Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
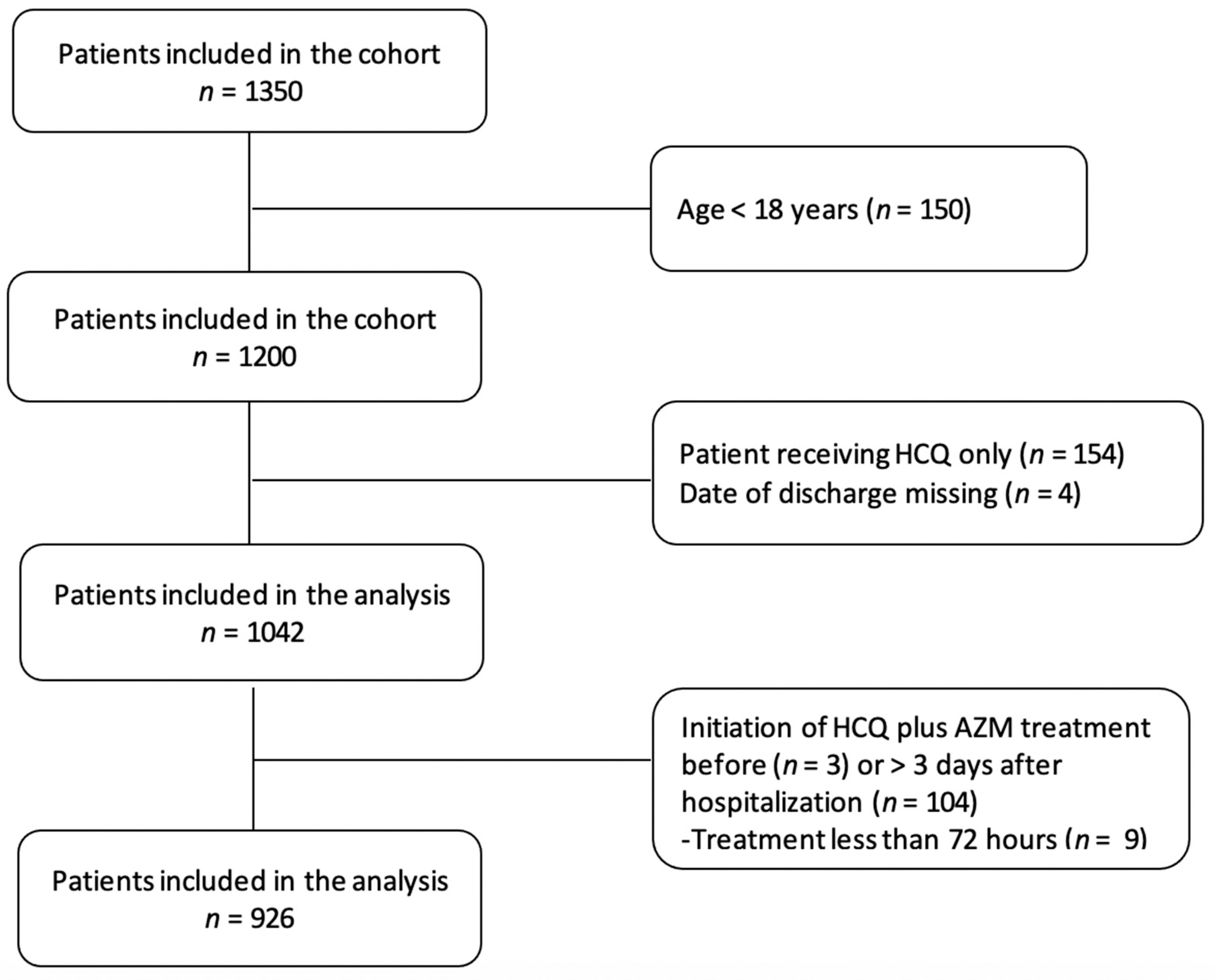
2.3. Participants
2.4. Data Sources
2.5. Variables Assessed
2.6. Illness Severity
2.7. End Point
2.8. Statistical Analysis
2.9. Ethical Aspects
3. Results
3.1. Characteristics of Patients
3.2. Characteristics of Patient at ITC Admission Time
3.3. Patients’ Characteristics during Hospitalization
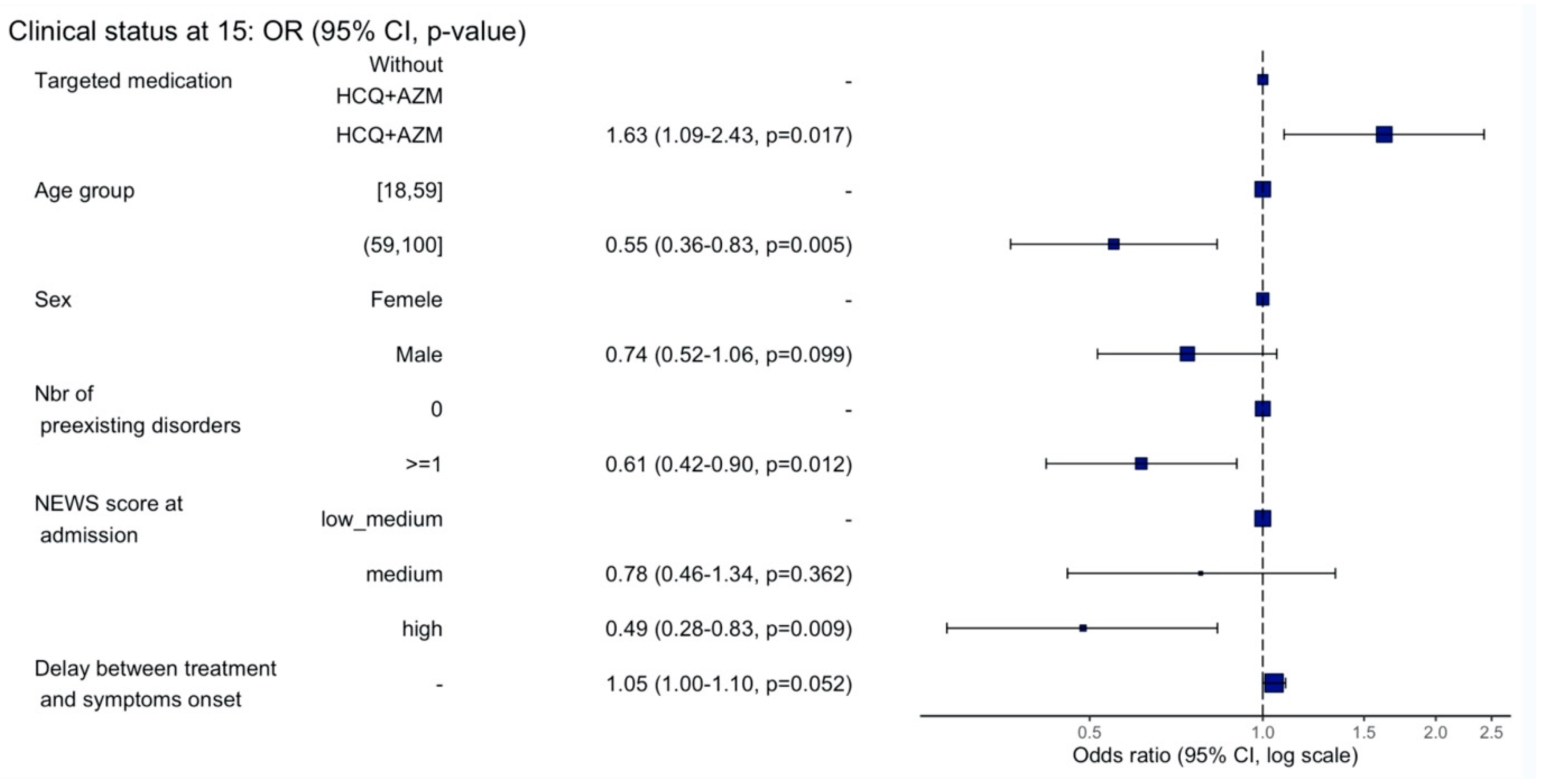
3.4. Clinical Status at Day 15
3.5. Reported Side Effects
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Novel Coronavirus–China. 12 January 2020. Available online: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (accessed on 1 June 2020).
- WHO WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 June 2020).
- SMoHaS, Coronavirus (COVID-19) Situation Dashboard. Available online: https://cartosantesen.maps.arcgis.com/apps/opsdashboard/index.html#/260c7842a77a48c191bf51c8b0a1d3f6 (accessed on 30 October 2020).
- Gautret, P.; Hoang, V.T.; Lagier, J.-C.; Raoult, D. Effect of hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, an update with an intention-to-treat analysis and clinical outcomes. Int. J. Antimicrob. Agents 2021, 57. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Million, M.; Gautret, P.; Colson, P.; Cortaredona, S.; Giraud-Gatineau, A.; Honoré, S.; Gaubert, J.-Y.; Fournier, P.-E.; Tissot-Dupont, H.; et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Dagens, A.; Sigfrid, L.; Cai, E.; Lipworth, S.; Cheung, V.; Harris, E.; Bannister, P.; Rigby, I.; Horby, P. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: Rapid review. BMJ 2020, 369, m1936. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.-M.; Colson, P.; Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Gautret, P.; Colson, P.; Roussel, Y.; Dubourg, G.; Chabriere, E.; Honore, S.; Rolain, J.-M.; Fenollar, F.; Fournier, P.-E.; et al. Clinical efficacy of chloroquine derivatives in COVID-19 infection: Comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020, 38. [Google Scholar] [CrossRef]
- Damle, B.; Vourvahis, M.; Wang, E.; Leaney, J.; Corrigan, B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020, 108, 201–211. [Google Scholar] [CrossRef]
- Andreani, J.; Le Bideau, M.; Duflot, I.; Jardot, P.; Rolland, C.; Boxberger, M.; Wurtz, N.; Rolain, J.-M.; Colson, P.; La Scola, B.; et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020, 145. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.-M.; Preziosi, M.-P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.-P.; et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M.; Tebé, C.; Peñafiel, J.; Tobias, A.; Ballana, E.; Alemany, A.; Riera-Martí, N.; Pérez, A.C.; et al. Hydroxychloroquine for Early Treatment of Adults with Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Us-tianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Bessière, F.; Roccia, H.; Delinière, A.; Charrière, R.; Chevalier, P.; Argaud, L.; Cour, M. Assessment of QT Intervals in a Case Series of Patients with Coronavirus Disease 2019 (COVID-19) Infection Treated with Hydroxychloroquine Alone or in Combination with Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020, 5, 1067–1069. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Clinical Management: Living Guidance 25 January 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Belayneh, A. Off-Label Use of Chloroquine and Hydroxychloroquine for COVID-19 Treatment in Africa Against WHO Recommendation. Res. Rep. Trop. Med. 2020, 11, 61–72. [Google Scholar] [CrossRef]
- Simpson, C.R.; Beever, D.; Challen, K.; De Angelis, D.; Fragaszy, E.; Goodacre, S.; Hayward, A.; Lim, W.S.; Rubin, G.J.; Semple, M.; et al. The UK’s pandemic influenza research portfolio: A model for future research on emerging infections. Lancet Infect. Dis. 2019, 19, e295–e300. [Google Scholar] [CrossRef] [Green Version]
- WHO Clinical Characterisation Protocol (CCP). ISARIC. Available online: https://isaric.org/research/covid-19-clinical-research-resources/clinical-characterisation-protocol-ccp/ (accessed on 1 June 2020).
- Hoehl, S.; Rabenau, H.; Berger, A.; Kortenbusch, M.; Cinatl, J.; Bojkova, D.; Behrens, P.; Böddinghaus, B.; Götsch, U.; Naujoks, F.; et al. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N. Engl. J. Med. 2020, 382, 1278–1280. [Google Scholar] [CrossRef]
- ISARIC. Novel Coronavirus (NCoV) Acute Respiratory Infection Clinical Characterisation Data Tool. Version 1.2 28 JAN 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Ilness Severity in the NHS. Updated Report of a Working Party; RCP: London, UK, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Guilbert, T.W.; Mauger, D.T.; Boehmer, S.J.; Beigelman, A.; Fitzpatrick, A.M.; Jackson, D.J.; Baxi, S.N.; Benson, M.; Burnham, C.-A.D.; et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children with a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015, 314, 2034–2044. [Google Scholar] [CrossRef]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nachega, J.B.; Ishoso, D.K.; Otokoye, J.O.; Hermans, M.P.; Machekano, R.N.; Sam-Agudu, N.A.; Nswe, C.B.-P.; Mbala-Kingebeni, P.; Madinga, J.N.; Mukendi, S.; et al. Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 in Africa: Early Insights from the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2020, 103, 2419–2428. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated with In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Jensen, M.; Mehlhorn, H. Seventy-five years of Resochin® in the fight against malaria. Parasitol. Res. 2009, 105, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.F. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001, 64, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.-F.; Tall, A.; Sokhna, C.; Ly, A.B.; Diagne, N.; Ndiath, O.; Mazenot, C.; Richard, V.; Badiane, A.; Dieye-Ba, F.; et al. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: A 22 year longitudinal study. Lancet Infect. Dis. 2014, 14, 476–488. [Google Scholar] [CrossRef]
- Trape, J.-F.; Pison, G.; Preziosi, M.-P.; Enel, C.; du Loû, A.D.; Delaunay, V.; Samb, B.; Lagarde, E.; Molez, J.-F.; Simondon, F. Impact of chloroquine resistance on malaria mortality. Comptes Rendus Acad. Sci. Ser. III Sci. Vie 1998, 321, 689–697. [Google Scholar] [CrossRef]
- Stokkermans, T.J.; Goyal, A.; Bansal, P.; Trichonas, G. Chloroquine and Hydroxychloroquine Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cardiac Effects and Toxicity of Chloroquine: A Short Update—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0924857920302272 (accessed on 5 June 2021).
- Huang, C.; Soleimani, J.; Herasevich, S.; Pinevich, Y.; Pennington, K.M.; Dong, Y.; Pickering, B.W.; Barwise, A.K. Clinical Characteristics, Treatment, and Outcomes of Critically Ill Patients With COVID-19: A Scoping Review. Mayo Clin. Proc. 2021, 96, 183–202. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
| Patient’s Characteristics | Without HCQ + AZM (n = 252) | HCQ + AZM (n = 674) | Total (n = 926) | p-Value |
|---|---|---|---|---|
| Age | <0.001 1 | |||
| Median | 57 | 42 | 45 | |
| Q1, Q3 | 36, 67 | 30, 58 | 31, 60 | |
| Age categories | <0.001 2 | |||
| 18–59 | 140 (55.6%) | 521 (77.3%) | 661 (71.4%) | |
| ≥60 | 112 (44.4%) | 153 (22.7%) | 265 (28.6%) | |
| Sex | 0.824 2 | |||
| Female | 114 (45.2%) | 298 (44.2%) | 412 (44.5%) | |
| Number of preexisting disorders | <0.001 2 | |||
| 0 | 118 (46.8%) | 464 (68.8%) | 582 (62.9%) | |
| 1 | 68 (27.0%) | 124 (18.4%) | 192 (20.7%) | |
| ≥2 | 66 (26.2%) | 86 (12.8%) | 152 (16.4%) | |
| Preexisting disorder | ||||
| Hypertension | 72 (30.1%) | 83 (12.5%) | 155 (17.1%) | <0.001 2 |
| Diabetes | 58 (23.8%) | 74 (11.1%) | 132 (14.5%) | <0.001 2 |
| Chronic lung disease * | 4 (1.6%) | 15 (2.2%) | 19 (2.1%) | 0.794 2 |
| Obesity | 17 (7.1%) | 21 (3.2%) | 38 (4.2%) | 0.014 2 |
| Chronic Heart dysfunction | 13 (5.2%) | 15 (2.2%) | 28 (3.0%) | 0.028 2 |
| Chronic hematologic dysfunction | 3 (1.2%) | 7 (1.0%) | 10 (1.1%) | 0.736 2 |
| Chronic renal disease | 4 (1.6%) | 1 (0.1%) | 5 (0.5%) | 0.020 2 |
| Others | 34 (13.7%) | 66 (9.9%) | 100 (10.9%) | 0.121 2 |
| Symptoms | ||||
| No COVID symptoms | 37 (14.7%) | 213 (32.2%) | 250 (27.4%) | <0.001 2 |
| Fever | 116 (49.2%) | 261 (39.5%) | 377 (42.1%) | 0.011 2 |
| Cough | 115 (48.9%) | 207 (31.6%) | 322 (36.1%) | <0.001 2 |
| Runny nose | 24 (10.3%) | 72 (11.0%) | 96 (10.8%) | 0.902 2 |
| Taste or smell lost | 33 (14.1%) | 107 (16.3%) | 140 (15.7%) | 0.465 2 |
| Myalgia | 67 (28.4%) | 144 (22.0%) | 211 (23.7%) | 0.050 2 |
| Asthenia | 47 (24.9%) | 80 (13.5%) | 127 (16.2%) | <0.001 2 |
| Diarrhea and/or Nausea | 19 (8.7%) | 44 (7.0%) | 63 (7.4%) | 0.373 2 |
| Median no. of days since symptom onse—median (IQR) | 0.359 1 | |||
| Median | 6 | 6 | 6 | |
| Q1, Q3 | 3, 8 | 3, 9 | 3, 8 | |
| Delay between first positive PCR and ITC admission, days—median (IQR) | 0.333 1 | |||
| Median | 3 | 3 | 3 | |
| Q1, Q3 | 2, 4 | 2, 4 | 2, 4 | |
| Systolic Blood Pressure (mmHg) | <0.001 1 | |||
| Median | 135 | 130 | 130 | |
| Q1, Q3 | 120, 150 | 120, 140 | 120, 145 | |
| Diastolic Blood Pressure (mmHg) | 0.697 1 | |||
| Median | 80 | 80 | 80 | |
| Q1, Q3 | 72, 90 | 77, 90 | 76, 90 | |
| Heart Rate (beats/min) | <0.001 1 | |||
| Median | 96 | 87 | 89 | |
| Q1, Q3 | 84, 107 | 76, 98 | 78, 100 | |
| Respiratory rate (breaths/min) | <0.001 1 | |||
| Median | 24 | 22 | 22 | |
| Q1, Q3 | 20, 30 | 20, 24 | 20, 26 | |
| NEWS score at admission time | <0.001 2 | |||
| Low | 135 (53.6%) | 519 (77.0%) | 654 (70.6%) | |
| Low medium | 16 (6.3%) | 60 (8.9%) | 76 (8.2%) | |
| Medium | 44 (17.5%) | 48 (7.1%) | 92 (9.9%) | |
| High | 57 (22.6%) | 47 (7.0%) | 104 (11.2%) | |
| Admitted to intensive care at admission (ICU) | 30 (11.9%) | 10 (1.5%) | 40 (4.3%) | <0.001 2 |
| Virological results at admission † | ||||
| Negative | - | - | 45 (4.86%) | |
| Positive | - | - | 881 (95.14%) | |
| Ct values—median (IQR) | - | - | 30 (23.7,33) |
| Without HCQ + AZM | HCQ + AZI | Total | |
|---|---|---|---|
| Abdominal discomfort | 14 (4.32%) | 6 (8.45%) | 20 (5.06%) |
| Cardiac rhythm disorder | 13 (4.01%) | 2 (2.82%) | 15 (3.80 %) |
| Death | 67 (20.68%) | 24 (33.80%) | 91 (23.04%) |
| Diarrhea | 18 (5.56%) | 19 (26.76%) | 37 (9.37%) |
| Headache | 78 (24.07%) | 4 (5.63%) | 82 (20.76%) |
| Hematologic disorders | 6 (1.85%) | 1 (1.41%) | 7 (1.77%) |
| Musculoskeletal disorders | 70 (21.60%) | 2 (2.82%) | 72 (18.23%) |
| Upset stomach | 43 (13.27%) | 5 (7.04%) | 48 (12.15%) |
| Vomit | 15 (4.63%) | 8 (11.27%) | 23 (5.82%) |
| Total side effect reported | 324 | 71 | 395 |
| Absence of side effect reported | 88 (9.5%) | 618 (66.74%) | 706 (76.24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taieb, F.; Mbaye, K.D.; Tall, B.; Lakhe, N.A.; Talla, C.; Thioub, D.; Ndoye, A.M.; Ka, D.; Gaye, A.; Cissé Diallo, V.M.-P.; et al. Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020. J. Clin. Med. 2021, 10, 2954. https://doi.org/10.3390/jcm10132954
Taieb F, Mbaye KD, Tall B, Lakhe NA, Talla C, Thioub D, Ndoye AM, Ka D, Gaye A, Cissé Diallo VM-P, et al. Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020. Journal of Clinical Medicine. 2021; 10(13):2954. https://doi.org/10.3390/jcm10132954
Chicago/Turabian StyleTaieb, Fabien, Khardiata Diallo Mbaye, Billo Tall, Ndèye Aïssatou Lakhe, Cheikh Talla, Daouda Thioub, Amadou Moustapha Ndoye, Daye Ka, Aboubacry Gaye, Viviane Marie-Pierre Cissé Diallo, and et al. 2021. "Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020" Journal of Clinical Medicine 10, no. 13: 2954. https://doi.org/10.3390/jcm10132954
APA StyleTaieb, F., Mbaye, K. D., Tall, B., Lakhe, N. A., Talla, C., Thioub, D., Ndoye, A. M., Ka, D., Gaye, A., Cissé Diallo, V. M.-P., Dia, N., Ba, P. S., Cissé, M., Diop, M., Diagne, C. T., Fortes, L., Diop, M., Fall, N. M., Sarr, F. D., ... Seydi, M. (2021). Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020. Journal of Clinical Medicine, 10(13), 2954. https://doi.org/10.3390/jcm10132954






