Abstract
Thyroid hormones may be a risk factor for the development of non-alcoholic fatty liver disease (NAFLD) and its progression to liver fibrosis. The aim of this study is to investigate the relationship between thyroid stimulating hormone (TSH) levels, NAFLD, and liver fibrosis in the general population. A descriptive cross-sectional study was performed in subjects aged 18–75 years randomly selected from primary care centers between 2012 and 2016. Each subject underwent clinical evaluation, physical examination, blood tests and transient elastography. Descriptive and multivariate logistic regression analyses were used to identify factors associated with NAFLD and fibrosis. We included 2452 subjects (54 ± 12 years; 61% female). Subjects with TSH ≥ 2.5 μIU/mL were significantly associated with obesity, atherogenic dyslipidemia, metabolic syndrome (MetS), hypertransaminasemia and altered cholesterol and triglycerides. The prevalence of NAFLD and liver fibrosis was significantly higher in subjects with TSH ≥ 2.5 (μIU/mL). We found a 1.5 times increased risk of NAFLD, 1.8 and 2.3 times increased risk of liver fibrosis for cut-off points of ≥8.0 kPa and ≥9.2 kPa, respectively, in subjects with TSH ≥ 2.5 μIU/mL compared with TSH < 2.5 μIU/mL (control group), independent of the presence of MetS. These findings remained significant when stratifying TSH, with values ≥ 10 μIU/mL.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a major public health problem in recent decades, being the most common liver disease worldwide, with a prevalence between 25% and 30% of the adult population [1]. The main cause of the increase in its prevalence is the close relationship between NAFLD and different metabolic disorders, such as obesity, type 2 diabetes (T2DM), hypertriglyceridemia or metabolic syndrome (MetS), which in turn affect a large number of subjects today [2]. The heterogenicity in the distribution of the disease according to sub-populations with metabolic risk factors has led to a rethinking, even in the nomenclature of NAFLD, with the aim of identifying those patients with a higher risk of liver progression [3].
The main characteristic of NAFLD is fatty infiltration in more than 5% of hepatocytes, which is known as simple steatosis, in the absence of other chronic liver diseases (viral, alcoholic, drug, autoimmune) [4]. Later, this disease can progress to steatohepatitis, with different degrees of affectation, leading to the development of advanced liver fibrosis in 5–8% of patients [5,6]. Detecting liver fibrosis early is crucial as the severity of fibrosis predicts the development of liver cirrhosis and long-term survival.
Among the multiple extrahepatic complications that have been described in NAFLD, where metabolic and endocrinological disorders predominate, are alterations in thyroid function [7]. Thyroid hormones (TH) are involved in glycemic and lipid metabolism, as well as insulin resistance. The participation of TH in some physiopathological processes of NAFLD, such as the beta oxidation of free fatty acids, the cascade of pro-inflammatory cytokines, oxidative stress reactions or activation of stellate liver cells that lead to a fibrogenic response, make a possible relationship plausible [8]. Some studies have shown that hypothyroidism is more common in subjects with NAFLD [9]; others, that a low thyroid function is related to the risk of developing NAFLD, independently of other metabolic factors [10]. TH levels have also been associated with NAFLD [11] and liver fibrosis [12]. However, due to the heterogenicity of the populations studied and the different diagnostic criteria for defining the alteration of thyroid function and NAFLD or liver fibrosis, other authors have not found such a relationship [13,14]. Therefore, there is still some controversy regarding this association and more studies are required to clarify the role of TH in NAFLD.
For this reason, the main objective of this study is to analyze the relationship between the levels of thyroid stimulating hormone (TSH), NAFLD, and liver fibrosis in the general population.
2. Methods
2.1. Study Design and Population
For the design of this study, the cohort of a previous project was used, which was carried out between 2012 and 2016, and whose results were recently published [15]. A descriptive, cross-sectional, multi-center, population-based study was carried out, which included subjects aged 18 to 75 years, randomly selected from the Primary Care Information System (SIAP). All subjects were invited to participate in the study by telephone and underwent a clinical interview, a physical examination, a blood test, and a transient liver elastography, with prior informed consent.
To carry out this sub-study, 3060 participants from the reference cohort were selected. Individuals with known chronic liver disease, hepatotoxic drug use, severe advanced disease, cognitive impairment, institutionalization, and death were previously excluded. For this sub-study, only subjects with information on TSH levels were included. The specific exclusion criteria were: incomplete laboratory data (n = 202), absence or invalidity of liver elastography measurements (n = 46), inability to calculate the Fatty Liver Index (FLI) (n = 50) and weekly alcohol consumption of ≥21 standard drink units (SDUs) in men and ≥14 SDUs in women (n = 310). Finally, the sample obtained for data analysis was 2452 individuals.
2.2. Clinical and Laboratory Parameters
The variables that were collected were the following: age, sex, height, weight, waist circumference (WC), body mass index (BMI), systolic and diastolic blood pressure (SBP and DBP), tobacco and alcohol consumption in SDU. Individuals were also questioned for the presence of previous co-morbidities: arterial hypertension (HBP), hypercholesterolemia, hypertriglyceridemia and T2DM. These data were compared with the records in the computerized medical history. Blood tests were performed after a 12-h fast, including the determination of a complete blood count, TSH, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), ferritin, total proteins, albumin, glycemia, glycosylated hemoglobin, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG). Hypertransaminasemia was defined with ALT and/or AST values > 35 U/L, using the cut-off point of our reference laboratory. Atherogenic dyslipidemia was established when TG levels ≥ 150 mg/dL and HDL levels < 40/50 mg/dL in men/women, respectively [16,17]. The normal range used for TSH values was 0.35–4.94 μIU/mL according to data from our reference laboratory.
2.3. Evaluation of MetS
The diagnosis of MetS was made according to the criteria established by the NCEP-ATPIII [18] when the subjects presented ≥ 3 of the following components: WC > 88 cm women and > 102 cm men; TG ≥ 150 mg/dL or on lipid-lowering treatment; HDL < 40 mg/dL in men and < 50 mg/dL in women or on lipid-lowering treatment; BP ≥ 130/85 mmHg or on hypotensive treatment; and baseline glycemia ≥ 100 mg/dL or on hypoglycemic treatment.
2.4. Evaluation of NAFLD
NAFLD was diagnosed using the FLI serological marker that includes the variables TG, BMI, GGT and WC; and is calculated from the following formula: FLI = (e0.953 × loge(TG) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × WC − 15.745)/(1 + e0.953 × loge(TG) + 0.139 × BMI + 0.718 × loge(GGT) + 0.053 × WC − 15.745) × 100. When the FLI score ≥ 60 the diagnosis is NAFLD, FLI between 30 and 60 the diagnosis is indeterminate, and if FLI < 30 no NAFLD [19].
2.5. Evaluation of Liver Fibrosis
Transient liver elastography (TE) was performed on each subject, using the M probe of the Fibroscan 402 apparatus (Echosens, Paris, France). Exclusion criteria were the inability to obtain 10 valid measurements and/or an interquartile range/liver stiffness (LS) measurement ratio greater than 30%. Two cut-off points, suggestive of significant liver fibrosis, were defined according to LS values: ≥8.0 kilopascals (kPa) and ≥9.2 kPa [15,20].
2.6. Statistical Analysis
For the descriptive analysis, the continuous variables were expressed as means and standard deviation, since they followed a normal distribution, and the categorical variables in frequencies and percentages. The prevalences were calculated with their respective 95% confidence intervals (95% CI). In the bivariate comparisons of categorical variables, the Chi-square test was used, for the continuous variables in two groups the Student’s t-test was used, and for comparisons in four groups analysis of variance was used.
The main outcome variables were, on the one hand, the presence of NAFLD (FLI ≥ 60), and on the other, liver fibrosis, for which the LS measured by TE was used at two cut-off points (≥8.0 kPa and ≥9.2 kPa) used independently for the analyses. Subjects were stratified into various groups, according to TSH levels, to assess the risk of presenting NAFLD or liver fibrosis. The main TSH groups (model 1) for the study of the outcome variables were: TSH < 2.5 μIU/mL, corresponding to the control group, and TSH ≥ 2.5 μIU/mL, as an explanatory variable. Furthermore, in order to better define the subjects with TSH ≥ 2.5 μIU/mL, they were additionally sub-classified into three groups (model 2): TSH 2.50–4.94 μIU/mL, TSH 4.95–9.99 μIU/mL and TSH ≥ 10 μIU/mL. Multivariate logistic regression analyses were used in several models adjusted for potential confounding factors. The corresponding odds ratio (OR) and their 95% CI were obtained. Statistical tests were performed with bilateral contrasts and statistical significance of p < 0.05. The analyses were carried out with the R package version 4.0.2 (R development Core Team, GNU, GPL) and Rstudio version 1.2.5019 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Basal Characteristics
Of the 2452 subjects included in this study, 61% were female, 94% Caucasian, and they had a mean age of 54 ± 12 years. The prevalence of the different metabolic factors in the global sample were the following: T2DM 10%, HBP 26%, hypercholesterolemia 38%, obesity 31%, hypertriglyceridemia 11% and abdominal obesity 47%. In addition, 27% of the subjects presented diagnostic criteria for MetS. Hypertransaminasemia affected 13% of the sample and NAFLD was found in 35% of the cases.
The individuals were classified according to TSH levels into two groups (model 1): 66% with TSH < 2.5 μIU/mL (n = 1619) and 44% with TSH ≥ 2.5 μIU/mL (n = 833). Additionally, subjects with TSH ≥ 2.5 μIU/mL were stratified in (model 2): 86% with TSH 2.50–4.94 μIU/mL (n = 718); 12% with TSH 4.95–9.99 μIU/mL (n = 96) and 2% with TSH ≥ 10 μIU/mL (n = 19).
The baseline characteristics of the study subjects are shown in Table 1. Obesity, both global and abdominal, hypercholesterolemia, atherogenic dyslipidemia, MetS, and hypertransaminasemia were significantly more prevalent in the group with TSH ≥ 2.5 μIU/mL compared to the control group. Likewise, in these subjects higher levels of BMI, WC, total cholesterol, TG, ALT, AST, and ALP were observed. Similar findings were observed in the stratification of the subjects with TSH ≥ 2.5 μIU/mL in model 2. Furthermore, in the group with TSH ≥ 10 μIU/mL, a higher prevalence of T2DM was found compared to the control group (16% vs. 9.8%) and higher LDL levels (p < 0.001). However, although the prevalence of MetS in subjects with TSH ≥ 10 μIU/mL was higher (32% vs. 25%), it was not significant.

Table 1.
Basal characteristics of the subjects according to TSH levels (μIU/mL).
3.2. Relationship between TSH and NAFLD
First, the relationship of TSH levels according to the presence of NAFLD was studied (Table 2). TSH levels were significantly higher in subjects with FLI ≥ 60 (2.9 vs. 2.3 μIU/mL). The prevalence of subjects with TSH ≥ 2.5 μIU/mL was higher in the presence of NAFLD compared to the group without NAFLD (39% vs. 31%; p < 0.001). The prevalence of TSH alteration, in the stratified groups of model 2, was also higher in the group with NAFLD (p < 0.001).

Table 2.
TSH levels and prevalence of TSH alteration according to presence of NAFLD.
Furthermore, the prevalence of NAFLD was analyzed according to TSH levels (Figure 1). NAFLD affected 40% of the subjects with TSH ≥ 2.5 μIU/mL (p = 0.001). Moreover, NAFLD occurred more frequently when TSH levels were higher (73.7% in subjects with TSH ≥ 10 μIU/mL).
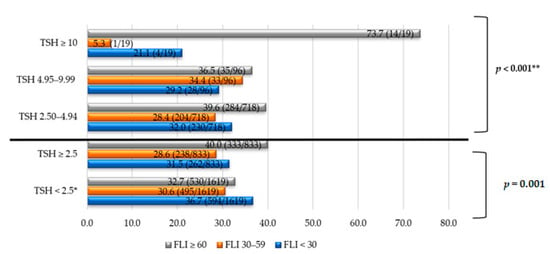
Figure 1.
NAFLD prevalence according to TSH levels in different groups. Note: The data are expressed in %. * Comparison/control group. ** p trend, comparing four groups of TSH: < 2.5, 2.50–4.94, 4.95–9.99, ≥ 10 μIU/mL. TSH units: μIU/mL. Abbreviations: NAFLD, non-alcoholic fatty liver disease; TSH, thyroid stimulating hormone; FLI, fatty liver index.
In multivariate analyses, the risk of presenting NAFLD according to TSH levels ≥ 2.5 μIU/mL, compared to the control group, was 1.5 times greater regardless of age, sex, alcohol consumption, obesity, cholesterol, or MetS; and 1.33 times greater, regardless of the MetS parameters (Figure 2a).
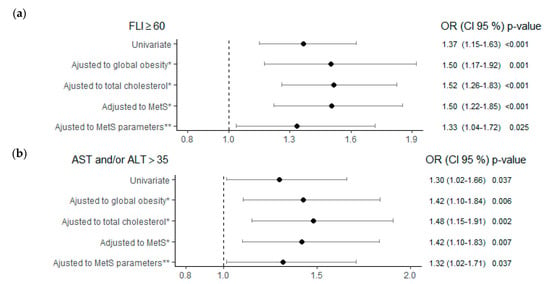
Figure 2.
Risk of NAFLD (a) and hypertransaminasemia (b) according to TSH ≥ 2.5 (μIU/mL) adjusted to different metabolic factors. Note: Control reference group for the analysis TSH < 2.5 (μIU/mL). * All multivariate analyses were adjusted also for age, sex and alcohol consumption. ** MetS parameters: WC > 88W/102M cm; TG ≥ 150 mg/dL; HDL < 50 W/40 M mg/dL; BP ≥ 130/85 mmHg; Glyc ≥ 100 mg/dL. Abbreviations: FLI, fatty liver index; MetS, metabolic syndrome; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TSH, thyroid stimulating hormone; NAFLD, non-alcoholic fatty liver disease; WC, waist circumference; TG, triglycerides; HDL, high-density lipoprotein; BP, blood pressure; Glyc, glycemia.
To better explain these findings, the risk of NAFLD was analyzed in the different stratified TSH groups of model 2. Both the subjects with TSH 2.50–4.94 μIU/mL and those with TSH ≥ 10 μIU/mL showed a significant increase in the risk of NAFLD of 1.31 and 8.22 times, respectively, in relation to the control group, independently of age, sex, alcohol consumption, and the different parameters of MetS (Table 3). In addition, an excess risk of FLI ≥ 60 with OR > 1 was also obtained in these two TSH groups, in the other multivariate analyses carried out in separate models and adjusted for global obesity, total cholesterol, and presence of MetS. Although the ORs for the TSH 4.95–9.99 μIU/mL group were >1, they did not reach statistical significance.

Table 3.
Analysis of NAFLD risk according to TSH group. Four different multivariate logistic regression models, adjusting for obesity, cholesterol, MetS and MetS parameters.
3.3. Relationship between TSH and Hypertransaminasemia
The prevalence of hypertransaminasemia (ALT and/or AST > 35 U/L) increased from 12%, in subjects with TSH < 2.5 μIU/mL, to 15% in subjects with TSH ≥ 2.5 μIU/mL or 26% when TSH ≥ 10 μIU/mL, in a significant way (p = 0.036 and p = 0.040, respectively). ALT and ALP values underwent a dose-dependent increase in relation to TSH levels (Table 1).
The risk of presenting hypertransaminasemia when TSH ≥ 2.5 μIU/mL was higher compared to the control group, and independent of age, sex, obesity, cholesterol, MetS, or MetS parameters (Figure 2b). In the multivariate analyses of model 2, the subjects with TSH 2.50–4.94 μIU/mL presented an increased risk of hypertransaminasemia of 1.34 times, compared to the control group, independently of the different parameters of MetS (p = 0.035). Furthermore, the risk of ALT and/or AST > 35 U/L in subjects with TSH ≥ 10 μIU/mL was 2.24 times higher compared to the TSH < 2.5 μIU/mL group, although these results were not significant (Table 4).

Table 4.
Analysis between TSH group and risk of hypertransaminasemia.
3.4. Association between TSH and Liver Fibrosis
LS values increased with TSH levels: from 4.8 ± 1.7 kPa in the group with TSH < 2.5 μIU/mL to 5.1 ± 2.7 kPa in subjects with TSH ≥ 2.5 μIU/mL (p = 0.003). A significant increase in LS was also observed in the group with TSH ≥ 10 μIU/mL (LS 5.3 ± 1.8 kPa, p = 0.017).
Regarding the prevalence of fibrosis, as seen in Figure 3, higher values are reached in all TSH groups compared to the control group. Some 7.1% and 4.3% of the subjects with TSH ≥ 2.5 μIU/mL obtained TE values ≥ 8.0 kPa and ≥9.2 kPa, respectively, higher than the control group. Furthermore, in subjects with TSH ≥ 10 μIU/mL, the prevalence of fibrosis was higher: Some 10.5% vs. 4.3% (control group) for TE values ≥ 8.0 kPa; and 5.3% vs. 2.0% (control group) for TE values ≥ 9.2 kPa. All of these findings were statistically significant.
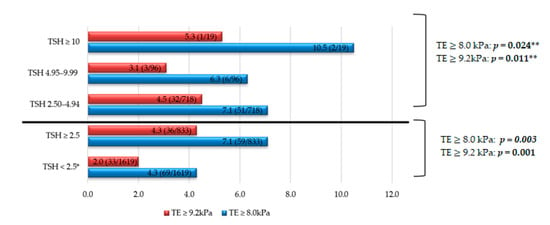
Figure 3.
Liver fibrosis prevalence according to TSH levels in different groups. Note: The data are expressed in %. * Comparison/control group. ** p trend, comparing four groups of TSH: <2.5, 2.50–4.94, 4.95–9.99, ≥10 μIU/mL. TSH units: μIU/mL. Abbreviations: TE, transient elastography; TSH, thyroid stimulating hormone.
The risk of fibrosis, at both TE cut-off points, in subjects with TSH ≥ 2.5 μIU/mL was higher when compared to the group with TSH < 2.5 μIU/mL, regardless of age, sex, obesity, cholesterol, and MetS (Figure 4). Subjects with TSH ≥ 2.5 μIU/mL showed an increased risk of fibrosis of 1.67 (Figure 4a) and 2.17 times (Figure 4b) for the cut-off points TE ≥ 8.0 kPa and ≥ 9.2 kPa respectively and independent of the different parameters of MetS.
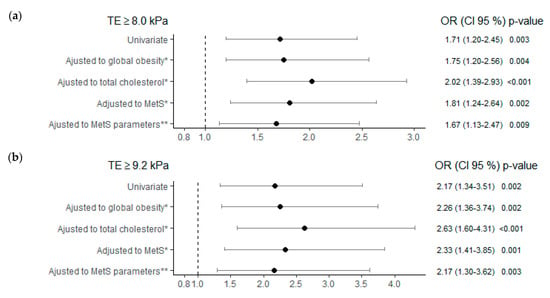
Figure 4.
Liver fibrosis risk, using two transient elastography cut-offs, TE ≥ 8.0kPa in (a) and TE ≥ 9.2 kPa in (b), as dependent variables, in TSH group ≥ 2.5 (μIU/mL), adjusted to different metabolic factors. Note: Control reference group for the analysis TSH < 2.5 (μIU/mL). * All multivariate analyses were adjusted also for age, sex and alcohol consumption. ** MetS parameters: WC > 88 W/102 M cm; TG ≥ 150 mg/dL; HDL < 50 W/40 M mg/dL; BP ≥ 130/85 mmHg; Glyc ≥ 100 mg/dL. Abbreviations: MetS, metabolic syndrome; TE, transient elastography; TSH, thyroid stimulating hormone; WC, waist circumference; TG, triglycerides; HDL, high-density lipoprotein; BP, blood pressure; Glyc, glycemia.
Likewise, in the multivariate analyses carried out with the different TSH groups (model 2), an increased risk of fibrosis was determined, both in TE ≥ 8.0 kPa and ≥ 9.2 kPa, in subjects with TSH 2.50–4.94 μIU/mL, significantly and independently of all the confounding factors studied (Table 5). Although in the TSH groups with higher levels an increased risk of fibrosis was also observed for both cut-off points, these findings were not significant for the TSH 4.95–9.99 μIU/mL groups or for TSH ≥ 10 μIU/mL, with the exception of the analysis adjusted for total cholesterol.

Table 5.
Analysis of liver fibrosis risk, using two elastography cut-offs as dependent variables, according to TSH group. Four different multivariate logistic regression models, adjusting for obesity, cholesterol, MetS and MetS parameters.
4. Discussion
The findings of this study demonstrate the association between TSH levels, NAFLD, and liver fibrosis. Specifically, those subjects with TSH levels ≥ 2.5 μIU/mL have a significantly increased risk of presenting NAFLD and fibrosis independently of the different metabolic factors studied.
The relationship between thyroid function or TH and NAFLD was analyzed in several studies in the last decade. There are multiple differences in the methodology used to study both pathologies. Thus, there are studies that have used serological markers, such as FLI, for the diagnosis of NAFLD [21], while others have used abdominal ultrasound [13] or pathological criteria [22]. The most accepted upper limit of TSH to define normality of thyroid function is between 4 and 5 μIU/mL [23], although there are authors who differentiate subjects with an upper limit of normality of TSH < 2.5 μIU/mL as those with strictly normal thyroid function [24]. Other factors, such as race, the presence of antithyroid antibodies, or the percentage of patients undergoing hormone replacement therapy, also influence the variability of the studies carried out.
Some studies have shown higher TSH levels [10] or a higher prevalence of hypothyroidism [25] in subjects with NAFLD. A study was recently published, with a large sample of subjects, which shows a higher incidence of hypothyroidism and autoimmune thyroiditis in the group with NAFLD, with a follow-up period of 10 years [26]. Other studies confirm an increased risk of presenting NAFLD based on TSH levels [27] or thyroid function [28]. Low T4 [29] or high T3 [30] levels have been correlated with liver steatosis. Furthermore, TSH levels have also been linked to NASH [31]. Likewise, there are studies that have demonstrated these associations independently of MetS [32] and, on the other hand, others that have not [33].
Furthermore, the relationship between TH and NAFLD has also been studied in specific subpopulations according to some metabolic conditioning factors. In the morbidly obese population, an increased risk of NAFLD, defined by serological markers, has been found in subjects with higher TSH and T3 values [34]. Higher TSH levels, lower T4 levels and a higher prevalence of positivity of anti-thyroid peroxidase antibodies were found in patients where T2DM and NAFLD coexist, compared to those without NAFLD [35]. In another similar study, carried out in a diabetic and euthyroid population, it was shown that subjects with higher levels of T3 and TSH had a higher risk of presenting NAFLD, with ORs of 3.02 and 1.58, respectively [36].
The meta-analyses available to date show some different results. In one by Guo et al., where 26 studies were included, the subjects with NAFLD/NASH reached significantly higher levels of TSH in relation to the control group. In addition, a 1.6 times increased risk of NAFLD was also demonstrated in hypothyroid subjects [37]. Along the same lines, Mantovani et al. demonstrated the association between hypothyroidism and NAFLD independently of age, sex, BMI, and other metabolic factors studied, in a study involving 44,140 individuals [38]. He et al. also found an increased risk of NAFLD of 1.81 and 1.63 times in subjects with overt and subclinical hypothyroidism, respectively [39]. In contrast, the study by Jaruvongvanich et al. did not demonstrate a relationship between thyroid hormones (TSH, T4, T3) and NAFLD, nor with hypothyroidism [40]. The findings of our study are in line with the above. On the one hand, we found a correlation between NAFLD and TSH levels; on the other hand, a higher prevalence of NAFLD in subjects with higher TSH and lastly, an increased risk of NAFLD of 1.5 times in subjects with TSH ≥ 2.5 μIU/mL independently of MetS. This last finding is of interest because it shows a higher risk of NAFLD in patients with TSH levels that are within normal parameters.
Although there is still some controversy in the relationship between TH and NAFLD, it is plausible to think that both entities are linked, either by a direct effect of TH or by an effect mediated by the components of MetS. A low thyroid function is associated with increases in cholesterol and TG levels, and with greater weight gain, which in turn are risk factors for the development of NAFLD [41]. We have demonstrated these same findings in our study. At the pathophysiological level, THs participate in intrahepatic lipolysis, through the activation of autophagy, and the beta oxidation of fatty acids [42], but when there is a low thyroid function, the activity of hepatic lipases decreases which entails accumulation of TG in hepatocytes [43]. Furthermore, it has been shown that, in hypothyroid subjects, adipocytokine levels are altered [44], which can contribute to liver inflammation processes. THs have also been linked to the regulation of micro-RNAs [45] and to some genetic polymorphisms related to NAFLD. For example, a recent study has shown that there is a significant association between euthyroid subjects with high-normal TSH (2.5 to 5.3 μIU/mL) and NASH, when they carry the risk of allele of PNPLA3 G [46].
THs have been correlated with moderate elevations in transaminase levels. In an observational study in our setting, where 10,116 subjects were included, it was found that the alteration in AST levels affected subjects with TSH ≥ 10 µIU/mL more frequently and significantly; it was also more prevalent in subjects with low T4 levels [41]. Along the same lines, Chung et al. demonstrated the association between alteration of ALT > 33/25 (men/women) and thyroid function, affecting 20.1% of subjects with subclinical hypothyroidism and 25.9% in clinical hypothyroidism [10]. Furthermore, ALT levels have also been linked to MetS [47]. Similarly, our study found a 1.32 times increased risk of having hypertransaminasemia in subjects with TSH ≥ 2.5 μIU/mL, regardless of MetS parameters. Although hypertransaminasemia is not a good predictive marker of NAFLD, since there may be steatosis with normal liver function, we believe that the relationship found between TH and hypertransaminasemia is due to the association with NAFLD.
Moreover, the role of TH in liver fibrosis has also been discussed. At the pathophysiological level, it is speculated that TH may be involved in the activation of stellate liver cells, a crucial step in the development of liver fibrosis [48]. In the same way as the studies that relate thyroid function and NAFLD, in this case, the methods used for the diagnosis of liver fibrosis are also different. While some authors use serological markers [34,49], others use TE [50] or liver biopsy [51]. In our case, we used TE, which has a high sensitivity and specificity and is the usual screening method in our setting. The findings that we encountered are similar to those present in the literature. Bano et al. found that higher TSH levels were associated with a 1.49 times increased risk of liver fibrosis, using TE values ≥ 8.0 kPa as the cut-off point [50]. Kim et al. also found a relationship between low thyroid function and liver fibrosis, using serological markers [49]. In another recent study, low-normal thyroid function was associated with TE values ≥ 8.0 kPa and ≥9.2 kPa, in euthyroid subjects, dependent on other metabolic factors [52]. Along the same lines, in a recently published meta-analysis, hypothyroidism, both sub-clinical and overt, was associated with an increased risk of liver fibrosis with OR > 2 [53]. In our study, we found an independent relationship of MetS parameters between subjects with TSH ≥ 2.5 μIU/mL and liver fibrosis. Still, these findings need to be confirmed in prospective studies.
Based on this evidence, in recent years it has been proposed to consider TH or the thyroid hormone receptor (THR) as therapeutic targets for NAFLD. In a population study with dyslipidemia and sub-clinical hypothyroidism, the use of low doses of levothyroxine decreased the prevalence of NAFLD [54]. In another, a 12% reduction in intrahepatic lipids was demonstrated in subjects with NAFLD and T2DM when using levothyroxine [55]. Treatments with agonists of THR-β, which is the main receptor for TH in the liver, have also been tried to reduce cholesterol levels [56] or for the treatment of non-alcoholic steatohepatitis [57]. Even so, no treatment has yet been approved for NAFLD and non-pharmacological measures aimed at controlling metabolic factors continue to be the gold standard in the treatment of these patients.
Finally, this study has some limitations. The gold standard method for the diagnosis of NAFLD and liver fibrosis is liver biopsy, but as it is an invasive test, it is not performed in routine clinical practice [4]. In this specific case, validated serological markers have been used for the diagnosis of NAFLD and liver stiffness measurements using transient liver elastography for the diagnosis of fibrosis. Furthermore, the recommended probe to measure liver stiffness in obese subjects is the XL, but in our study all the examinations were performed with the M probe as it was the only probe available. Due to the design of the reference cohort used, we do not know the percentage of subjects who were being treated with hormone replacement therapy, and we do not have information on T4 or T3 levels, the presence of antithyroid antibodies or the previous diagnosis of thyroid cancer. Finally, the cross-sectional design of this study does not allow for determining causal relationships.
5. Conclusions
In conclusion, the findings of this study demonstrate that TSH levels ≥ 2.5 μIU/mL are associated with a higher risk of NAFLD and liver fibrosis in the general population, independently of the MetS parameters. Furthermore, individuals with TSH ≥ 10 μIU/mL have an additional increased risk of NAFLD. Although more prospective design studies are required, we propose the need for stricter control of TSH levels in those subjects with coexisting metabolic risk factors for developing NAFLD and liver fibrosis.
Author Contributions
Conceptualization, A.M.-E. and L.C.; methodology, A.M.-E., G.P. and L.C.; validation, A.M.-E., G.P., P.T.-M. and L.C.; investigation, A.M.-E., L.R., I.A. and C.E.-M.; supervision, A.M.-E., L.R., I.A. and C.E.-M.; formal analysis and data curation, G.P. and A.C.-G.; writing—original draft preparation, A.M.-E.; writing—review and editing, A.M.-E. and L.C.; funding acquisition, P.T.-M. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the project “High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study” and obtained funding from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded on the 2011 call under the Health Strategy Action 2013–2016, within the National Research Program oriented to Societal Challenges, with reference PI11/0267 (PI Llorenç Caballería). Alba Martínez-Escudé received a predoctoral and research grant from IDIAP Jordi Gol 2017 and 2020.
Institutional Review Board Statement
The protocol was approved by the Ethics Committee of IDIAP Jordi Gol Institute with reference P11/58. The study was conducted according to the guidelines of the Declaration of Helsinki and the data were treated according to the state data protection law.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. S1), 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; the American Association for the Study of Liver Diseases NASH Task Force. NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 2021, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Masarone, M.; Dallio, M.; Federico, A.; Aglitti, A.; Persico, M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 3415. [Google Scholar] [CrossRef] [Green Version]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 5927. [Google Scholar] [CrossRef]
- Pagadala, M.R.; Zein, C.O.; Dasarathy, S.; Yerian, L.M.; Lopez, R.; McCullough, A.J. Prevalence of Hypothyroidism in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2012, 57, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.-H.; Lee, H.-S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, X.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, T.; Wang, X.; et al. High-Normal Thyroid Function Predicts Incident Nonalcoholic Fatty Liver Disease Among Middle-Aged and Older Euthyroid Subjects. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, glab037. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Dai, Y.; Qin, J. Serum Thyroid Hormones Levels are Significantly Associated with Nonalcoholic Fatty Liver Disease in Euthyroid Chinese Population. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Eshraghian, A.; Dabbaghmanesh, M.H.; Eshraghian, H.; Fattahi, M.R.; Omrani, G.R. Nonalcoholic fatty liver disease in a cluster of Iranian population: Thyroid status and metabolic risk factors. Arch. Iran. Med. 2013, 16, 584–589. [Google Scholar]
- Lee, K.W.; Bang, K.B.; Rhee, E.J.; Kwon, H.J.; Lee, M.Y.; Cho, Y.K. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin. Mol. Hepatol. 2015, 21, 372–378. [Google Scholar] [CrossRef]
- Caballería, L.; Pera, G.; Arteaga, I.; Rodríguez, L.; Alumà, A.; Morillas, R.M.; de la Ossa, N.; Díaz, A.; Expósito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e5. [Google Scholar] [CrossRef] [Green Version]
- Ascaso, J.F.; Millán, J.; Hernández-Mijares, A.; Blasco, M.; Brea, Á.; Díaz, Á.; Pedro-Botet, J.; Pintó, X. Dislipidemia aterogénica 2019. Consensus document of the Atherogenic Dyslipidaemia Group of the Spanish Arteriosclerosis Society. Clín. Investig. Arterioscler. 2020, 32, 120–125. [Google Scholar] [CrossRef]
- Julián, M.T.; Pera, G.; Soldevila, B.; Caballería, L.; Julve, J.; Puig-Jové, C.; Morillas, R.; Torán, P.; Expósito, C.; Puig-Domingo, M.; et al. Atherogenic dyslipidemia, but not hyperglycemia, is an independent factor associated with liver fibrosis in subjects with type 2 diabetes and NAFLD: A population-based study. Eur. J. Endocrinol. 2021, 184, 587–596. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP); Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [Green Version]
- Berg, E.H.V.D.; van Tienhoven-Wind, L.J.; Amini, M.; Schreuder, T.C.; Faber, K.N.; Blokzijl, H.; Dullaart, R.P. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: The Lifelines Cohort Study. Metabolism 2017, 67, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carulli, L.; Ballestri, S.; Lonardo, A.; Lami, F.; Violi, E.; Losi, L.; Bonilauri, L.; Verrone, A.M.; Odoardi, M.R.; Scaglioni, F.; et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern. Emerg. Med. 2011, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Klose, M. Clinical Strategies in the Testing of Thyroid Function. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285558/ (accessed on 20 November 2020).
- Baloch, Z.; Carayon, P.; Conte-Devolx, B.; Demers, L.M.; Feldt-Rasmussen, U.; Henry, J.-F.; LiVosli, A.V.; Niccoli-Sire, P.; John, R.; Ruf, J.; et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003, 13, 3–126. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Phadke, A.; Sawant, P. Prevalence of hypothyroidism in nonalcoholic fatty liver disease in patients attending a tertiary hospital in western India. Indian J. Gastroenterol. 2015, 34, 169–173. [Google Scholar] [CrossRef]
- Loosen, S.H.; Demir, M.; Kostev, K.; Luedde, T.; Roderburg, C. Incidences of hypothyroidism and autoimmune thyroiditis are increased in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Tahara, K.; Akahane, T.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kaji, K.; Takaya, H.; Sawada, Y.; Shimozato, N.; Sato, S.; et al. Thyroid-stimulating hormone is an independent risk factor of non-alcoholic fatty liver disease. JGH Open 2019, 4, 400–404. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.; Jo, K.; Lim, D.-J.; Lee, J.-M.; Chang, S.-A.; Kang, M.-I.; Cha, B.-Y.; Kim, M.-H. Male-specific association between subclinical hypothyroidism and the risk of non-alcoholic fatty liver disease estimated by hepatic steatosis index: Korea National Health and Nutrition Examination Survey 2013 to 2015. Sci. Rep. 2018, 8, 15145. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Gu, H.; Wu, J.; Sui, J. Thyroid function is associated with non-alcoholic fatty liver disease in euthyroid subjects. Endocr. Res. 2015, 40, 74–78. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, X.; Guan, L.; Jiang, Z.; Lin, H.; Jiang, Q.; Zhang, N.; Zhang, Y.; Zhang, X.; Yu, C.; et al. Free triiodothyronine levels are positively associated with non-alcoholic fatty liver disease in euthyroid middle-aged subjects. Endocr. Res. 2014, 40, 188–193. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.; Mi, Y.; Liu, Y.; Liu, Y.; Zhang, P. Thyroid-stimulating hormone is associated with nonalcoholic steatohepatitis in patients with chronic hepatitis B. Medicine 2019, 98, e17945. [Google Scholar] [CrossRef]
- Grewal, H.; Joshi, S.; Sharma, R.; Mittal, P.; Goel, A. Non-alcoholic fatty liver disease in patients with hypothyroidism presenting at a rural tertiary care centre in north India. Trop. Doct. 2021, 51, 181–184. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Cesena, F.H.; Valente, V.A.T.; Conceição, R.D.D.O.; Santos, R.D.; Bittencourt, M.S. Association between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent from Metabolic Syndrome Criteria. Eur. Thyroid. J. 2018, 7, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. Thyroid Function and the Risk of Non-Alcoholic Fatty Liver Disease in Morbid Obesity. Front. Endocrinol. 2020, 11, 572128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, Q.; Lv, H.; Li, Q.; Ma, Y.; Tan, J.; Liu, C. Elevated TPOAb is a Strong Predictor of Autoimmune Development in Patients of Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Case–Control Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4369–4378. [Google Scholar] [CrossRef]
- Huang, B.; Yang, S.; Ye, S. Association between Thyroid Function and Nonalcoholic Fatty Liver Disease in Euthyroid Type 2 Diabetes Patients. J. Diabetes Res. 2020, 2020, 6538208. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.-A. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid. J. 2017, 6, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Martínez Escudé, A.; Pera, G.; Arteaga, I.; Expósito, C.; Rodríguez, L.; Torán, P.; Caballeria, L. Relationship between hypothyroidism and non-alcoholic fatty liver disease in the Spanish population. Med. Clín. 2020, 154, 1–6. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.-H.; Zhou, J.; Siddique, M.M.; Bay, B.-H.; Zhu, X.; Privalsky, M.L.; Cheng, S.-Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, C.; Claudel, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014, 25, 576–585. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Sinha, R.A.; Yen, P.M. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. Int. J. Mol. Sci. 2018, 19, 3284. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.-S.; Zhu, S.-H.; Liu, W.-Y.; Pan, X.-Y.; Zhu, P.-W.; Li, Y.-Y.; Zheng, I.K.; Ma, H.-L.; You, J.; Targher, G.; et al. PNPLA3 polymorphism influences the association between high-normal TSH level and NASH in euthyroid adults with biopsy-proven NAFLD. Diabetes Metab. 2020, 46, 496–503. [Google Scholar] [CrossRef]
- Dullaart, R.P.; Berg, E.H.V.D.; Van Der Klauw, M.M.; Blokzijl, H. Low normal thyroid function attenuates serum alanine aminotransferase elevations in the context of metabolic syndrome and insulin resistance in white people. Clin. Biochem. 2014, 47, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Gionfra, F.; De Vito, P.; Pallottini, V.; Lin, H.-Y.; Davis, P.J.; Pedersen, J.Z.; Incerpi, S. The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer. Front. Endocrinol. 2019, 10, 532. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Yoo, E.R.; Li, A.; Fernandes, C.; Tighe, S.; Cholankeril, G.; Hameed, B.; Ahmed, A. Low-Normal Thyroid Function Is Associated With Advanced Fibrosis Among Adults in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2379–2381. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Plompen, E.P.C.; Hofman, A.; Dehghan, A.; Franco, O.H.; Janssen, H.L.A.; Murad, S.D.; Peeters, R.P. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2016, 101, 3204–3211. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, R.; Campi, I.; Maggioni, M.; Perbellini, R.; Giammona, E.; Stucchi, R.; Borghi, M.; Degasperi, E.; De Silvestri, A.; Persani, L.; et al. The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD). PLoS ONE 2021, 16, e0249614. [Google Scholar] [CrossRef]
- Martínez-Escudé, A.; Pera, G.; Rodríguez, L.; Arteaga, I.; Expósito-Martínez, C.; Torán-Monserrat, P.; Caballería, L. Risk of Liver Fibrosis According to TSH Levels in Euthyroid Subjects. J. Clin. Med. 2021, 10, 1350. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Muka, T.; Mattace-Raso, F.U.S.; Bally, L.; Franco, O.H.; Peeters, R.P.; Razvi, S. Thyroid Function and the Risk of Fibrosis of the Liver, Heart, and Lung in Humans: A Systematic Review and Meta-Analysis. Thyroid 2020, 30, 806–820. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Zhao, M.; Zheng, D.; Zhang, X.; Guan, Q.; Xu, C.; Gao, L.; Zhao, J.; Zhang, H. Benefits of Levothyroxine Replacement Therapy on Nonalcoholic Fatty Liver Disease in Subclinical Hypothyroidism Patients. Int. J. Endocrinol. 2017, 2017, 5753039. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.-A.; Leow, M.K.S.; et al. Low-Dose Levothyroxine Reduces Intrahepatic Lipid Content in Patients With Type 2 Diabetes Mellitus and NAFLD. J. Clin. Endocrinol. Metab. 2018, 103, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Ladenson, P.W.; Kristensen, J.D.; Ridgway, E.C.; Olsson, A.G.; Carlsson, B.; Klein, I.; Baxter, J.D.; Angelin, B. Use of the Thyroid Hormone Analogue Eprotirome in Statin-Treated Dyslipidemia. N. Engl. J. Med. 2010, 362, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Harrison, A.S.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, A.B.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).