Effectiveness of Acceptance and Commitment Therapy in Central Pain Sensitization Syndromes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
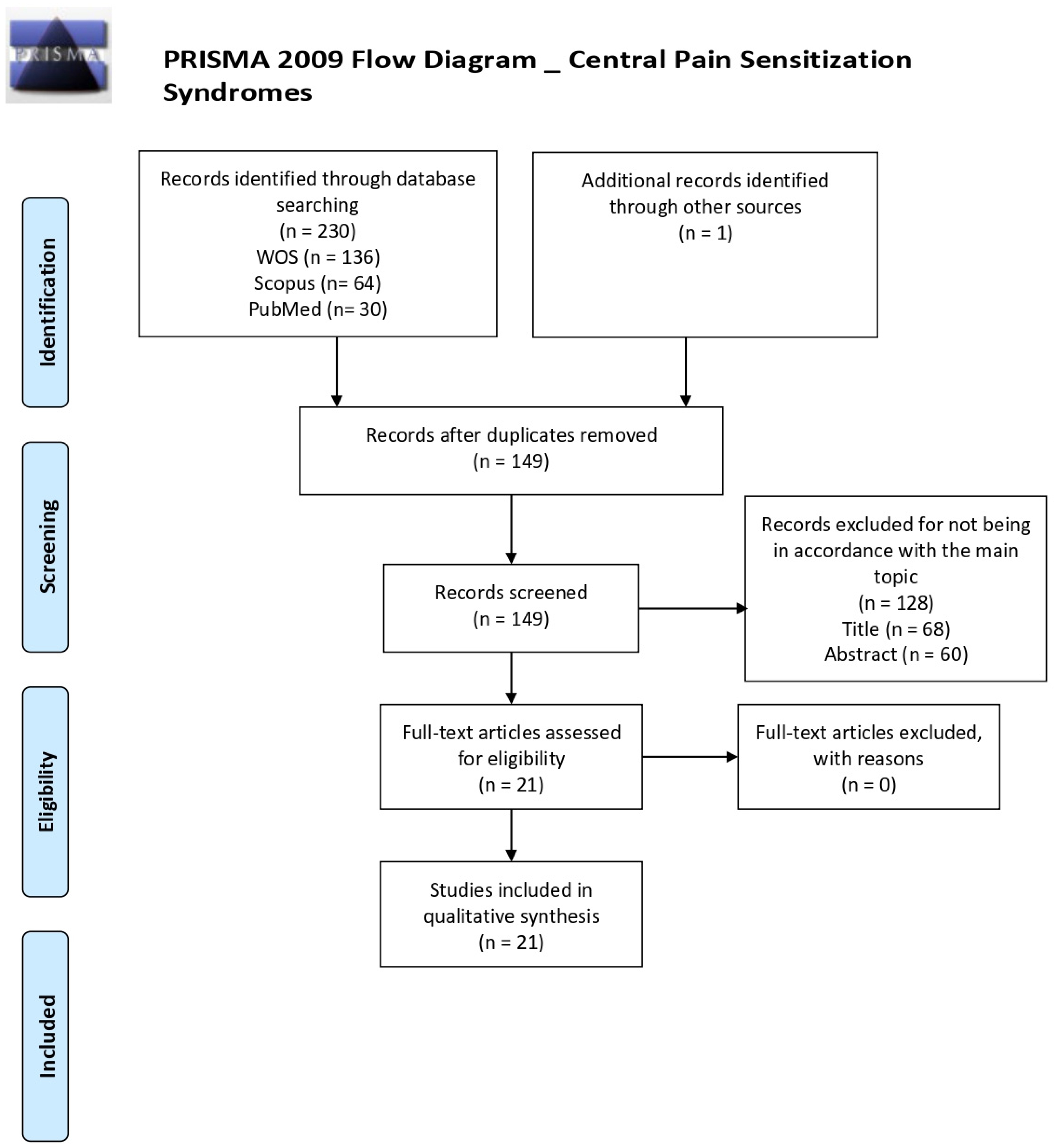
3. Results
3.1. Literature Search and Study Characteristics
3.2. Participants
3.3. Effectiveness of Acceptance and Commitment Therapy in the Treatment of Central Pain Sensitization Syndromes
3.3.1. Fibromyalgia Syndrome
3.3.2. Irritable Bowel Syndrome
3.3.3. Migraine
3.3.4. Risk of Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Acceptance and Commitment Therapy. |
| CNS | Central Nervous System |
| CPSS | Central Pain Sensitization Syndrome. |
| CTTH | Chronic tension-type headache. |
| FMS | Fibromyalgia Syndrome. |
| HRQoL | Health Related Quality of Life. |
| IBS | Irritable Bowel Syndrome. |
| IC | Interstitial Cystitis. |
| ICHD-3 beta | International Classification of Headache Disorders, third edition (beta version). |
| TMD | Temporomandibular disorder. |
| TTH | Tension-Type Headache. |
| USA | United States of America. |
References
- Woolf, C.J.; Salter, M.W. Neuronal plasticity: Increasing the gain in pain. Science 2000, 288, 1765–1768. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheumatol. 2002, 46, 1333–1343. [Google Scholar] [CrossRef]
- Montoya, P.; Sitges, C.; García-Herrera, M.; Rodríguez-Cotes, A.; Izquierdo, R.; Truyols, M.; Collado, D. Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis Rheumatol. 2006, 54, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Staud, R. Neurobiology of fibromyalgia syndrome. J. Rheumatol. Suppl. 2005, 75, 22–28. [Google Scholar] [PubMed]
- Gebhart, G.F. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004, 27, 729–737. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Julien, N.; Goffaux, P.; Arsenault, P.; Marchand, S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005, 11, 295–302. [Google Scholar] [CrossRef]
- Yunus, M.B. Central sensitivity syndromes: An overview. J. Musculoskelet. Pain 2009, 17, 400–408. [Google Scholar] [CrossRef]
- Yunus, M.B. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin. Arthritis Rheumatol. 2007, 36, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. Editorial review (thematic issue: An update on central sensitivity syndromes and the issues of nosology and psychobiology). Curr. Rheumatol. Rev. 2015, 11, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheumatol. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. The pathogenesis of chronic pain and fatigue syndromes, with special reference to fibromyalgia. Med. Hypotheses 1995, 44, 369–378. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Pulgar, A.; Duschek, S.; Garrido, S. Cognitive impairment in fibromyalgia syndrome: The impact of cardiovascular regulation, pain, emotional disorders and medication. Eur. J. Pain 2012, 16, 421–429. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Wolfe, F.; Ross, K.; Anderson, J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheumatol. 1995, 38, 19–28. [Google Scholar] [CrossRef]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; de la Coba, P.; Duschek, S.; Reyes del Paso, G.A. Reliability, factor structure and predictive validity of the Widespread Pain Index and Symptom Severity scales of the 2010 American College of Rheumatology criteria of fibromyalgia. J. Clin. Med. 2020, 9, 2460. [Google Scholar] [CrossRef]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef]
- White, K.P.; Harth, M. Classification, epidemiology, and natural history of fibromyalgia. Curr. Pain Headache Rep. 2001, 5, 320–329. [Google Scholar] [CrossRef]
- Buskila, D. Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res. Ther. 2009, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Sundgren, P.C.; Craig, A.D.; Kirshenbaum, E.; Sen, A.; Napadow, V.; Clauw, D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheumatol. 2009, 60, 3146–3152. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Graven-Nielsen, T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr. Pain Headache Rep. 2003, 7, 355–361. [Google Scholar] [CrossRef]
- Jensen, K.B.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.R.; Choy, E.; Giesecke, T.; Mainguyd, Y.; et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009, 144, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Vierck, C.J.; Cannon, R.L.; Mauderli, A.P.; Price, D.D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001, 91, 165–175. [Google Scholar] [CrossRef]
- De la Coba, P.; Bruehl, S.; Duschek, S.; Reyes del Paso, G.A. Blood pressure-related pain modulation in fibromyalgia: Differentiating between static versus dynamic pain indicators. Int. J. Psychophysiol. 2018, 134, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J.; Pillemer, S.R.; Kalogeras, K.T.; Cash, J.M.; Michelson, D.; Mitchel, A.K.; Sternberg, E.M.; Gold, P.W.; Chrousos, G.P.; Wilder, R.L. Hypothalamic–pituitary–adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheumatol. 1994, 37, 1583–1592. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25. [Google Scholar] [CrossRef]
- Martinez-Lavin, M. Fibromyalgia: When distress becomes (un) sympathetic pain. Pain Res. Treat. 2012, 2012, 981565. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; de la Coba, P. Reduced activity, reactivity and functionality of the sympathetic nervous system in fibromyalgia: An electrodermal study. PLoS ONE 2020, 15, e0241154. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: An overview. Am. J. Med. 2009, 122, S3–S13. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Defrees, D.N.; Bailey, J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim. Care 2017, 44, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Coffin, B.; Bouhassira, D.; Sabate, J.M.; Barbe, L.; Jian, R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut 2004, 53, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Sturniolo, G.C.; Zaninotto, G.; D’Incà, R.; Polo, R.; Naccarato, R.; Ancona, E. Altered esophageal pain threshold in irritable bowel syndrome. Dig. Dis. Sci. 1993, 38, 206–212. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Laganà, A.S.; Vitale, S.G.; Butticè, S.; Noventa, M.; Gizzo, S.; Valenti, G.; Rapisarda, A.M.C.; La Rosa, V.L.; Magno, C.; et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch. Gynecol. Obstet. 2017, 295, 1341–1359. [Google Scholar] [CrossRef]
- Nickel, J.C.; Tripp, D.A.; Pontari, M.; Moldwin, R.; Mayer, R.; Carr, L.K.; Doggweiler, R.; Yang, C.C.; Mishra, N.; Nordling, J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J. Urol. 2010, 184, 1358–1363. [Google Scholar] [CrossRef]
- Tony-Buffington, C.A. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J. Urol. 2004, 172, 1242–1248. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Whitmore, K.; Stanford, E.; Moldwin, R.; O’Leary, M.P. Interstitial cystitis: Bladder pain and beyond. Expert Opin. Pharmacother. 2008, 9, 2979–2994. [Google Scholar] [CrossRef]
- Stanford, E.J.; Dell, J.R.; Parsons, C.L. The emerging presence of interstitial cystitis in gynecologic patients with chronic pelvic pain. Urology 2007, 69, S53–S59. [Google Scholar] [CrossRef]
- Leppilahti, M.; Tammela, T.L.; Huhtala, H.; Auvinen, A. Prevalence of symptoms related to interstitial cystitis in women: A population based study in Finland. J. Urol. 2002, 168, 139–143. [Google Scholar] [CrossRef]
- Rosenberg, M.T.; Hazzard, M. Prevalence of interstitial cystitis symptoms in women: A population based study in the primary care office. J. Urol. 2005, 174, 2231–2234. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Gardner, V.; Ness, T.J.; Gereau, R.W. Segmental hyperalgesia to mechanical stimulus in interstitial cystitis/bladder pain syndrome: Evidence of central sensitization. J. Urol. 2014, 191, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Schmidt, M.; Radulovic, D.; Singer, A.; Katz, P.; Bresette, J. The relationship between fibromyalgia and interstitial cystitis. J. Psychiatr. Res. 1997, 31, 125–131. [Google Scholar] [CrossRef]
- Ouanounou, A.; Goldberg, M.; Haas, D.A. Pharmacotherapy in Temporomandibular Disorders: A Review. J. Can. Dent. Assoc. 2017, 83, h7. [Google Scholar] [PubMed]
- Herb, K.; Cho, S.; Stiles, M.A. Temporomandibular joint pain and dysfunction. Curr. Pain Headache Rep. 2006, 10, 408–414. [Google Scholar] [CrossRef]
- McNeill, C. History and evolution of TMD concepts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 51–60. [Google Scholar] [CrossRef]
- Wright, E.F. Manual of Temporomandibular Disorders, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Fernández-de-las-Peñas, C.; Svensson, P. Myofascial Temporomandibular Disorder. Curr. Rheumatol. Rev. 2016, 12, 40–54. [Google Scholar] [CrossRef]
- Sarlani, E.; Greenspan, J. Evidence for generalized hyperalgesia in temporo-mandibular disorders patients. Pain 2003, 10, 221–226. [Google Scholar] [CrossRef]
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.B.; Scher, A.I.; Steiner, T.J.; Zwart, J.A. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headaches. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headaches. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988, 8 (Suppl. 7), 1–96. [Google Scholar]
- Yu, S.; Han, X. Update of chronic tension-type headache. Curr. Pain Headache Rep. 2015, 19, 469. [Google Scholar] [CrossRef] [PubMed]
- Lyngberg, A.C.; Rasmussen, B.K.; Jorgensen, T.; Jensen, R. Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. Eur. J. Epidemiol. 2005, 20, 243–249. [Google Scholar] [CrossRef]
- Russell, M.B.; Levi, N.; Saltyte-Benth, J.; Fenger, K. Tension-type headache in adolescents and adults: A population based study of 33,764 twins. Eur. J. Epidemiol. 2006, 21, 153–160. [Google Scholar] [CrossRef]
- Lance, J.W.; Curran, D.A. Treatment of chronic tension headache. Lancet 1964, 1, 1236–1239. [Google Scholar] [CrossRef]
- Russell, M.B.; Ostergaard, S.; Bendtsen, L.; Olesen, J. Familial occurrence of chronic tension-type headache. Cephalalgia 1999, 19, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Svensson, D.A.; Ekbom, K.; Larsson, B.; Waldenlind, E. Lifetime prevalence and characteristics of recurrent primary headaches in a population-based sample of Swedish twins. Headache 2002, 42, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri-Sani, M.; Silver, N. Headache (chronic tension-type). BMJ Clin. Evid. 2016, 5, 1205. [Google Scholar]
- International Headache Society. The international classification of headache disorders, 3rd ed. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef]
- Kelman, L. Pain characteristics of the acute migraine attack. Headache 2006, 46, 942–953. [Google Scholar] [CrossRef]
- GBD. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- GBD. Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Noseda, R.; Kainz, V.; Jakubowski, M.; Gooley, J.J.; Saper, C.B.; Digre, K.; Burstein, R. A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 2010, 13, 239–245. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Larrosa, D.; Ramón, C.; Vega, J.; Martínez-Camblor, P.; Pascual, J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.; Wilson, K.G. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change; Guilford Press: New York, NY, USA, 1999. [Google Scholar]
- Hayes, S.C.; Barnes-Holmes, D.; Roche, B. Relational Frame Theory: A Post-Skinnerian Account of Human Language and Cognition; Plenum Press: New York, NY, USA, 2001. [Google Scholar]
- Hayes, S.C.; Pistorello, J.; Levin, M.E. Acceptance and commitment therapy as a unified model of behavior change. Couns. Psychol. 2012, 40, 976–1002. [Google Scholar] [CrossRef]
- Bodenlos, J.S.; Hawes, E.S.; Burstein, K.M.; Arroyo, K.M. Association of cognitive fusion with domains of health. J. Contextual Behav. Sci. 2020, 18, 9–15. [Google Scholar] [CrossRef]
- Fledderus, M.; Bohlmeijer, E.T.; Pieterse, M.E. Does experiential avoidance mediate the effects of maladaptive coping styles on psychopathology and mental health? Behav. Modif. 2010, 34, 503–519. [Google Scholar] [CrossRef]
- Costa, J.; Pinto-Gouveia, J. The mediation effect of experiential avoidance between coping and psychopathology in chronic pain. Clin. Psychol. Psychother. 2011, 18, 34–47. [Google Scholar] [CrossRef]
- Gillanders, D.T.; Sinclair, A.K.; MacLean, M.; Jardine, K. Illness cognitions, cognitive fusion, avoidance and self-compassion as predictors of distress and quality of life in a heterogeneous sample of adults, after cancer. J. Contextual Behav. Sci. 2015, 4, 300–311. [Google Scholar] [CrossRef]
- Yu, L.; Norton, S.; McCracken, L.M. Change in “self-as-context” (“perspective-taking”) occurs in acceptance and commitment therapy for people with chronic pain and is associated with improved functioning. J. Pain 2017, 18, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Olsson, G.L.; Hayes, S.C. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain 2011, 152, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Solé, E.; Tomé-Pires, C.; de la Vega, R.; Racine, M.; Castarlenas, E.; Jensen, M.P.; Miró, J. Cognitive fusion and pain experience in young people. Clin. J. Pain 2016, 32, 602–608. [Google Scholar] [CrossRef]
- Veehof, M.M.; Oskam, M.J.; Schreurs, K.M.G.; Bohlmeijer, E.T. Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain 2011, 152, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Veehof, M.M.; Trompetter, H.R.; Bohlmeijer, E.T.; Schreurs, K.M. Acceptance-and mindfulness-based interventions for the treatment of chronic pain: A meta-analytic review. Cogn. Behav. Ther. 2016, 45, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; Wetherell, J.L.; Sorrell, J.T. Targeting acceptance, mindfulness, and values-based action in chronic pain: Findings of two preliminary trials of an outpatient group-based intervention. Cogn. Behav. Pract. 2009, 16, 49–58. [Google Scholar] [CrossRef]
- McCracken, L.M.; Vowles, K.E. Acceptance and commitment therapy and mindfulness for chronic pain: Model, process, and progress. Am. Psychol. 2014, 69, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Aytur, S.A.; Ray, K.L.; Meier, S.K. Neural mechanisms of acceptance and commitment therapy for chronic pain: A network-based fMRI approach. Front. Hum. Neurosci. 2021, 15, 587018. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.K.; Ray, K.L.; Waller, N.C.; Gendron, B.C.; Aytur, S.A.; Robin, D.A. Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain. Brain Sci. 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ferreira, N.B.; Gillanders, D.; Morris, P.G.; Eugenicos, M.P. Pilot study of acceptance and commitment therapy for irritable bowel syndrome: A preliminary analysis of treatment outcomes and processes of change. Clin. Psychol. 2018, 22, 241–250. [Google Scholar] [CrossRef]
- Gillanders, D.; Ferreira, N.B.; Angioni, E.; Carvalho, S.A.; Eugenicos, M.P. An implementation trial of ACT-based bibliotherapy for irritable bowel syndrome. J. Contextual Behav. Sci. 2017, 6, 172–177. [Google Scholar] [CrossRef]
- Gómez-Pérez, M.C.; García-Palacios, A.; Castilla, D.; Zaragozá, I.; Suso-Ribera, C. Brief Acceptance and Commitment Therapy for Fibromyalgia: Feasibility and Effectiveness of a Replicated Single-Case Design. Pain Res. Manag. 2020, 2020, 7897268. [Google Scholar] [CrossRef]
- Grazzi, L.; Bernstein, C.; Raggi, A.; Sansone, E.; Grignani, E.; Searl, M.; Rizzoli, P. ACT for migraine: Effect of acceptance and commitment therapy (ACT) for high-frequency episodic migraine without aura: Preliminary data of a phase-II, multicentric, randomized, open-label study. Neurol. Sci. 2019, 40 (Suppl. 1), 191–192. [Google Scholar] [CrossRef]
- Ljótsson, B.; Atterlöf, E.; Lagerlöf, M.; Andersson, E.; Jernelöv, S.; Hedman, E.; Kemani, M.; Wicksell, R.K. Internet-delivered acceptance and values-based exposure treatment for fibromyalgia: A pilot study. Cogn. Behav. Ther. 2014, 43, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Kosek, E.; Wicksell, R.; Kemani, M.; Olsson, G.; Merle, J.V.; Kadetoff, D.; Ingvar, M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgeia. Pain 2012, 153, 1495–1503. [Google Scholar] [CrossRef]
- Luciano, J.V.; Guallar, J.A.; Aguado, J.; López-Del-Hoyo, Y.; Olivan, B.; Magallón, R.; Alda, M.; Serrano-Blanco, A.; Gili, M.; Garcia-Campayo, J. Effectiveness of group acceptance and commitment therapy for fibromyalgia: A 6-month randomized controlled trial (EFFIGACT study). Pain 2014, 155, 693–702. [Google Scholar] [CrossRef]
- Pedersen, H.F.; Agger, J.L.; Frostholm, L.; Jensen, J.S.; Ørnbøl, E.; Fink, P.; Schröder, A. Acceptance and Commitment group Therapy for patients with multiple functional somatic syndromes: A three-armed trial comparing ACT in a brief and extended version with enhanced care. Psychol. Med. 2019, 49, 1005–1014. [Google Scholar] [CrossRef]
- Vasiliou, V.S.; Karademas, E.C.; Christou, Y.; Papacostas, S.; Karekla, M. Acceptance and Commitment Therapy for Primary Headache Sufferers: A Randomized Controlled Trial of Efficacy. J. Pain 2020, 17, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Kemani, M.; Jensen, K.; Kosek, E.; Kadetoff, D.; Sorjonen, K.; Ingvar, M.; Olsson, G.L. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Eur. J. Pain 2013, 17, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Dindo, L.; Recober, A.; Marchman, J.; O’Hara, M.W.; Turvey, C. One-day behavioral intervention in depressed migraine patients: Effects on headache. Headache 2014, 54, 528–538. [Google Scholar] [CrossRef]
- Grazzi, L.; Rizzoli, P. Acceptance and Commitment Therapy (ACT) vs Erenumab for High Frequency Episodic Migraine Without Aura: Time to Take the Gloves Off! Headache 2020, 60, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Dindo, L.; Recober, A.; Marchman, J.N.; Turvey, C.; O’Hara, M.W. One-day behavioral treatment for patients with comorbid depression and migraine: A pilot study. Behav. Res. Ther. 2012, 50, 537–543. [Google Scholar] [CrossRef]
- Dindo, L.; Recober, A.; Calarge, C.A.; Zimmerman, B.M.; Weinrib, A.; Marchman, J.N.; Turvey, C. One-Day Acceptance and Commitment Therapy Compared to Support for Depressed Migraine Patients: A Randomized Clinical Trial. Neurotherapeutics 2020, 17, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Bogusch, L.; Bigatti, S.M. Values-based action in fibromyalgia: Results from a randomized pilot of acceptance and commitment therapy. Health Psychol. Res. 2013, 1, 176–181. [Google Scholar] [CrossRef]
- Aghalar, S.; Moradi Manesh, F.; Saraj Khorami, N.; Hafezi, F. The effectiveness of acceptance-and commitment-based therapy on perception of disease in patients with irritable bowel syndrome. Int. Arch. Health Sci. 2020, 7, 137–142. [Google Scholar]
- Kamalinejad, F.; Amiri, A. The Efficacy of Acceptance and Commitment Therapy on Psychological Well-Being and Optimism of Patients with Irritable Bowel Syndrome. Int. J. Body Mind Cult. 2019, 1, 97–103. [Google Scholar]
- Mirsharifa, S.M.; Mirzaian, B.; Dousti, Y. The efficacy of Acceptance and Commitment Therapy (ACT) Matrix on depression and psychological capital of the patients with irritable bowel syndrome. Open Access Maced. J. Med. Sci. 2019, 7, 421. [Google Scholar] [CrossRef]
- Mo’tamedi, H.; Rezaiemaram, P.; Tavallaie, A. The effectiveness of a group-based acceptance and commitment additive therapy on rehabilitation of female outpatients with chronic headache: Preliminary findings reducing 3 dimensions of headache impact. Headache 2012, 52, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Simister, H.D.; Tkachuk, G.A.; Shay, B.L.; Vincent, N.; Pear, J.J.; Skrabek, R.Q. Randomized controlled trial of online acceptance and commitment therapy for fibromyalgia. J. Pain 2018, 19, 741–753. [Google Scholar] [CrossRef]
- Ito, M.; Muto, T. Effectiveness of acceptance and commitment therapy for irritable bowel syndrome non-patients: A pilot randomized waiting list controlled trial. J. Contextual Behav. Sci. 2020, 15, 85–91. [Google Scholar] [CrossRef]
- Kanter, G.; Komesu, Y.M.; Qaedan, F.; Jeppson, P.C.; Dunivan, G.C.; Cichowski, S.B.; Rogers, R.G. Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: A randomized controlled trial. IUJ 2016, 27, 1705–1711. [Google Scholar]
- McKernan, L.C.; Walsh, C.G.; Reynolds, W.S.; Crofford, L.J.; Dmochowski, R.R.; Williams, D.A. Psychosocial co-morbidities in interstitial cystitis/bladder pain syndrome (IC/BPS): A systematic review. Neurourol. Urodyn. 2018, 37, 926–941. [Google Scholar] [CrossRef]
- Haugmark, T.; Hagen, K.B.; Smedslund, G.; Zangi, H.A. Mindfulness- and acceptance-based interventions for patients with fibromyalgia–A systematic review and meta-analyses. PLoS ONE 2019, 14, e0221897. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Vetvik, K.G.; MacGregor, E.A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. The Lancet. Neurology 2017, 16, 76–87. [Google Scholar]
- Zarcone, D.; Corbetta, S. Shared mechanisms of epilepsy, migraine and affective disorders. Neurol. Sci. 2017, 38 (Suppl. 1), 73–76. [Google Scholar] [CrossRef] [PubMed]
- IsHak, W.W.; Wen, R.Y.; Naghdechi, L.; Vanle, B.; Dang, J.; Knosp, M.; Dascal, J.; Marcia, L.; Gohar, Y.; Eskander, L.; et al. Pain and Depression: A Systematic Review. Harv. Rev. Psychiatry 2018, 26, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Yalinay Dikmen, P.; Yavuz, B.G.; Aydinlar, E.I. The relationships between migraine, depression, anxiety, stress, and sleep disturbances. Acta Neurol. Belg. 2015, 115, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, H.; Heath, A.C.; Madden, P.A.; Martin, N.G.; Nyholt, D.R. Shared Genetic Factors Underlie Migraine and Depression. Twin Res. Hum. Genet. 2016, 19, 341–350. [Google Scholar] [CrossRef]
- Breslau, N.; Lipton, R.B.; Stewart, W.F.; Schultz, L.R.; Welch, K.M. Comorbidity of migraine and depression: Investigating potential etiology and prognosis. Neurology 2003, 60, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.M.; Tepper, S.J.; Robbins, L.D.; Manack Adams, A.; Buse, D.C.; Orejudos, A.; Silberstein, S. Effects of onabotulinumtoxin. A treatment for chronic migraine on common comorbidities including depression and anxiety. J. Neurol. Neurosurg. Psychiatry 2019, 90, 353–360. [Google Scholar] [CrossRef]
- Inadomi, J.M.; Fennerty, M.B.; Bjorkman, D. Systematic review: The economic impact of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 18, 671–682. [Google Scholar] [CrossRef]
- Mearin, F.; Caballero, A.M.; Serra, J.; Brotons, C.; Tantiñà, A.; Fort, E.; Martínez-Cerezo, F.J.; Perelló, A.; Sánchez-Antolín, G.; Rey, E.; et al. A retrospective and prospective 12-month observational study of the socioeconomic burden of moderate to severe irritable bowel syndrome with constipation in Spain. Gastroenterol. Hepatol. 2019, 42, 141–149. [Google Scholar] [CrossRef]
- Poulsen, C.H.; Eplov, L.F.; Hjorthøj, C.; Hastrup, L.H.; Eliasen, M.; Dantoft, T.M.; Schröder, A.; Jørgensen, T. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: A long-term population-based study. Scand. J. Public Health 2019, 47, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, N.; Harnett, J.; Melkonian, A.; Lynch, W.; Kaplan-Machlis, B.; Silverman, S.L. Burden of fibromyalgia and comparisons with osteoarthritis in the workforce. J. Occup. Environ. Med. 2009, 51, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, A.; Bourgault, P.; Choinière, M. Fibromyalgia-related costs and loss of productivity: A substantial societal burden. BMC Musculoskelet. Disord. 2016, 17, 168. [Google Scholar] [CrossRef]
- Skaer, T.L. Fibromyalgia: Disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics 2014, 32, 457–466. [Google Scholar] [CrossRef]
- Linde, M.; Steiner, T.J.; Chisholm, D. Cost-effectiveness analysis of interventions for migraine in four low- and middle-income countries. J. Headache Pain 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Manack, A.N.; Buse, D.C.; Lipton, R.B. Chronic migraine: Epidemiology and disease burden. Curr. Pain Headache Rep. 2011, 15, 70–78. [Google Scholar] [CrossRef] [PubMed]
| Fibromyalgia Syndrome | ||||
|---|---|---|---|---|
| First Author (Publication Year), Study Name, Country | Study Design/ Diagnostic Technique | Sample Size, Age (Mean ± SD) | Period and Treatment Characteristics | Variables and Results |
| Jensen et al., 2012. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Sweden. | Randomized controlled clinical trial with follow-up. A previous diagnosis of FMS by primary care physicians. according to the 1990 ACR diagnostic criteria. | N = 43 women with FMS. ACT group = 25 (44.50 ± 1.50). WL group = 18 (46.90 ± 1.10). | Twelve weekly ACT sessions in groups of six patients. Improvement of functioning and life satisfaction by increasing the participants’ ability to behave in accordance with their values in the presence of interference (pain and distress). Follow-up: 3-month assessment. | Primary Outcomes: Anxiety: STAI */** Depression: BDI */** Event-related potentials (ERP)-P50 Functional magnetic resonance imaging (fMRI) during pressure-evoked pain: - Insula * - Cerebellum * - Thalamus and caudate * - Hippocampus * Pain intensity: 0-100 visual analog scale *; pressure pain thresholds |
| Steiner et al., 2013. Values-based action in fibromyalgia: results from a randomized pilot of acceptance and commitment therapy. USA. | Randomized controlled trial (pilot study). A diagnosis of FMS by a physician. | N = 28 women with FMS. ACT group = 18 (47.82 ± 12.91). Psycho-education group = 10 (50.00 ± 13.62). | Eight weekly sessions of ACT intervention based on the manual “Living Beyond Your Pain: Using Acceptance and Commitment Therapy to Ease Chronic Pain”. Follow-up: 12-week assessment. | Secondary Outcomes: Values: CVPI: - (1) Family */** (d = 0.75/0.81) - (2) Intimate relationships */** (d = 0.64/0.53) - (3) Friends - (4) Health - (5) Work * (d = 0.64) - (6) Personal growth and learning |
| Wicksell et al., 2013. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Sweden. | Randomized controlled clinical trial with follow-up. A previous diagnosis of FMS by primary care physicians according to the 1990 ACR diagnostic criteria + a weekly self-reported average pain intensity of >40 on a visual analogue scale (0–100). | N = 40 women with FMS (45.10 ± 6.60). ACT group = 23. WL group = 17. | Twelve weekly 90-min group ACT sessions with six participants per group. ACT intervention was organized into four phases: (1) preparing for behavioral change. (2) shifting perspective. (3) values-oriented behavior activation. (4) acceptance and cognitive diffusion. Follow-up: 3-month assessment. | Primary Outcomes: Anxiety: STAI: */** - State anxiety */** (d = 0.51/0.55) - Trait anxiety */** (d = 0.73/0.74) Depression: BDI */**. (d = 0.44/0.64) Impact of FMS: FIQ */**: (d = 0.41/0.66) Quality of life: SF-36 */**: - Mental quality of life */** (d = 0.84/1.06) - Physical quality of life */** (d = 0.19/0,28) Pain disability: PDI */**. (d = 0.75/0.73) Pain intensity: 0-100 pain numeric rating scale */**. (d = 0.38/0.82) Secondary Outcomes: Psychological inflexibility: PIPS. */** (d = 1.06/0.72) Self-efficacy: SES */**. (d = 0.74/0.38) |
| Ljótsson et al., 2014. Internet-Delivered Acceptance and Values-Based Exposure Treatment for Fibromyalgia: A Pilot Study. Sweden. | Uncontrolled trial (pilot study) with follow-up. A diagnosis of FMS confirmed by a physician. | N = 41 women with FMS (52.00 ± 9.00). | Ten weekly online treatment included acceptance, mindfulness, work on life values, and systematic exposure to FMS symptoms and FMS-related situations + regular contact with an assigned online therapist. Follow-up: 6-month assessment. | Primary Outcomes: Anxiety: HADS */**. (d = 0.75/0.90) Depression: HADS */**. (d = 0.80/1.03) Pain disability: PDI. */** (d = 0.82/0.87) Fatigue: FSS. */** (d = 0.75/0.62) Impact of FMS: FIQ */**: - General */** (d = 0.71/0.96) - Pain */** (d = 0.62 / 1.22) Quality of life: SF-12 */**: - Mental quality of life */** (d = 0.63/0.86) - Physical quality of life */** (d = 0.85/0.68) Secondary Outcomes: Psychological Inflexibility: PIPS */**. (d = 1.56/2.01) |
| Luciano et al., 2014. Effectiveness of group acceptance and commitment therapy for fibromyalgia: A 6-month randomized controlled trial (EFFIGACT study). Spain. | Randomized controlled clinical trial with follow-up. Self-rated fulfillment of the ACR 1990 criteria for FMS at a screening visit to a primary health care center. | N = 156 women with FMS. Final sample = 136. ACT group = 51 ACT (48.88 ± 5.94) Final sample = 45. Pharmacologic group = 52 (47.77 ± 5.87) Final sample = 44. WL group = 53 WL (48.28 ± 5.71) Final sample = 47. | Eight weekly sessions with exercises based on ACT and mindfulness practice. Follow-up: 6-month assessment. | Primary Outcomes: Anxiety: HADS */**. (d = 0.36/0.39) Catastrophizing: PCS */**. (d = 0.76/0.69) Clinical pain: visual analog scale. */** (d = 0.62/0.47) Depression: HADS. */** (d = 0.43/0.37) Impact of FMS: FIQ */**. (d = 1.43/1.43) Quality of life: EQ-5D */**. (d = 0.85/0.66) Secondary Outcomes: Acceptance of chronic pain: CPAQ */**. (d = 1.05/1.01) Effect sizes for the comparisons between ACT and pharmacologic group (the differences between ACT and WL were even larger). |
| Pedersen et al., 2018. Acceptance and Commitment Group Therapy for patients with multiple functional somatic syndromes: a three-armed trial comparing ACT in a brief and extended version with enhanced care. Denmark. † | Randomized controlled clinical trial. Diagnosis made by a physician using the Bodily Distress Syndrome (BDS) checklist. The diagnosis was established by a medical doctor after a thorough physical and psychological Assessment, including the SCAN diagnostic interview. | N = 180 patients with CPSS. Final sample = 139: Sample of patients suffering from one or more central sensitization syndromes (>70% FMS; >50% tension headache; >35% IBS). Extended ACT group = 59 (38.80 ± 8.00) [80% women]. Final sample = 44. Brief ACT group = 61 (38.70 ± 8.60) [87% women]. Final sample = 49. Enhanced care group = 60 (40.10 ± 8.50) [87% women]. Final sample = 46. | Extended ACT: Nine weekly 3-h ACT sessions during a 3-month period led by two therapists. Treatment based on hexaflex model. Brief ACT: A workshop involving up to 15 patients providing information about illness and an introduction to ACT concepts through psycho-education, experiential exercises and group discussions. Enhanced care: A 1–1.5-h session/consultation for enhancing the patient’s understanding of their symptoms and diagnosis, and to optimize the treatment initiatives. Follow-up: 6-, 14- and 20-month assessments. | Clinical improvement: CGI * Disability: WHODAS 2.0. Distress: SCL-92: - Anxiety. - Depression. Illness worry: 7-item Whiteley checklist. Quality of life: SF-36: - Mental quality of life. - Physical quality of life. |
| Simister et al., 2018. Randomized Controlled Trial of Online Acceptance and Commitment Therapy for Fibromyalgia. Canada. | Randomized controlled clinical trial with follow-up. A diagnosis of FMS by a medical professional according to the 1990 ACR diagnostic criteria for FMS. | N = 67 FMS patients (39.70 ± 9.36) [95% women]. ACT + TAU group = 34. TAU group = 33. | 2-month online ACT protocol on a virtual platform. Seven treatment modules including a written unit with 5-8 pages on metaphors, experiential exercises, and introductory and recurring vignettes describing typical FMS experiences, along with videos and experiential homework tasks. * TAU = analgesics and other treatments like physiotherapy or physical exercise. Follow-up: 3-month assessment. | Primary Outcomes: Aerobic capacity: 6-min walk test. Catastrophizing: PCS.Clinical pain: SFMPQ. */** (d = 0.84/0.11) Depression: CES-D */**. (d = 0.87/0.56) Impact of FMS: FIQ */**. (d = 1.26/1.59) Kinesiophobia: TSK-11 */**. (d = 0.95/0.64) Physical exercise tolerance: 1-min sit-to-stand test. Sleep: PSQI. (d = 0.79/0.53) Secondary Outcomes: Acceptance of chronic pain: CPAQ */**. (d = 0.84/0.80) Cognitive fusion: CFQ */**. (d = 0.51/0.55) Mindfulness: FFMQ. Valued living: VLQ */**. (d = 0.51/0.46) |
| Gómez-Pérez et al., 2020. Brief Acceptance and Commitment Therapy for Fibromyalgia: Feasibility and Effectiveness of a Replicated Single-Case Design. Spain. | Quasi-experimental, replicated single-case/small group design. A previous diagnosis of FMS by a rheumatologist. | N = 7 women with FMS. Group ACT intervention = 4 (59.75 ± 7.27). Individual ACT intervention = 3 (65.00 ± 2.65). | Five weekly ACT sessions (a brief treatment protocol created by the group LabPsiTec): -1-h session of individual therapy. -1.5-h group session. Follow-up: None. | Primary Outcomes: Pain monitoring app: - Interference with sleep - Social activities - Fatigue - Sadness - Pain intensity |
| Irritable Bowel Syndrome | ||||
| Gillanders et al., 2017. An implementation trial of ACT-based bibliotherapy for irritable bowel syndrome. United Kingdom. | Uncontrolled pre/post-test study. Participants were diagnosed using the ROME III criteria for IBS by a consultant gastroenterologist. | N = 24 IBS patients (49.30 ± 14.90) [women = 19]. Final sample = 21. | A self-help book, “Better Living with IBS” (Ferreira & Gillanders, 2012), and the accompanying audio exercises on CD Follow-up: 2- and 6-month assessments. | Avoidance behaviors: BRQ. Diagnostic criteria for IBS: ROME III criteria **. (not estimated effect size) Quality of life: SF36. Symptom severity: SSS */** (ηp2 = 0.09/d = 0.33) Pain acceptance: AAQ-II: * (ηp2 = 0.08) - Activity engagement. - Willingness * (ηp2 = 0.14) Visceral sensitivity: VSI *. (ηp2 = 0.07) |
| Ferreira et al., 2018. Pilot study of acceptance and commitment therapy for irritable bowel syndrome: A preliminary analysis of treatment outcomes and processes of change. United Kingdom. | Uncontrolled pre/post-test study. Participants were diagnosed using the ROME III criteria for IBS by a consultant gastroenterologist. | N = 56 IBS patients (47.60 ± 13.00) [women = 52]. Final sample = 40. | One-day workshop about IBS (6 h) + a self-help book, “Better Living with IBS” (Ferreira & Gillanders, 2012), and the accompanying exercises in audio format. Follow-up: 6-month assessment. | Avoidance behaviors: BRQ */**. (d = 0.32/0.39) Quality of life: SF36 */** (d = 0.41/0.55) Symptoms severity: SSS */** (d = 0.41/0.47) Pain acceptance: AAQ-II */** (d = 0.32/0.50) Visceral sensitivity: VSI */**. (d = 0.76/1.10) |
| Kamalinejad et al., 2019. The Efficacy of Acceptance and Commitment Therapy on Psychological Well-Being and Optimism of Patients with Irritable Bowel Syndrome. Iran. | Controlled pre/post-test study. IBS patients referred from health centers in Tehran (Iran). | N= 60 IBS patients. ACT group = 30. Non-intervention group = 30. | Nine 90-min sessions of ACT therapy aimed at improving psychological well-being and optimism. Control group received no intervention. Follow-up: one assessment, timing not specified. | Optimism: LOT */**. Pessimism: LOT */**. Psychological well-being: RSPWB.*/** - Positive relationships */** - Autonomy */** - Environmental domination */** - Personal growth */** - Purposefulness in life */** - Admission */** |
| Mirsharifa et al., 2019. The Efficacy of Acceptance and Commitment Therapy (ACT) Matrix on Depression and Psychological Capital of the Patients with Irritable Bowel Syndrome. Iran. | Controlled pre/post-test study. Diagnosis of irritable bowel syndrome by a gastroenterology specialist. | N = 30 IBS patients between 19–60 years old (31.93) [19 women]. ACT group = 15. Non-intervention group = 15. | Six weekly 90-min ACT sessions based on the six principles of psychological flexibility. Two main elements comprised the intervention: (1) Reality vs. mental experience. (2) Behavior in line with values vs. behavior for escaping worries. Follow-up: none. | Depression: BDI *. (ηp2 = 0.08) Psychological capital (hope, efficacy, resilience and optimism): PCQ *. (ηp2 = 0.29) |
| Ito et al., 2020. Effectiveness of acceptance and commitment therapy for irritable bowel syndrome non-patients: A pilot randomized waiting list controlled trial. Japan. | Pilot randomized controlled trial. Japanese version of the IBS Severity Index (IBSSI). | N = 17 IBS patients [11 women]. ACT group = 9 (19.89 ± 1.36) [6 women]. WL group = 8 (19.63 ± 0.92) [5 women]. | One-day ACT program consisting of a group session and 2-month self-help program + online value adherence quizzes. Follow-up: 2-month assessment. | Anxiety: STAI. Depression: BDI **. (d = 1.10) Cognitive fusion: CFQ. Mindfulness: FFMQ. Quality of life: SF-36; IBS-QOL. Pain acceptance and related actions: AAQ-II. Symptom severity: SSS |
| Aghalar et al., 2020. The Effectiveness of Acceptance and Commitment-Based Therapy on Perception of Disease in Patients with Irritable Bowel Syndrome. Iran. | Controlled pre/post-test study. The Rome III criteria (2006) for irritable bowel syndrome were used by a gastroenterology specialist for the diagnosis. | N = 30 IBS patients. ACT group= 15 (44.80 ± 4.72). Non-intervention group = 15 (33.43 ± 7.66). | Eight weekly 90-min ACT sessions based on an adaptation of the Zatel treatment protocol. Each session had different goals, techniques, and practices. At the end of each session, patients were required to practice at home, and progress was checked at the beginning of the next session. Follow-up: none. | Illness perception: BIPQ *: - Illness sequences * (d = 0.46) - Illness duration - Personal control * (d = 0.43) - Nature of illness * (d = 0.43) - Control through treatment * (d = 0.44) - Worrying about illness * (d = 0.40) - Knowing about illness - Affective response to illness * (d = 0.63) |
| Migraine | ||||
| Mo’tamedi et al., 2012. The effectiveness of a group-based acceptance and commitment additive therapy on rehabilitation of women outpatients with chronic headache: preliminary findings reducing 3 dimensions of headache impact. Iran. | Randomized pre/post-test control group design. ICDH, 2nd edition (2004). | N= 30 women outpatients diagnosed with primary chronic (migraine and tension-type) headache. ACT group = 15 (34.18 ± 7.39). - 8 chronic tension-type patients. - 7 chronic migraines (without aura) patients. Final sample = 11. TAU group = 15 (37.87 ± 8.74): - 10 chronic tension-type patients. - 5 chronic migraine (without aura) patients. | The ACT group, in addition to TAU, completed eight weekly sessions over 2 months. On average, each session lasted 90 min. A brief ACT orientation session was completed by each participant in the ACT group. The baseline and outcome assessment periods were 2 days in duration. The TAU group was given the opportunity to speak with the therapist and other participants about the problems they experienced with medication use, as well as other psychological problems. The therapist also provided the participants with guidance on problem-solving. Follow-up: none. | Primary Outcomes: Anxiety: STAI-Trait *. (d = 2.54) Clinical pain: Short-form McGill pain questionnaire: - Sensory * (d = 0.28) - Evaluative - Miscellaneous - Affective * (d = 1.35) - Total Migraine disability: MIDAS *. (d = 0.93) |
| Dindo et al., 2012. One-day behavioral treatment for patients with comorbid depression and migraine: a pilot study. USA. | Pilot study. A diagnosis of migraine made by a physician. | N= 45 patients with comorbid depression and migraine. ACT-ED group = 31 (32.50 ± 13.30) [97% women). WL/TAU group = 14 (33.50 ± 12.90) [86% women]. | The ACT-ED group completed a 5-h workshop based on ACT and migraine education. Each ACT-ED workshop involved 5-8 patients and emphasized three topics. The education component (1 h) provided education about migraine. The ACT component (4 h) included training on acceptance and behavioral change. The WL/TAU group waited at least 12 weeks for treatment. Although no treatment was provided by the investigators during this time, the participants in the WL/TAU group completed the same clinical assessments as the ACT-ED group. Follow-up: 2, 6, and 12 weeks after the workshop. | Primary Outcomes: Depression: HRSD ** (d = 1.18); major depressive disorder criteria of SCID-IV **; IDAS ** (d = 0.87). Disability: WHO-DAS **. (d = 0.98) Headache disability: HDI **. (d = 1.03) Health related quality of life: SF-36 **. (d = 0.69) ** Significant results at the 12-weeks follow-up. |
| Dindo et al., 2014. One-day behavioral intervention in depressed migraine patients: effects on headache. USA. | Pilot study. Treatment trial. A diagnosis of migraine made by a physician. | N= 60 patients with comorbid migraine and depression. ACT-ED group = 38 (32.50 ± 12.60) [95% women] TAU group = 22 (29.60 ± 11.70) [91% women]. | The ACT-ED group completed a 5-h workshop based on ACT and migraine education. Each ACT-ED workshop involved 5–8 patients. The migraine education component (1 h) provided education about migraine. Patients in the TAU group completed the same clinical assessments as the ACT-ED group. Follow-up: 3 months. | Primary Outcomes: Daily headache: headache frequency **/severity **, medication use**, disability **, and visits to a healthcare professional **. |
| Grazzi et al., 2019. ACT for migraine: effect of acceptance and commitment therapy (ACT) for high-frequency episodic migraine without aura: preliminary data of a phase-II, multicentric, randomized, open-label study. Italy. | Multicentre, phase-II, open, randomized trial. Diagnosis of high-frequency migraine without aura. ICHD-3 beta, 3rd edition (2013). | N= 50 patients with high-frequency episodic migraine without aura (18-65 years old). Still ongoing (24 patients). | Patients were randomized to one of the following treatment arms: Condition A: education of patients, followed by pharmacological prophylaxis for migraine. Condition B: education of patients, followed by pharmacological prophylaxis for migraine plus ACT. The ACT protocol consisted of 6 90-m weekly sessions and two supplementary sessions separated by a 15-day interval, wherein patients were trained in mindfulness and pain management. Patients were trained in small groups (5/8) by a therapist, and were instructed to practice at home for at least 10 min per day. Reported follow-up: 3 months. | Primary Outcomes: Daily headache diary: decrease in days of headache/month ** and medication intake/month **. Not reported Outcomes: Anxiety: HADS. Depression: HADS. Headache impact: HIT6. Migraine disability: MIDAS. Pain catastrophizing: PCS. |
| Dindo et al., 2020. One-Day Acceptance and Commitment Therapy Compared to Support for Depressed Migraine Patients: a Randomized Clinical Trial. USA. | Randomized clinical trial. ID Migraine: Brief self-administered migraine screening test (Lipton et al., 2003). A medical chart diagnosis of migraine. | N = 103 patients with comorbid depression and migraine. ACT-ED group = 56 (36.90 ± 14.90) [47 women] S-ED group = 47 (34.40 ± 12.60) [38 women]. | ACT-ED. Two 1-day (5- to 6-h) interventions. The ACT-ED workshops lasted 5–6 h, involved 4–8 patients, and provided training in ACT and education about migraine. The S-ED workshop also lasted about 5–6 h and involved 4–8 patients. The same educational topics listed above about migraine were covered. Follow-up: 3 and 6 months after treatment. | Primary Outcomes: Anxiety: SIGH-A **. (d = 0.74) Depression: HRSD **. (d = 0.46) Disability: WHO-DAS**. (d = 0.23) Headache disability: HDI **. (d = 0.48) Quality of life: WHO-QOL **: - Psychological well-being ** (d = 0.44) - Social relationships - Environment - Physical health ** (d = 0.24) ** Significant results at the 6-month follow-up. |
| Vasiliou et al., 2020. Acceptance and Commitment Therapy for Primary Headache Sufferers: A Randomized Controlled Trial of Efficacy. Cyprus and Greece. | Randomized clinical trial. ICHD-3 beta, 3rd edition (2013) and a psychological evaluation by a doctoral-level clinical psychology trainee. | N = 94 patients with an 87.35% migraine diagnosis rate (43.00 ± 10.35) [84% women]. ACT group = 47 (42.89 ± 10.27) [74.53% women]. Final sample = 31. WL group = 47 (44.92 ± 10.43) [92.58% women]. Final sample = 30. | The authors developed an updated ACT process-based treatment guide consisting of three components: a therapist’s manual, a participants’ workbook, and 2 CDs. The 8 weekly, 1.5-h treatment sessions were conducted in groups of approximately 8 to 10 participants and two co-therapists. There was also an additional, final last session wherein participants were accompanied by their significant others. Follow-up: 3-, 6- and 12-month assessments (the final two were only for the ACT group). | Primary Outcomes: Anxiety: HADS. Clinical pain: GBPI: - Pain severity ** (RCI = 47%) Cognitive screening: MMSE. Depression: HADS. Headache disability: HDI-Func ** (RCI = 48%); HDI-Em ** (RCI = 33%). Medical utilization. Migraine-specific quality of life: MSQ v 2.1 **: - Role restrictive ** (RCI = 42%) - Emotional role ** (RCI = 32%) - Role preventive ** (RCI = 23%) ** Significant results at the 12-month follow-up. Secondary Outcomes: Chronic pain acceptance: Greek CPAQ **. (ηp2 = 0.14) Cognitive affective mindfulness: CAMS. Committed action: CAQ. Psychological inflexibility: Greek PIPS-II **: - Pain fusion ** (ηp2 = 0.13) - Pain avoidance ** (ηp2 = 0.12) Values: VQ: - Value progress - Barriers to value adherence ** (ηp2 = 0.04) ** Significant results at the 3-month follow-up in relation to post-test outcomes. |
| Grazzi et al., 2020. Acceptance and Commitment Therapy (ACT) vs Erenumab for High-Frequency Episodic Migraine Without Aura: Time to Take the Gloves Off! USA. | Longitudinal study. ICDH. | N= 40 patients with HFEM without aura. ACT group: 13 (42.10 ± 11.60). TAU group: 11 (41.80 ± 11.10). Erenumab group: 16 (45.70 ± 9.70). | The ACT protocol consisted of six 90-min small group sessions (once per week for 6 weeks) followed by two “booster” sessions. Training sessions involved structured behavioral education, experiential exercises and home assignments. The erenumab group was treated with 70 mg erenumab (per month as an adjunct to pharmacological prophylaxis. This group was not included in the original ACT project, and served as a further comparison group. Follow-up: 3- and 6-month assessments. | Primary Outcomes: Monthly migraine days **. Monthly medication intake **. Significant differences between ACT and TAU group (regarding the comparisons between ACT and Erenumab, there were hardly any differences between them). ** Significant results at the 6-month follow-up. |
| First Author (Year) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | General Assessment (Low, Medium, High) |
|---|---|---|---|---|---|---|---|---|
| Fibromyalgia Syndrome | ||||||||
| Jensen et al., 2012. | L | L | M | L | M | L | Yes | Medium |
| Steiner et al., 2013. | M | L | H | H | H | H | Yes | Low |
| Wicksell et al., 2013. | L | L | L | L | M | L | Yes | High |
| Ljóntsson et al., 2014. | M | H | M | H | L | L | Yes | Low |
| Luciano et al., 2014. | L | L | M | L | L | L | Yes | High |
| Pedersen et al., 2018. † | M | L | H | H | H | H | Yes | Low |
| Simister et al., 2018. | L | L | L | L | L | L | Yes | High |
| Gómez-Pérez et al., 2020. | H | L | H | H | H | M | Yes | Low |
| Irritable Bowel Syndrome | ||||||||
| Gillanders et al., 2017. | M | L | H | H | M | L | Yes | Moderate |
| Ferreira et al., 2018. | M | L | H | H | M | L | Yes | Moderate |
| Kamali-Nedjad et al., 2019. | H | M | H | H | H | H | Yes | Low |
| Mirsharifa et al., 2019. | H | L | H | H | H | H | Yes | Low |
| Aghalar et al., 2020. | H | L | H | H | M | H | Yes | Low |
| Ito et al., 2020. | M | L | H | H | M | L | Yes | Moderate |
| Migraine | ||||||||
| Mo’tamedi et al., 2012. | L | H | H | H | L | L | Yes | Low |
| Dindo et al., 2012. | H | H | H | H | L | L | Yes | Low |
| Dindo et al., 2014. | H | H | H | H | L | L | Yes | Low |
| Grazzi et al., 2019. | L | H | H | H | H | H | Yes | Low |
| Dindo et al., 2020. | L | L | L | H | L | L | Yes | Moderate |
| Vasiliou et al., 2020. | L | L | L | L | L | L | No | High |
| Grazzi et al., 2020. | L | H | H | H | H | H | Yes | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Sánchez, C.M.; Montoro, C.I.; Moreno-Padilla, M.; Reyes del Paso, G.A.; de la Coba, P. Effectiveness of Acceptance and Commitment Therapy in Central Pain Sensitization Syndromes: A Systematic Review. J. Clin. Med. 2021, 10, 2706. https://doi.org/10.3390/jcm10122706
Galvez-Sánchez CM, Montoro CI, Moreno-Padilla M, Reyes del Paso GA, de la Coba P. Effectiveness of Acceptance and Commitment Therapy in Central Pain Sensitization Syndromes: A Systematic Review. Journal of Clinical Medicine. 2021; 10(12):2706. https://doi.org/10.3390/jcm10122706
Chicago/Turabian StyleGalvez-Sánchez, Carmen M., Casandra I. Montoro, María Moreno-Padilla, Gustavo A. Reyes del Paso, and Pablo de la Coba. 2021. "Effectiveness of Acceptance and Commitment Therapy in Central Pain Sensitization Syndromes: A Systematic Review" Journal of Clinical Medicine 10, no. 12: 2706. https://doi.org/10.3390/jcm10122706
APA StyleGalvez-Sánchez, C. M., Montoro, C. I., Moreno-Padilla, M., Reyes del Paso, G. A., & de la Coba, P. (2021). Effectiveness of Acceptance and Commitment Therapy in Central Pain Sensitization Syndromes: A Systematic Review. Journal of Clinical Medicine, 10(12), 2706. https://doi.org/10.3390/jcm10122706







