Variations of Thiol–Disulfide Homeostasis Parameters after Treatment with H1-Antihistamines in Patients with Chronic Spontaneous Urticaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Tests
- DS/NT (-S-S- * 100/-SH);
- DS/TT (-S-S- * 100/-SH + -S-S-);
- NT/TT (-SH * 100/-SH + -S-S-);
- NT: (-SH); TT: (-SH + -S-S-); DS: (-S-S).
2.3. Statistical Analysis
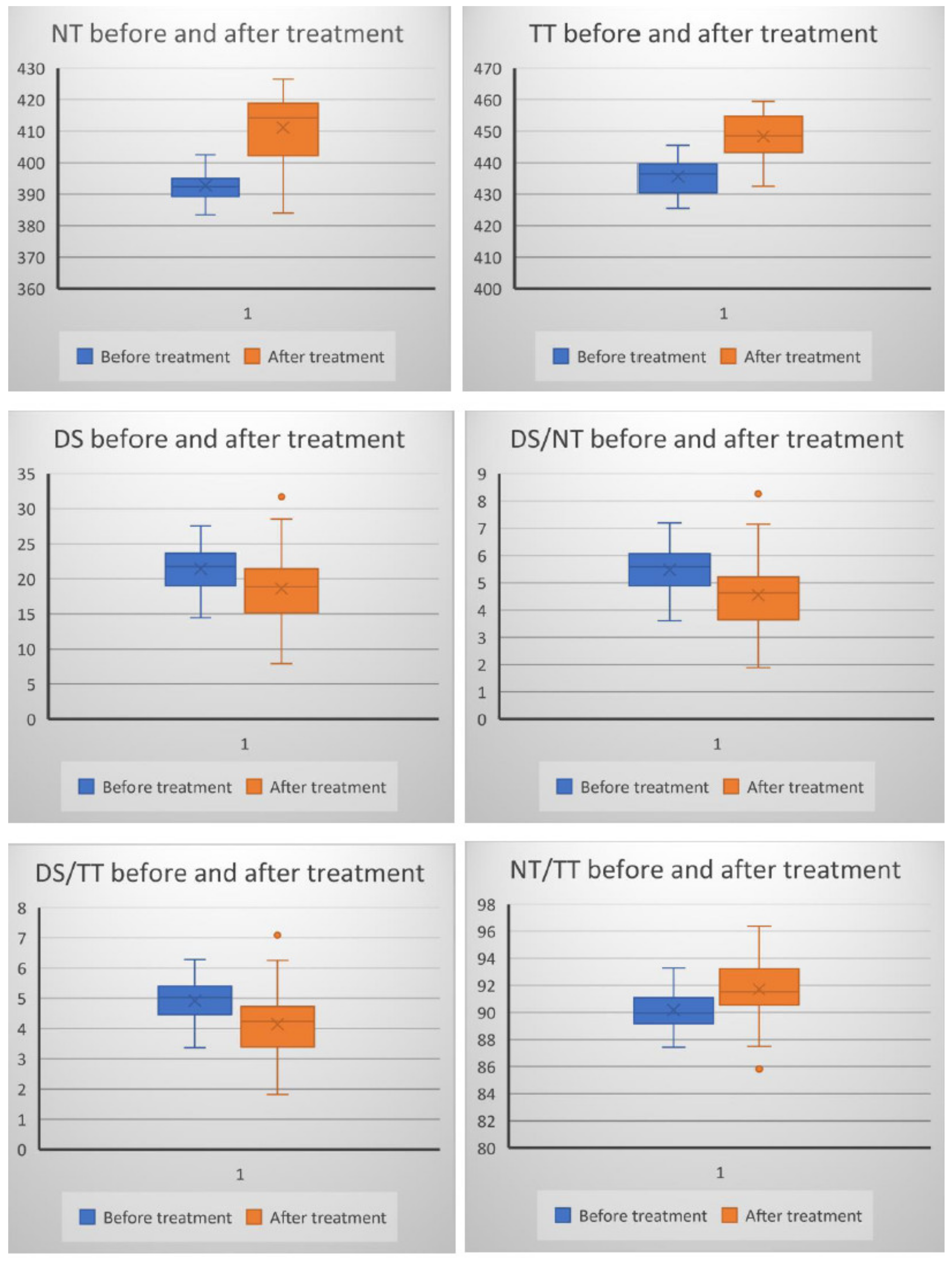
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baek, J.; Lee, M.-G. Oxidative Stress and Antioxidant Strategies in Dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox Modifications of Protein-Thiols: Emerging Roles in Cell Signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The Thiol Pool in Human Plasma: The Central Contribution of Albumin to Redox Processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Poole, L.B. The Basics of Thiols and Cysteines in Redox Biology and Chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Laer, K.; Hamilton, C.J.; Messens, J. Low-Molecular-Weight Thiols in Thiol-Disulfide Exchange. Antioxid. Redox Signal. 2013, 18, 1642–1653. [Google Scholar] [CrossRef]
- Kalkan, G.; Emre, S.; Alisik, M.; Aktaş, A.; Baran, P. Dynamic Thiol/Disulfide Homeostasis in Patients with Lichen Planus. J. Clin. Lab. Anal. 2019, 33, e22642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sener, S.; Akbas, A.; Kilinc, F.; Baran, P.; Erel, O.; Aktas, A. Thiol/Disulfide Homeostasis as a Marker of Oxidative Stress in Rosacea: A Controlled Spectrophotometric Study. Cutan. Ocul. Toxicol. 2019, 38, 55–58. [Google Scholar] [CrossRef] [PubMed]
- McLeay, Y.; Stannard, S.; Houltham, S.; Starck, C. Dietary Thiols in Exercise: Oxidative Stress Defence, Exercise Performance, and Adaptation. J. Int. Soc. Sports Nutr. 2017, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dirican, N.; Dirican, A.; Sen, O.; Aynali, A.; Atalay, S.; Bircan, H.A.; Oztürk, O.; Erdogan, S.; Cakir, M.; Akkaya, A. Thiol/Disulfide Homeostasis: A Prognostic Biomarker for Patients with Advanced Non-Small Cell Lung Cancer? Redox Rep. 2016, 21, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.; Hansen, J.M. Oxidative Stress, Thiols, and Redox Profiles. Methods Mol. Biol. 2012, 889, 325–346. [Google Scholar] [CrossRef]
- Aksoy, M.; Çelik, H. Dynamic Thiol/Disulphide Homeostasis in Vitiligo Patients. Postep. Dermatol. Alergol. 2018, 35, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Ene, C.D.; Tampa, M.; Matei, C.; Benea, V.; Nicolae, I. Oxidative stress-related markers and alopecia areata through latex turbidimetric immunoassay method. Mater. Plast. 2016, 53, 522–526. [Google Scholar]
- Persson, B.; Andersson, A.; Hultberg, B.; Hansson, C. The Redox State of Glutathione, Cysteine and Homocysteine in the Extracellular Fluid in the Skin. Free Radic. Res. 2002, 36, 151–156. [Google Scholar] [CrossRef]
- Kalkan, G.; Seçkin, H.Y.; Duygu, F.; Akbaş, A.; Ozyurt, H.; Sahin, M. Oxidative Stress Status in Patients with Acute Urticaria. Cutan. Ocul. Toxicol. 2014, 33, 109–114. [Google Scholar] [CrossRef]
- Gol, M.; Özkaya, B.; Yildirim, C.; Bal, R. Regular Exercise, Overweight/Obesity and Sedentary Lifestyle Cause Adaptive Changes in Thiol-Disulfide Homeostasis. An. Acad. Bras. Ciênc. 2019, 91, e20180547. [Google Scholar] [CrossRef]
- Nicolae, I.; Ene, C.D.; Georgescu, S.R.; Tampa, M.; Matei, C.; Ceausu, E. Effects of UV Radiation and Oxidative DNA Adduct 8-Hydroxy-2′-Deoxiguanosine on the Skin Diseases. Rev. Chim. 2014, 65, 1036–1041. [Google Scholar]
- Sogut, I.; Aydin, A.S.; Gokmen, E.S.; Atak, P.G.; Erel, O.; DeGrigo, U.G. Evaluation of Oxidative Stress and Thiol-Disulfide Parameters According to the Body Mass Index in Adult Individuals. Erciyes Med. J. 2018, 40. [Google Scholar] [CrossRef]
- Üstüner, P.; Balevi, A.; Özdemir, M.; Olmuşçelik, O.; Ülfer, G.; Yiğitbaşı, T. The role of thiol/disulfide homeostasis in psoriasis: Can it be a new marker for inflammation? Turkderm 2018, 52, 120–125. [Google Scholar] [CrossRef]
- Akbaş, A.; Kılınç, F.; Sener, S.; Akın, A.; Bıçer, C.; Şen, O. Research on the Balance of Thiol-Disulfide in Blood Serum in Women with Telogen Effluvium. Int. J. Trichol. 2019, 11, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, G.; Neselioglu, S.; Karaismailoglu, E.; Aktas, A.; Erel, O. Plasma Thiol Levels Are Associated with Disease Severity in Nonsegmental Vitiligo. Indian J. Dermatol. 2018, 63, 323–327. [Google Scholar] [CrossRef]
- Emre, S.; Demirseren, D.D.; Alisik, M.; Aktas, A.; Neselioglu, S.; Erel, O. Dynamic Thiol/Disulfide Homeostasis and Effects of Smoking on Homeostasis Parameters in Patients with Psoriasis. Cutan. Ocul. Toxicol. 2017, 36, 393–396. [Google Scholar] [CrossRef]
- Pektaş, S.D.; Alataş, E.T.; Doğan, G.; Neşelioğlu, S.; Erel, Ö. A Marker for Evaluation of Oxidative Stress in Patients with Alopecia Areta: Thiol-Disulphide Homeostasis. Meandros Med. Dent. J. 2018, 19, 205. [Google Scholar] [CrossRef] [Green Version]
- Demirseren, D.D.; Cicek, C.; Alisik, M.; Demirseren, M.E.; Aktaş, A.; Erel, O. Dynamic Thiol/Disulphide Homeostasis in Patients with Basal Cell Carcinoma. Cutan. Ocul. Toxicol. 2017, 36, 278–282. [Google Scholar] [CrossRef]
- Kilinc, F.; Akbas, A.; Sener, S.; Ergin, M.; Baran, P.; Metin, A. The Effect of Tinea Versicolor on Thiol/Disulphide Homeostasis. Postep. Dermatol. Alergol. 2018, 35, 299–303. [Google Scholar] [CrossRef]
- Uysal, P.; Avcil, S.; Neşelioğlu, S.; Biçer, C.; Çatal, F. Association of Oxidative Stress and Dynamic Thiol-Disulphide Homeostasis with Atopic Dermatitis Severity and Chronicity in Children: A Prospective Study. Clin. Exp. Dermatol. 2018, 43, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Karacan, G.; Ercan, N.; Bostanci, I.; Alisik, M.; Erel, O. A Novel Oxidative Stress Marker of Atopic Dermatitis in Infants: Thiol-Disulfide Balance. Arch. Dermatol. Res. 2020, 312, 697–703. [Google Scholar] [CrossRef]
- Mitran, M.I.; Nicolae, I.; Tampa, M.; Mitran, C.I.; Caruntu, C.; Sarbu, M.I.; Ene, C.D.; Matei, C.; Georgescu, S.R.; Popa, M.I. Reactive Carbonyl Species as Potential Pro-Oxidant Factors Involved in Lichen Planus Pathogenesis. Metabolites 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurel, G.; Karadol, M.; Bal, C.; Iptec, B.; Clgecen, E. Evaluation of thiol/disulfide homeostasis in patients with acne vulgaris. J. Clin. Anal. Med. 2019, 10, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Akbas, A.; Kilinc, F.; Sener, S.; Aktaş, A.; Baran, P.; Ergin, M. Investigation of Thiol-Disulphide Balance in Patients with Acute Urticaria and Chronic Spontaneous Urticaria. Cutan. Ocul. Toxicol. 2017, 36, 205–210. [Google Scholar] [CrossRef]
- Akdag, S.; Ozmen, S.; Ercan, N.; Bostanci, I.; Neselioglu, S. Assessment of Thiol/Disulphide Homoeostasis and Ischaemia-Modified Albumin and Their Relationship with Disease Severity in Children with Chronic Urticaria. Cutan. Ocul. Toxicol. 2020, 39, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Aydin, İ.E.; Savrun, Ş.T.; Savrun, A.; Önder, S.; NeşeliOğlu, S.; Erel, Ö.; Kaşko Arici, Y. Assessment of Oxidative Stress with Thiol Disulfide Homeostasis and Ischemia-Modifıed Albumin Level in Acute Urticaria. Middle Black Sea J. Health Sci. 2021, 7, 115–121. [Google Scholar] [CrossRef]
- Saini, S.S.; Kaplan, A.P. Chronic Spontaneous Urticaria: The Devil’s Itch. J. Allergy Clin. Immunol. Pract. 2018, 6, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Antia, C.; Baquerizo, K.; Korman, A.; Bernstein, J.A.; Alikhan, A. Urticaria: A Comprehensive Review: Epidemiology, Diagnosis, and Work-Up. J. Am. Acad. Dermatol. 2018, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Dinu, L.; Nicolae, I.; Tampa, M.; Matei, C.; Georgescu, S.; Christian, H.J.; Babes, V. The serum Levels of 8-Hydroxy-Deoxyguanosine under the Chemicals Influence. Rev. Chim. 2014, 65, 1319–1326. [Google Scholar]
- Georgescu, S.R.; Ene, C.D.; Nicolae, I.; Mitran, M.; Musetescu, A.; Matei, C.; Rusu, L.C.; Tampa, M. Reflectometric Analysis for Identification of Various Pathological Conditions Associated with Lichen Planus. Rev. Chim. 2017, 68, 1103–1108. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, M.J.; Krämer, A.C.; Miotto, G.; Zaccarin, M.; Zhang, H.; Ursini, F. Protein Cysteine Oxidation in Redox Signaling: Caveats on Sulfenic Acid Detection and Quantification. Arch. Biochem. Biophys. 2017, 617, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, D.A.; Forman, H.J. Cellular Glutathione and Thiols Metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Winther, J.R.; Thorpe, C. Quantification of Thiols and Disulfides. Biochim. Biophys. Acta 2014, 1840, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of New Oxidative Stress Markers in Chronic Spontaneous Urticaria. Postep. Dermatol. Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Cannavò, S.P.; Riso, G.; Di Salvo, E.; Casciaro, M.; Giuffrida, R.; Minciullo, P.L.; Guarneri, F.; Nettis, E.; Gangemi, S. Oxidative Stress Involvement in Urticaria. J. Biol. Regul. Homeost. Agents 2020, 34, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Yorulmaz, A.; Erdogan, S.; Cakmak, S.K.; Guney, E.; Sen, O.; Erel, O. An Evaluation of Thiol/Disulphide Homeostasis in Patients with Psoriasis. Postep. Dermatol. Alergol. 2017, 34, 464–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emre, S.; Kalkan, G.; Erdoğan, S.; Aktaş, A.; Ergin, M. Dynamic Thiol/Disulfide Balance in Patients with Seborrheic Dermatitis: A Case-Control Study. Saudi J. Med. Med. Sci. 2020, 8, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gaffrey, M.J.; Li, X.; Qian, W.-J. Characterization of Cellular Oxidative Stress Response by Stoichiometric Redox Proteomics. Am. J. Physiol. Cell Physiol. 2021, 320, C182–C194. [Google Scholar] [CrossRef]
- Aksoy, M.; Kirmit, A. Thiol/Disulphide Balance in Patients with Psoriasis. Postep. Dermatol. Alergol. 2020, 37, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient Characteristic | Value |
|---|---|
| Age (years)—mean ± SD Female/male ratio | 29.73 ± 3.19 1.5/1 |
| History of angioedema | 33.3% |
| Disease duration (months)—mean ± SD | 9.2 ± 3.4 |
| Parameter | Before Treatment n = 30 | After Treatment n = 26 | ||
|---|---|---|---|---|
| rho | p | rho | p | |
| NT | −0.74 | 0.0001 | −0.85 | 0.0001 |
| TT | −0.73 | 0.0001 | −0.80 | 0.0001 |
| DS | 0.10 | 0.58 | 0.01 | 0.9 |
| DS/NT | 0.46 | 0.009 | 0.41 | 0.02 |
| DS/TT | 0.46 | 0.008 | 0.43 | 0.02 |
| NT/TT | −0.47 | 0.007 | −0.43 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, C.; Georgescu, S.R.; Nicolae, I.; Ene, C.D.; Mitran, C.I.; Mitran, M.I.; Tampa, M. Variations of Thiol–Disulfide Homeostasis Parameters after Treatment with H1-Antihistamines in Patients with Chronic Spontaneous Urticaria. J. Clin. Med. 2021, 10, 2980. https://doi.org/10.3390/jcm10132980
Matei C, Georgescu SR, Nicolae I, Ene CD, Mitran CI, Mitran MI, Tampa M. Variations of Thiol–Disulfide Homeostasis Parameters after Treatment with H1-Antihistamines in Patients with Chronic Spontaneous Urticaria. Journal of Clinical Medicine. 2021; 10(13):2980. https://doi.org/10.3390/jcm10132980
Chicago/Turabian StyleMatei, Clara, Simona Roxana Georgescu, Ilinca Nicolae, Corina Daniela Ene, Cristina Iulia Mitran, Madalina Irina Mitran, and Mircea Tampa. 2021. "Variations of Thiol–Disulfide Homeostasis Parameters after Treatment with H1-Antihistamines in Patients with Chronic Spontaneous Urticaria" Journal of Clinical Medicine 10, no. 13: 2980. https://doi.org/10.3390/jcm10132980
APA StyleMatei, C., Georgescu, S. R., Nicolae, I., Ene, C. D., Mitran, C. I., Mitran, M. I., & Tampa, M. (2021). Variations of Thiol–Disulfide Homeostasis Parameters after Treatment with H1-Antihistamines in Patients with Chronic Spontaneous Urticaria. Journal of Clinical Medicine, 10(13), 2980. https://doi.org/10.3390/jcm10132980








