A Systematic Review of the Efficacy and Safety of Direct Oral Anticoagulants in Atrial Fibrillation Patients with Diabetes Using a Risk Index
Abstract
:1. Introduction
2. Methods
3. Statistical Analysis
4. Main Results
5. Discussion
6. Study Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, preva-lence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.S.H.; Goncalves, E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of an-ti-diabetic therapies. Diabetes Obes. Metab. 2019, 21, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Haywood, L.J.; Ford, C.E.; Crow, R.S.; Davis, B.R.; Massie, B.M.; Einhorn, P.T.; Williard, A. Atrial Fibrillation at Baseline and During Follow-Up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). J. Am. Coll. Cardiol. 2009, 54, 2023–2031. [Google Scholar] [CrossRef] [Green Version]
- Aksnes, T.A.; Schmieder, R.E.; Kjeldsen, S.E.; Ghani, S.; Hua, T.A.; Julius, S. Impact of new-onset diabetes mellitus on develop-ment of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial). Am. J. Cardiol. 2008, 101, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.F.; Simpson, C.; Jhund, P.; Stewart, S.; Kirkpatrick, M.; Chalmers, J.; Macintyre, K.; McMurray, J.J.V. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 2007, 93, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Eckhardt, L.L.; Chen, L.Y.; Ahmed, H.M.; Gopinathannair, R.; Joglar, J.A.; Noseworthy, P.A.; Pack, Q.R.; Sanders, P.; Trulock, K.M. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e750–e772. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giacomello, R.; Stel, G.; Motz, E.; Taboga, C.; Tonutti, L.; Pirisi, M.; Falleti, E.; Bartoli, E. Hyperglycemia-induced thrombin formation in diabetes. The possible role of oxidative stress. Diabetes 1995, 44, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ay, C.; Kopp, C.W.; Panzer, S.; Gremmel, T. Impaired glucose metabolism is associated with increased thrombin generation potential in patients undergoing angioplasty and stenting. Cardiovasc. Diabetol. 2018, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Baraka-Vidot, J.; Guerin-Dubourg, A.; Bourdon, E.; Rondeau, P. Impaired drug-binding capacities of in vitro and in vivo glycated albumin. Biochimie 2012, 94, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.W.; Desai, S.; Damaraju, C.V.; Lu, L.; Fields, L.E.; Wildgoose, P.; Schein, J.R. International normalized ratio stability in warfarin-experienced patients with nonvalvular atrial fbrillation. Am. J. Cardiovasc. Drugs 2015, 15, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Acanfora, D.; Ciccone, M.M.; Scicchitano, P.; Ricci, G.; Acanfora, C.; Uguccioni, M.; Casucci, G. Efficacy and Safety of Direct Oral Anticoagulants in Patients With Atrial Fibrillation and High Thromboembolic Risk. A Systematic Review. Front. Pharmacol. 2019, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, M.; Terranova, A.; Di Lullo, L. Valutazione delle reazioni avverse agli anticoagulanti orali diretti registrate nella Rete Nazionale di Farmacovigilanza mediante uno specifico indice di rischio. Ital. Cardiol. 2018, 19, 3–11. [Google Scholar]
- Du, X.; Ninomiya, T.; De Galan, B.; Abadir, E.; Chalmers, J.; Pillai, A.; Woodward, M.; Cooper, M.; Harrap, S.; Hamet, P.; et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: Results of the ADVANCE study. Eur. Hear. J. 2009, 30, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Patti, G.; Cavallari, I.; Andreotti, F.; Calabrò, P.; Cirillo, P.; Denas, G.; Galli, M.; Golia, E.; Maddaloni, E.; Marcucci, R.; et al. Prevention of atherothrombotic events in patients with diabetes mellitus: From antithrombotic therapies to new-generation glucose-lowering drugs. Nat. Rev. Cardiol. 2019, 16, 113–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, G.; Di Gioia, G.; Cavallari, I.; Nenna, A. Safety and efficacy of nonvitamin K antagonist oral anticoagulants versus warfarin in diabetic patients with atrial fibrillation: A study-level meta-analysis of phase III randomized trials. Diabetes Metab. Res. Rev. 2017, 33, e2876. [Google Scholar] [CrossRef] [PubMed]
- Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology 2007, 69, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog. Cardiovasc. Dis. 2019, 62, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiong, Q.; Chen, Z.; Fu, L.; Hu, J.; Chen, Q.; Tu, W.; Xu, C.; Xu, G.; Li, J.; et al. Factors Associated with a Large Decline in Renal Function or Progression to Renal Insufficiency in Hospitalized Atrial Fibrillation Patients with Early-Stage CKD. Int. Hear. J. 2020, 61, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Tangri, N.; Gersh, B.J.; Sangaralingham, L.R.; Shah, N.D.; Nath, K.A.; Noseworthy, P.A. Renal Outcomes in Anticoagulated Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Acanfora, D.; Casucci, G.; Ciccone, M.M.; Scicchitano, P.; Montefusco, G.; Lanzillo, A.; Acanfora, C.; Lanzillo, B. Direct Oral Anti-coagulants, Bleeding Risk in Patients with Atrial Fibrillation, CHADS2 ≥ 3 or HAS-BLED ≥ 3. Cardiovasc. Pharm. 2018, 240, 2–4. [Google Scholar]
- Pacholczak-Madej, R.; Bazan-Socha, S.; Zaręba, L.; Undas, A.; Dropiński, J. Direct oral anticoagulants in the prevention of stroke in breast cancer patients with atrial fibrillation during adjuvant endocrine therapy: A cohort study. Int. J. Cardiol. 2021, 324, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zadok, O.I.B.; Eisen, A. Use of non-vitamin K oral anticoagulants in people with atrial fibrillation and diabetes mellitus. Diabetes Med. 2018, 35, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, C.A.; Nassar Gorra, L.; Rodriguez, A.L.; Trepka, M.J.; Veledar, E.; Madhivanan, P. Evaluation of the inclusion of studies identified by the FDA as having falsified data in the results of meta-analyses: The example of the apixaban trials. JAMA Int. Med. 2019, 179, 582–584. [Google Scholar] [CrossRef] [PubMed]
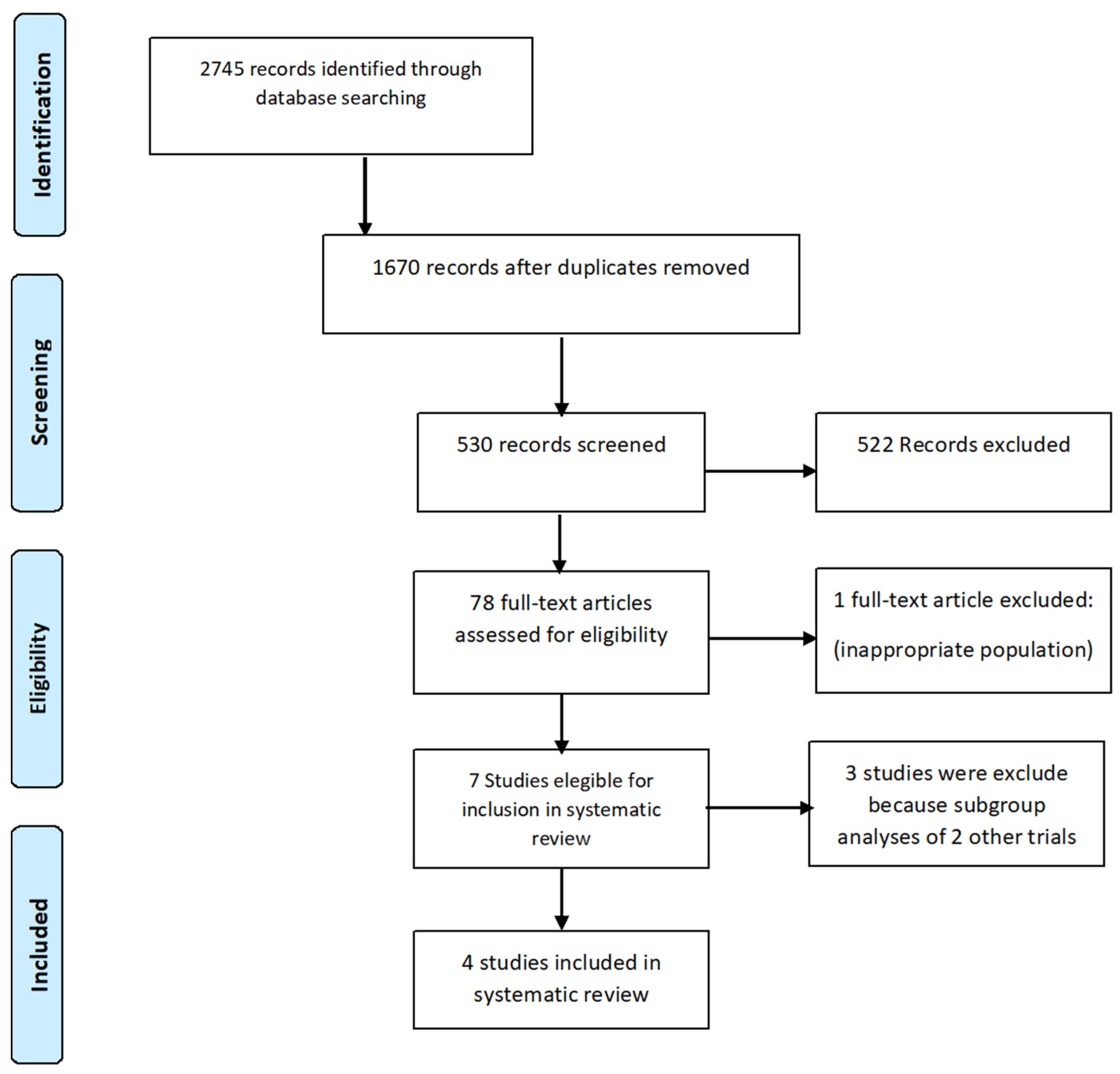

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Patients with NVAF receiving any of the treatments below. All studies in the SLR must include ≥ 90% patients with NVAF. SLRs including studies with < 90% patients with NVAF must report data separately for the NVAF studies | Not a population of interest (ie, non-NVAF patients) Studies of patients receiving ablation, cardioversion, or left-atrial appendage closure |
| Intervention/comparator | DOACs (apixaban, dabigatran, rivaroxaban, edoxaban) and warfarin studies need to have compared 1 or more DOACs and/or warfarin | Studies not reporting outcomes for population of interest |
| Outcome | Clinical outcomes:
Apixaban: 5 or 2.5 mg a Rivaroxaban: 20 or 15 mg Dabigatran: 150 or 110 mg Edoxaban: 30 or 60 mg | SLRs/NMAs of observational studies, nonsystematic reviews, primary research trials, primary observational studies, case reports, case series, narrative reviews Letters to the editor, guidelines, meeting abstracts In vitro pharmacodynamic or pharmacokinetic studies only, animal studies, genetic studies only |
| Study design | SLR of randomized controlled trials |
| ROCKET AF Rivaroxaban | ARISTOTLE Apixaban | RE-LY Dabigatran | ENGAGE Edoxaban | |
|---|---|---|---|---|
| Effect | Anti-Xa | Anti-Xa | Anti-IIa | Anti-Xa |
| Dose | 20/15 mg QD | 5/2.5 mg BID | 150/110 mg BID | 60/30 mg QD |
| Mean CHADS2 score | 3.5 | 2.1 | 2.1 | 2.8 |
| Target INR (Warfarin arm) | 2–3 | 2–3 | 2–3 | 2–3 |
| TTR (%) | 58 | 62 | 64 | 68 |
| Asia Pacific Region N (%) | 2109 (14.8%) | 2916 (16%) | 3854 (21%) | 3383 (16%) |
| Median Follow-up duration | 1.9 y | 1.8 y | 2 y | 2.8 y |
| RCTs | Pts on DOACs (N) | Diabetes N (%) | No Diabetes N (%) | Pts on Warfarin (N) | Diabetes N (%) | No Diabetes N (%) |
|---|---|---|---|---|---|---|
| ROCKET AF | 7131 | 2878 (40.3%) | 4253 (59.7%) | 7133 | 2817 (39.5%) | 4316 (60.5%) |
| ARISTOTLE | 9120 | 2284 (25.0%) | 6836 (75%) | 9087 | 2263 (24.9%) | 6818 (75.1%) |
| RE-LY 110 mg | 6015 | 1409 (23.4%) | 4606 (66.6%) | 6022 | 1410 (23.4%) | 4612 (66.6%) |
| RE-LY 150 mg | 6076 | 1402 (23.1%) | 4674 (63.9%) | 6022 | 1410 (23.4%) | 4612 (66.6%) |
| ENGAGE 30 mg | 7034 | 2544 (36.2%) | 4490 (63.8%) | 7036 | 2521 (35.8%) | 4515 (64.2%) |
| ENGAGE 60 mg | 7035 | 2529 (35.9%) | 4476 (64.1%) | 7036 | 2521 (35.8%) | 4515 (64.2%) |
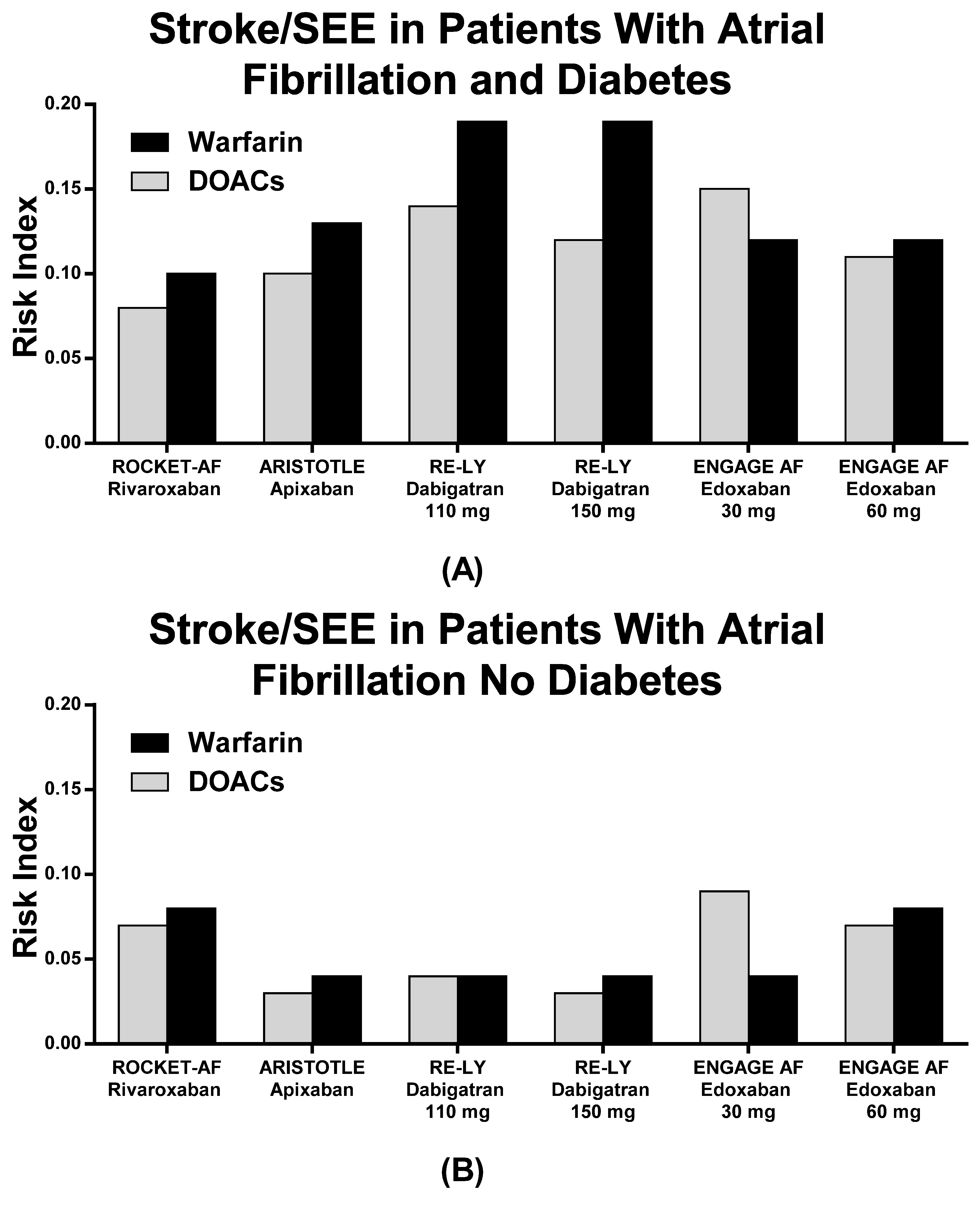
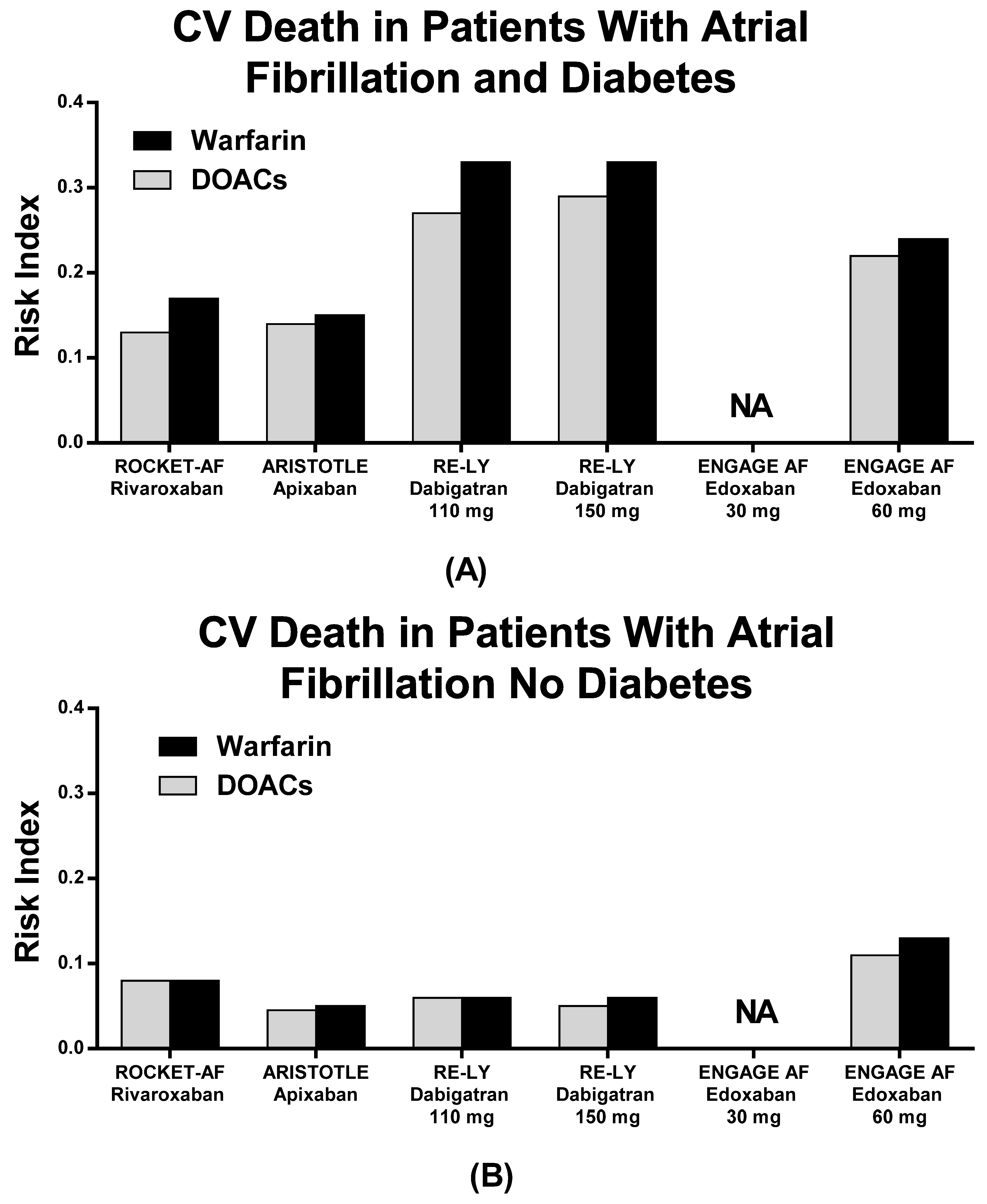
| Diabetes | ||||||
| RCTs | Stroke o SEE (N)% | MB (N)% | CV Death N (%) | |||
| DOACs | Warfarin | DOACs | Warfarin | DOACs | Warfarin | |
| ROCKET AF | 95 (3.3%) | 114 (4.0%) | 165 (5.7%) | 169 (6.0%) | 152 (5.3%) | 192 (6.8%) |
| ARISTOTLE | 57 (2.5%) | 75 (3.3%) | 112 (4.9%) | 114 (5.0%) | 79 (3.5%) | 88 (3.9%) |
| RE-LY 110 | 49 (3.5%) | 64 (4.5%) | 106 (7.5%) | 114 (8.0%) | 91 (6.5%) | 109 (7.7%) |
| RE-LY 150 | 40 (2.9%) | 129 (9.2%) | 95 (6.8%) | |||
| ENGAGE 30 mg | 135 (5.3%) | 107 (4.2%) | 123 (4.9%) | 278 (11%) | NA | NA |
| ENGAGE 60 mg | 102 (4.0%) | 219 (8.6%) | 209 (8.1%) | 219 (8.6%) | ||
| No Diabetes | ||||||
| RCTs | Stroke o SEE (N)% | MB (N)% | CV Death N (%) | |||
| ROCKET AF | 174 (4.0%) | 192 (4.4%) | 230 (5.4%) | 217(5.0%) | 223 (5.2%) | 209 (4.8%) |
| ARISTOTLE | 155 (2.3%) | 190 (2.8%) | 215 (3.2%) | 348 (5.1%) | 229 (3.4%) | 256 (3.7%) |
| RE-LY 110 | 134 (2.9%) | 138 (2.9%) | 236 (5.1%) | 307 (6.6%) | 198 (4.3%) | 208 (4.5%) |
| RE-LY 150 | 94 (2.0%) | 271 (5.8%) | 179 (3.8%) | |||
| ENGAGE 30 mg | NA | NA | NA | NA | NA | NA |
| ENGAGE 60 mg | 207 (4.6%) | 248 (5.4%) | 335 (7.5%) | 417 (9.2%) | 331 (7.4%) | 406 (8.9%) |
| Diabetes | ||||||
| RCTs | Stroke o SEE | MB | CV Death | |||
| DOACs | Warfarin | DOACs | Warfarin | DOACs | Warfarin | |
| ROCKET AF | 0.08 | 0.10 | 0.14 | 0.15 | 0.13 | 0.17 |
| ARISTOTLE | 0.10 | 0.13 | 0.19 | 0.20 | 0.14 | 0.15 |
| RE-LY 110 | 0.14 | 0.19 | 0.32 | 0.34 | 0.27 | 0.33 |
| RE-LY 150 | 0.12 | 0.40 | 0.29 | |||
| ENGAGE 30 mg | 0.15 | 0.12 | 0.13 | 0.30 | NA | NA |
| ENGAGE 60 mg | 0.11 | 0.23 | 0.22 | 0.24 | ||
| No Diabetes | ||||||
| RCTs | Stroke o SEE | MB | CV Death | |||
| ROCKET AF | 0.06 | 0.07 | 0.09 | 0.08 | 0.08 | 0.08 |
| ARISTOTLE | 0.03 | 0.03 | 0.04 | 0.06 | 0.04 | 0.04 |
| RE-LY 110 | 0.04 | 0.04 | 0.07 | 0.09 | 0.06 | 0.06 |
| RE-LY 150 | 0.03 | 0.09 | 0.06 | |||
| ENGAGE 30 mg | NA | NA | NA | NA | NA | NA |
| ENGAGE 60 mg | 0.07 | 0.08 | 0.11 | 0.14 | 0.11 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acanfora, D.; Ciccone, M.M.; Carlomagno, V.; Scicchitano, P.; Acanfora, C.; Bortone, A.S.; Uguccioni, M.; Casucci, G. A Systematic Review of the Efficacy and Safety of Direct Oral Anticoagulants in Atrial Fibrillation Patients with Diabetes Using a Risk Index. J. Clin. Med. 2021, 10, 2924. https://doi.org/10.3390/jcm10132924
Acanfora D, Ciccone MM, Carlomagno V, Scicchitano P, Acanfora C, Bortone AS, Uguccioni M, Casucci G. A Systematic Review of the Efficacy and Safety of Direct Oral Anticoagulants in Atrial Fibrillation Patients with Diabetes Using a Risk Index. Journal of Clinical Medicine. 2021; 10(13):2924. https://doi.org/10.3390/jcm10132924
Chicago/Turabian StyleAcanfora, Domenico, Marco Matteo Ciccone, Valentina Carlomagno, Pietro Scicchitano, Chiara Acanfora, Alessandro Santo Bortone, Massimo Uguccioni, and Gerardo Casucci. 2021. "A Systematic Review of the Efficacy and Safety of Direct Oral Anticoagulants in Atrial Fibrillation Patients with Diabetes Using a Risk Index" Journal of Clinical Medicine 10, no. 13: 2924. https://doi.org/10.3390/jcm10132924
APA StyleAcanfora, D., Ciccone, M. M., Carlomagno, V., Scicchitano, P., Acanfora, C., Bortone, A. S., Uguccioni, M., & Casucci, G. (2021). A Systematic Review of the Efficacy and Safety of Direct Oral Anticoagulants in Atrial Fibrillation Patients with Diabetes Using a Risk Index. Journal of Clinical Medicine, 10(13), 2924. https://doi.org/10.3390/jcm10132924






