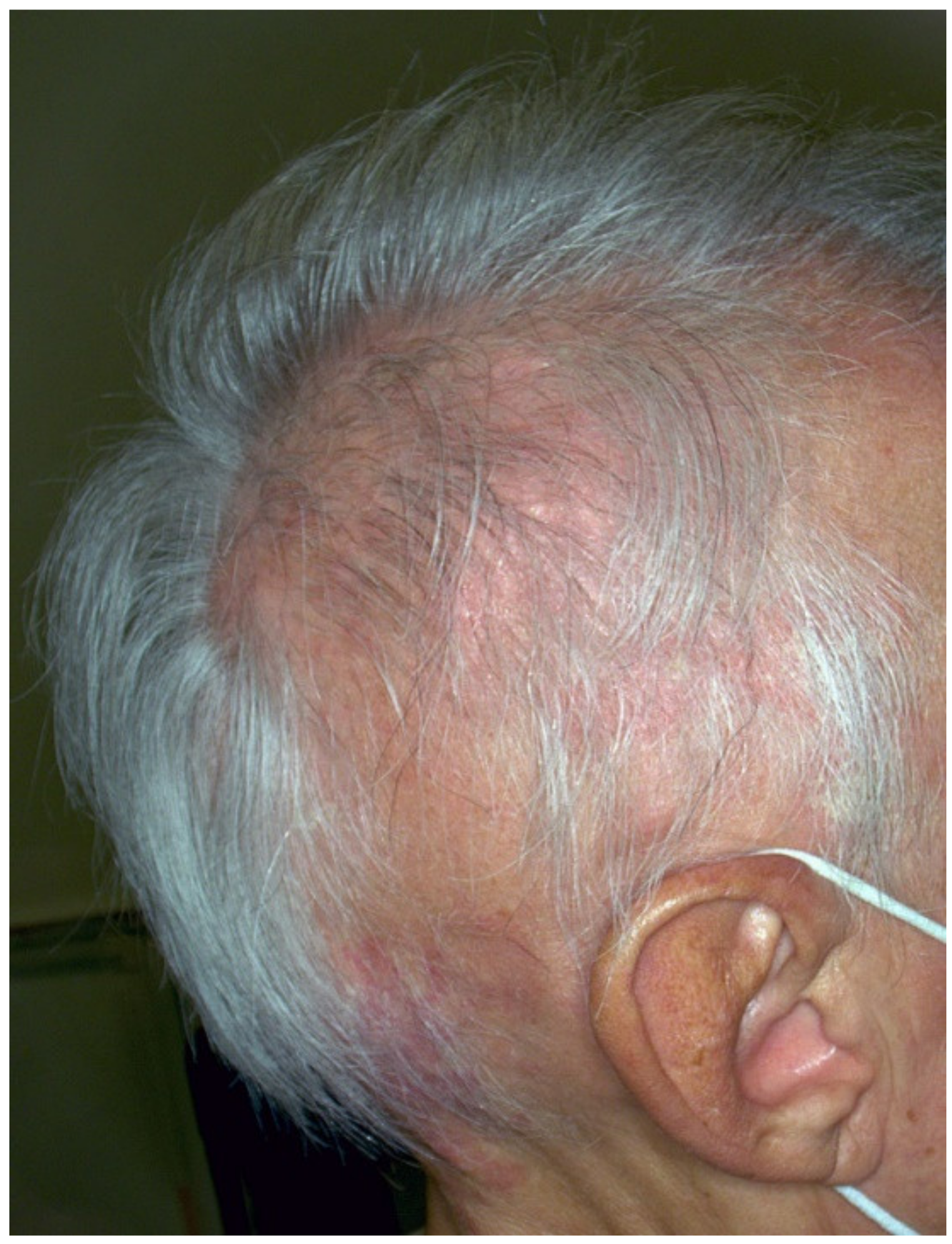
3. Halo Nevus
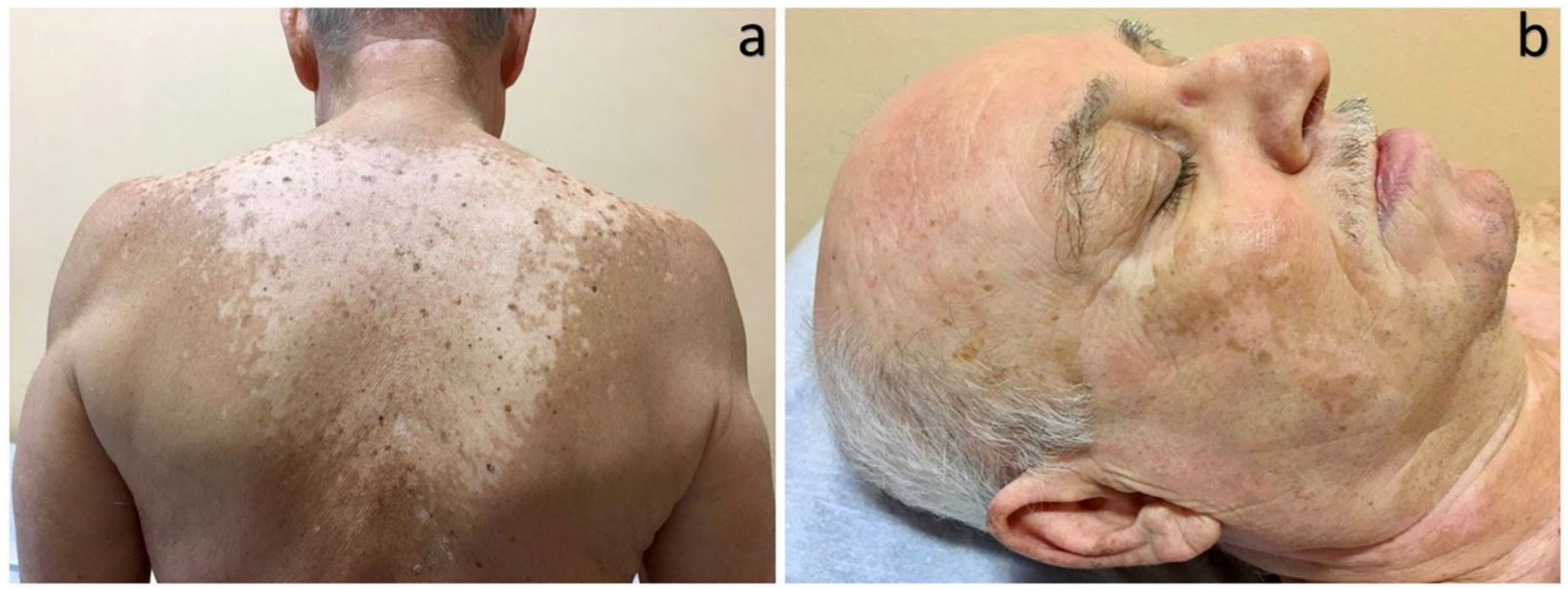
4. Melanosis
5. Hair and Nail Toxicities
Funding
Conflicts of Interest
References
- Guan, X.; Wang, H.; Ma, F.; Qian, H.; Yi, Z.; Xu, B. The Efficacy and Safety of Programmed Cell Death 1 and Programmed Cell Death 1 Ligand Inhibitors for Advanced Melanoma. Medicine 2016, 95, e3134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, J. Efficacy and safety of ipilimumab for treating advanced melanoma: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2019, 44, 420–429. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19 (Suppl. 1), 31–39. [Google Scholar] [CrossRef]
- Larsabal, M.; Marti, A.; Jacquemin, C.; Rambert, J.; Thiolat, D.; Dousset, L.; Taieb, A.; Dutriaux, C.; Prey, S.; Boniface, K.; et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J. Am. Acad. Dermatol. 2017, 76, 863–870. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Manga, P.; Lin, K.; Elbuluk, N. Vitiligo and Melanoma-Associated Vitiligo: Understanding Their Similarities and Differences. Am. J. Clin. Dermatol. 2020, 21, 669–680. [Google Scholar] [CrossRef]
- Cui, J.; Bystryn, J.C. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch. Dermatol. 1995, 131, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Marenco, F.; Osella-Abate, S.; Cappello, N.; Ortoncelli, M.; Salomone, B.; Fierro, M.T.; Savoia, P.; Bernengo, M.G. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: Results from a single-institution hospital-based observational cohort study. Ann. Oncol. 2010, 21, 409–414. [Google Scholar] [CrossRef]
- Teulings, H.-E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-Like Depigmentation in Patients with Stage III-IV Melanoma Receiving Immunotherapy and Its Association With Survival: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef]
- Phan, G.Q.; Yang, J.C.; Sherry, R.M.; Hwu, P.; Topalian, S.L.; Schwartzentruber, D.J.; Restifo, N.P.; Haworth, L.R.; Seipp, C.A.; Freezer, L.J.; et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2003, 100, 8372–8377. [Google Scholar] [CrossRef]
- Dika, E.; Ravaioli, G.M.; Fanti, P.A.; Piraccini, B.M.; Lambertini, M.; Chessa, M.A.; Baraldi, C.; Ribero, S.; Andrea, A.; Melotti, B.; et al. Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: A prospective study. Eur. J. Dermatol. 2017, 27, 266–270. [Google Scholar] [CrossRef]
- Belum, V.; Benhuri, B.; Postow, M.; Hellmann, M.; Lesokhin, A.; Segal, N.; Motzer, R.; Wu, S.; Busam, K.; Wolchok, J.; et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur. J. Cancer 2016, 60, 12–25. [Google Scholar] [CrossRef]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e1. [Google Scholar] [CrossRef]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Asami, Y.; Teramoto, Y.; Imamura, T.; Sato, S.; Maruyama, H.; Fujisawa, Y.; Matsuya, T.; Fujimoto, M.; et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Babai, S.; Voisin, A.-L.; Bertin, C.; Gouverneur, A.; Le-Louet, H. Occurrences and Outcomes of Immune Checkpoint Inhibitors-Induced Vitiligo in Cancer Patients: A Retrospective Cohort Study. Drug Saf. 2019, 43, 111–117. [Google Scholar] [CrossRef]
- Hwang, S.J.E.; Park, J.; Wakade, D.; Chou, S.; Byth, K.; Peñas, P.F. Cutaneous adverse events of anti-programmed death 1 antibodies combined with anti-cytotoxic T-lymphocyte-associated protein 4 therapy use in patients with metastatic melanoma. Melanoma Res. 2019, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.T.; Dewan, A.K.; Davis, E.J.; Ancell, K.K.; Fan, R.; Ye, F.; Johnson, D.B. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects With Outcomes in Patients With Advanced Melanoma. JAMA Oncol. 2019, 5, 906–908. [Google Scholar] [CrossRef]
- Hodi, F.S.; Sileni, V.C.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Epstein, W.L.; Sagebeil, R.; Spitler, L.; Wybran, J.; Reed, W.B.; Blois, M.S. Halo nevi and melanoma. JAMA 1973, 225, 373–377. [Google Scholar] [CrossRef]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2019, 145, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Turk, M.J. New Perspectives on the Role of Vitiligo in Immune Responses to Melanoma. Oncotarget 2011, 2, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Ramondetta, A.; Ribero, S.; Conti, L.; Fava, P.; Marra, E.; Broganelli, P.; Caliendo, V.; Picciotto, F.; Guida, M.; Fierro, M.; et al. Clinical and Pathological Relevance of Drug-induced Vitiligo in Patients Treated for Metastatic Melanoma with Anti-PD1 or BRAF/MEK Inhibitors. Acta Derm. Venereol. 2020, 100, adv00001. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Floris, A.; Novelli, M.; Gammaitoni, L.; Giraudo, L.; Macagno, M.; Leuci, V.; Rotolo, R.; Donini, C.; et al. BRAF and MEK Inhibitors Increase PD-1-Positive Melanoma Cells Leading to a Potential Lymphocyte-Independent Synergism with Anti–PD-1 Antibody. Clin. Cancer Res. 2018, 24, 3377–3385. [Google Scholar] [CrossRef]
- Daneshpazhooh, M.; Shokoohi, A.; Dadban, A.; Raafat, J. The course of melanoma-associated vitiligo: Report of a case. Melanoma Res. 2006, 16, 371–373. [Google Scholar] [CrossRef]
- Nardin, C.; Pelletier, F.; Puzenat, E.; Aubin, F. Vitiligo Repigmentation with Melanoma Progression During Pembrolizumab Treatment. Acta Derm. Venereol. 2019, 99, 913–914. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Brugués, A.; Ribero, S.; Silva, V.; Aguilera, P.; Garcia, A.; Alós, L.; Malvehy, J.; Puig, S.; Carrera, C. Sutton Naevi as Melanoma Simulators: Can Confocal Microscopy Help in the Diagnosis? Acta Derm. Venereol. 2020, 100, adv00134. [Google Scholar] [CrossRef]
- Moretti, S.; Spallanzani, A.; Pinzi, C.; Prignano, F.; Fabbri, P. Fibrosis in regressing melanoma versus nonfibrosis in halo nevus upon melanocyte disappearance: Could it be related to a different cytokine microenvironment? J. Cutan. Pathol. 2007, 34, 301–308. [Google Scholar] [CrossRef]
- Lorentzen, H. Eruptive Halo Naevi: A Possible Indicator of Malignant Disease in a Case Series of Post-Adolescent Patients. Acta Derm. Venereol. 2020, 100, adv00228. [Google Scholar] [CrossRef]
- Schwager, Z.; Laird, M.E.; Latkowski, J.-A. Regression of pigmented lesions in a patient with metastatic melanoma treated with immunotherapy. JAAD Case Rep. 2018, 4, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Libon, F.; Arrese, J.E.; Rorive, A.; Nikkels, A.F. Ipilimumab induces simultaneous regression of melanocytic naevi and melanoma metastases. Clin. Exp. Dermatol. 2012, 38, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Plaquevent, M.; Greliak, A.; Pinard, C.; Duval-Modeste, A.-B.; Joly, P. Simultaneous long-lasting regression of multiple nevi and melanoma metastases after ipilimumab therapy. Melanoma Res. 2019, 29, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, M.R.; Ma, M.W.; A Casey, M.; Amin, B.D.; Jacobson, M.; Cheng, H.; McLellan, B. Development of Halo Nevi in a Lung Cancer Patient: A Novel Immune-Related Cutaneous Event from Atezolizumab. J. Drugs Dermatol. 2017, 16, 1047–1049. [Google Scholar] [PubMed]
- Jurgens, A.; Guru, S.; Guo, R.; Brewer, J.; Bridges, A.; Jakub, J.; Comfere, N. Tumoral Melanosis in the Setting of Targeted Immunotherapy for Metastatic Melanoma—A Single Institutional Experience and Literature Review. Am. J. Dermatopathol. 2021, 43, 9–14. [Google Scholar] [CrossRef]
- George, E.V.; Kalen, J.E.; Kapil, J.P.; Motaparthi, K. Comparison of the Inflammatory Infiltrates in Tumoral Melanosis, Regressing Nevi, and Regressing Melanoma. Am. J. Dermatopathol. 2019, 41, 480–487. [Google Scholar] [CrossRef]
- Emanuel, P.O.; Mannion, M.; Phelps, R.G. Complete Regression of Primary Malignant Melanoma. Am. J. Dermatopathol. 2008, 30, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Relvas, M.; Alves, F.; Mariano, A.; Cardoso, J.; Coutinho, I. Tumoral melanosis after immunotherapy with pembrolizumab—A response sign mimicking melanoma. Dermatol. Online J. 2020, 26, 13030/qt4xc955rq. [Google Scholar] [CrossRef]
- Staser, K.; Chen, D.; Solus, J.; Rosman, I.; Schaffer, A.; Cornelius, L.; Linette, G.; Fields, R. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br. J. Dermatol. 2016, 175, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Bari, O.; Cohen, P.R. Tumoral Melanosis Associated with Pembrolizumab-Treated Metastatic Melanoma. Cureus 2017, 9, 1026. [Google Scholar] [CrossRef]
- Guo, R.; Jakub, J.; Bridges, A. Tumoral melanosis as a therapeutic response to PD1 inhibitor in advanced melanoma: Report of three cases. In Proceedings of the Poster Presentation at: 53rd Annual Meeting of the American Society of Dermatopathology, Chicago, IL, USA, 27 October 2016. [Google Scholar]
- Helm, M.F.; Bax, M.J.; Bogner, P.N.; Chung, C.G. Metastatic melanoma with features of blue nevus and tumoral melanosis identified during pembrolizumab therapy. JAAD Case Rep. 2017, 3, 135–137. [Google Scholar] [CrossRef][Green Version]
- Woodbeck, R.; Metelitsa, A.I.; Naert, K.A. Granulomatous Tumoral Melanosis Associated With Pembrolizumab Therapy: A Mimicker of Disease Progression in Metastatic Melanoma. Am. J. Dermatopathol. 2018, 40, 523–526. [Google Scholar] [CrossRef]
- Thiem, A.; Schummer, P.; Ueberschaar, S.; Kerstan, A.; Kneitz, H.; Schrama, D.; Appenzeller, S.; Meierjohann, S.; Schilling, B.; Goebeler, M.; et al. Early onset of diffuse melanosis cutis under pembrolizumab therapy illustrates the limitations of anti-PD-1 checkpoint inhibitors. Melanoma Res. 2018, 28, 465–468. [Google Scholar] [CrossRef]
- Alessandrini, A.; Bruni, F.; Piraccini, B.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Wang, X.; Marr, A.; Breitkopf, T.; Leung, G.; Hao, J.; Wang, E.; Kwong, N.; Akhoundsadegh, N.; Chen, L.; Mui, A.; et al. Hair Follicle Mesenchyme-Associated PD-L1 Regulates T-Cell Activation Induced Apoptosis: A Potential Mechanism of Immune Privilege. J. Investig. Dermatol. 2014, 134, 736–745. [Google Scholar] [CrossRef]
- Zarbo, A.; Belum, V.; Sibaud, V.; Oudard, S.; Postow, M.; Hsieh, J.; Motzer, R.; Busam, K.; Lacouture, M. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2017, 176, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Mangana, J.; Dummer, R. Hair Depigmentation and Hair Loss in Advanced Melanoma Treated with Combined Immunotherapy and Targeted Therapy. Acta Derm. Venereol. 2020, 100, adv00007. [Google Scholar] [CrossRef]
- Adle, M.A.; Chastagner, M.; Mansard, S.; Dalle, S. Image Gallery: Unilateral eyebrow depigmentation. Br. J. Dermatol. 2019, 180, e107. [Google Scholar] [CrossRef]
- Burzi, L.; Parietti, M.; Agostini, A.; Marra, E.; Fierro, M.; Ribero, S.; Quaglino, P. Eyelashes poliosis as first sign of metastatic melanoma. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 459. [Google Scholar] [CrossRef]
- Ediriwickrema, L.S.; Liu, C.Y.; Kikkawa, D.O.; Korn, B.S. Development of Poliosis Following Checkpoint Inhibitor Treatment for Cutaneous Melanoma. Ophthalmic Plast. Reconstr. Surg. 2019, 35, e121–e122. [Google Scholar] [CrossRef]
- Ocampo, M.M.; Lerner, J.; Dasanu, C.A. Bluish-gray fingernail discoloration due to the use of nivolumab. J. Oncol. Pharm. Pract. 2021, 27, 457–459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burzi, L.; Alessandrini, A.M.; Quaglino, P.; Piraccini, B.M.; Dika, E.; Ribero, S. Cutaneous Events Associated with Immunotherapy of Melanoma: A Review. J. Clin. Med. 2021, 10, 3047. https://doi.org/10.3390/jcm10143047
Burzi L, Alessandrini AM, Quaglino P, Piraccini BM, Dika E, Ribero S. Cutaneous Events Associated with Immunotherapy of Melanoma: A Review. Journal of Clinical Medicine. 2021; 10(14):3047. https://doi.org/10.3390/jcm10143047
Chicago/Turabian StyleBurzi, Lorenza, Aurora Maria Alessandrini, Pietro Quaglino, Bianca Maria Piraccini, Emi Dika, and Simone Ribero. 2021. "Cutaneous Events Associated with Immunotherapy of Melanoma: A Review" Journal of Clinical Medicine 10, no. 14: 3047. https://doi.org/10.3390/jcm10143047
APA StyleBurzi, L., Alessandrini, A. M., Quaglino, P., Piraccini, B. M., Dika, E., & Ribero, S. (2021). Cutaneous Events Associated with Immunotherapy of Melanoma: A Review. Journal of Clinical Medicine, 10(14), 3047. https://doi.org/10.3390/jcm10143047








