Characteristics, Location, and Clinical Outcomes of Gastrointestinal Bleeding in Patients Taking New Oral Anticoagulants Compared to Vitamin K Antagonists
Abstract
1. Introduction
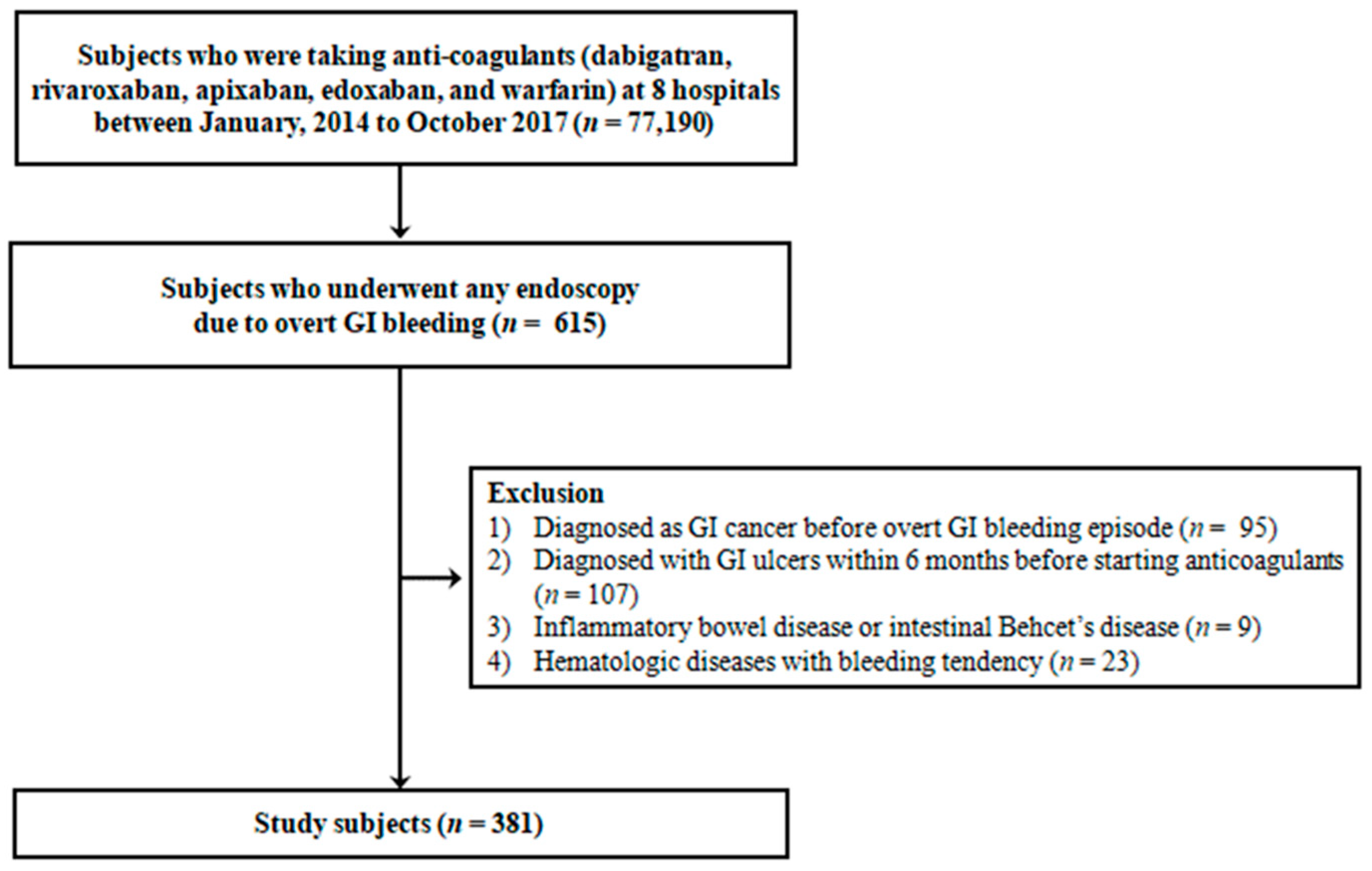
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definition of Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Subjects
3.2. Source, Lesion, and Location of Acute GI Bleeding in Patients on NOACs or VKA
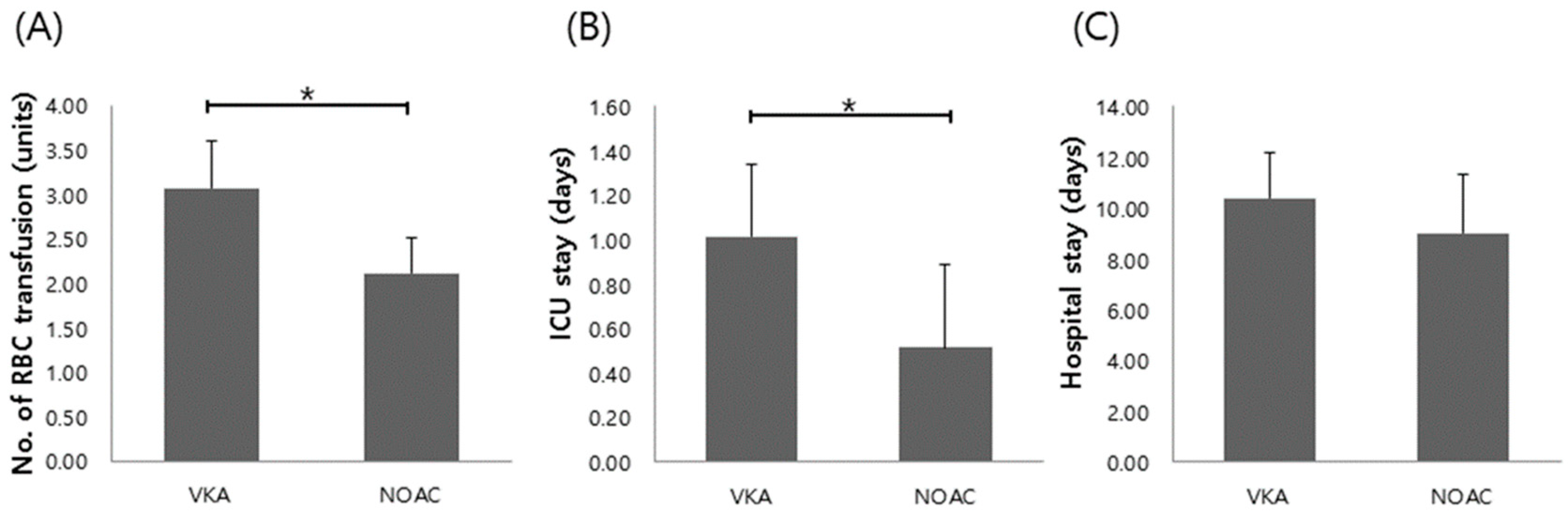
3.3. Comparison of Clinical Outcomes in Patients on NOACs vs. VKA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk. Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Greety, T.; Kumar, K.K.; Jayapraksah, K. A review on novel oral anticoagulants. Hygeia J. D Med. 2015, 7, 51–56. [Google Scholar]
- Kuznetsov, S.; Barcelona, R.; Josephson, R.A.; Mohan, S.K.M. The Role of Nonvitamin K Antagonist Oral Anticoagulants (NOACs) in Stroke Prevention in Patients with Atrial Fibrillation. Curr. Neurol. Neurosci. Rep. 2016, 16. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Hearth Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Ingrasciotta, Y.; Crisafulli, S.; Pizzimenti, V.; Marcianò, I.; Mancuso, A.; Andò, G.; Corrao, S.; Capranzano, P.; Trifirò, G. Pharmacokinetics of new oral anticoagulants: Implications for use in routine care. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Hogg, K.; Weitz, J.I. Overview of the new oral anticoagulants: Opportunities and challenges. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1056–1065. [Google Scholar] [CrossRef]
- Ciurus, T.; Sobczak, S.; Cichocka-Radwan, A. New oral anticoagulants—A practical guide. Kardiochir. Torakochirurgia Pol. 2015, 12, 111–118. [Google Scholar]
- Helms, T.M.; Silber, S.; Schäfer, A.; Masuhr, F.; Palm, F.; Darius, H.; Schrör, K.; Bänsch, D.; Bramlage, P.; Hankowitz, J.; et al. Consensus statement: Management of oral anticoagulation for stroke prevention in patients with nonvalvular atrial fibrillation. Herzschrittmacherther Elektrophysiol. 2016, 27, 295–306. [Google Scholar] [CrossRef]
- Senoo, K.; Lip, G. Comparative Efficacy and Safety of the Non–Vitamin K Antagonist Oral Anticoagulants for Patients with Nonvalvular Atrial Fibrillation. Semin. Thromb. Hemost. 2015, 41, 146–153. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Koretsune, Y.; Yamashita, T.; Yang, Y.; Chen, S.A.; Chung, N.; Giugliano, R.P. Edoxaban versus warfarin in east-asian (including Japanese) patients with atrial fibrillation―An engage AF-TIMI 48 sub-analysis. Circ. J. 2016, 80, 860–869. [Google Scholar]
- Caldeira, D.; Barra, M.; Pinto, F.J.; Ferreira, J.J.; Costa, J. Intracranial hemorrhage risk with the new oral anticoagulants: A systematic review and meta-analysis. J. Neurol. 2015, 262, 516–522. [Google Scholar] [CrossRef]
- Skaistis, J.; Tagami, T. Risk of Fatal Bleeding in Episodes of Major Bleeding with New Oral Anticoagulants and Vitamin K Antagonists: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0137444. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Schellinger, P.D.; Köhrmann, M.; Filippatou, A.; Gurol, M.E.; Caso, V.; Paciaroni, M.; Perren, F.; Alexandrov, A.V.; Tsivgoulis, G. Fatal oral anticoagulant-related intracranial hemorrhage: A systematic review and meta-analysis. Eur. J. Neurol. 2018, 25, 1299–1302. [Google Scholar] [CrossRef]
- Almutairi, A.R.; Zhou, L.; Gellad, W.F.; Lee, J.K.; Slack, M.K.; Martin, J.R.; Lo-Ciganic, W.H. Effectiveness and safety of non–vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: A systematic review and meta-analyses. Clin. Ther. 2017, 39, 1456–1478. [Google Scholar] [CrossRef]
- Caldeira, D.; Rodrigues, F.B.; Barra, M.; Santos, A.T.; de Abreu, D.; Gonçalves, N.; Pinto, F.J.; Ferreira, J.J.; Costa, J. Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: A systematic review and meta-analysis. Heart 2015, 101, 1204–1211. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Camm, A.J.; Amarenco, P.; Haas, S.; Hess, S.; Kirchhof, P.; Kuhls, S.; Van Eickels, M.; Turpie, A.G. XANTUS: A real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur. Hearth J. 2016, 37, 1145–1153. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost 2005, 3, 692–694. [Google Scholar]
- Chahal, D.; Lee, J.G.; Ali-Mohamad, N.; Donnellan, F. High rate of re-bleeding after application of Hemospray for upper and lower gastrointestinal bleeds. Dig. Liver Dis. 2020, 52, 768–772. [Google Scholar] [CrossRef]
- Wetwittayakhlang, P.; Wonglhow, J.; Netinatsunton, N.; Chamroonkul, N.; Piratvisuth, T. Re-bleeding and its predictors after capsule en-doscopy in patients with obscure gastrointestinal bleeding in long-term follow-up. BMC Gastroenterol. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Westendorf, J.; Förster, K.; Pannach, S.; Ebertz, F.; Gelbricht, V.; Thieme, C.; Michalski, F.; Köhler, C.; Werth, S.; Sahin, K.; et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: Results from the Dresden NOAC registry. Blood 2014, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.E.; Lim, W.-H.; Ahn, J.; Cha, M.-J.; Park, J.; Lee, K.H.; Park, H.-C.; Choi, E.-K.; Joung, B. The 2018 Korean Heart Rhythm Society Practical Guidelines on the use of Non-Vitamin K-Antagonist Oral Anticoagulants: Bleeding Control and Perioperative Management. Korean J. Med. 2019, 94, 40–56. [Google Scholar] [CrossRef]
- Suh, D.-C.; Nelson, W.W.; Choi, J.C.; Choi, I. Risk of Hemorrhage and Treatment Costs Associated with Warfarin Drug Interactions in Patients with Atrial Fibrillation. Clin. Ther. 2012, 34, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Snipelisky, D.; Kusumoto, F. Current strategies to minimize the bleeding risk of warfarin. J. Blood Med. 2013, 4, 89–99. [Google Scholar] [CrossRef][Green Version]
- Pannach, S.; Goetze, J.; Marten, S.; Schreier, T.; Tittl, L.; Beyer-Westendorf, J. Management and outcome of gastrointestinal bleeding in patients taking oral anticoagulants or antiplatelet drugs. J. Gastroenterol. 2017, 52, 1211–1220. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Heidbuchel, H. The Significance of Drug–Drug and Drug–Food Interactions of Oral Anticoagulation. Arrhythmia Electrophysiol. Rev. 2018, 7, 55–61. [Google Scholar] [CrossRef]
- Walborn, A.; Williams, M.; Fareed, J.; Hoppensteadt, D. International Normalized Ratio Relevance to the Observed Coagulation Abnormalities in Warfarin Treatment and Disseminated Intravascular Coagulation. Clin. Appl. Thromb. 2018, 24, 1033–1041. [Google Scholar] [CrossRef]
- Brodie, M.M.; Newman, J.C.; Smith, T.; Rockey, D.C. Severity of Gastrointestinal Bleeding in Patients Treated with Direct-Acting Oral Anticoagulants. Am. J. Med. 2018, 131, 573. [Google Scholar] [CrossRef]
- Gerson, L.B.; Fidler, J.L.; Cave, D.R.; Leighton, J.A. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am. J. Gastroenterol. 2015, 110, 1265–1287. [Google Scholar] [CrossRef]
- Diamantopoulou, G.; Konstantakis, C.; Skroubis, G.; Theocharis, G.; Theopistos, V.; Triantos, C.; Thomopoulosa, K. Acute Lower Gastrointestinal Bleeding in Patients Treated with Non-Vitamin K Antagonist Oral Anticoagulants Compared With Warfarin in Clinical Practice: Characteristics and Clinical Outcome. Gastroenterol. Res. 2019, 12, 21–26. [Google Scholar] [CrossRef]
- Desai, J.; Kolb, J.M.; Weitz, J.I.; Aisenberg, J. Gastrointestinal bleeding with the new oral anticoagulants–defining the issues and the management strategies. Thromb. Haemost. 2013, 110, 205–212. [Google Scholar] [CrossRef]
- Black, S.A.; Cohen, A.T. Anticoagulation strategies for venous thromboembolism: Moving towards a personalised approach. Thromb. Haemost. 2015, 114, 660–669. [Google Scholar]
- Hakeam, H.A.; Al-Sanea, N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs). J. Thromb. Thrombolysis 2017, 43, 343–351. [Google Scholar] [CrossRef]
- Guo, W.Q.; Chen, X.H.; Tian, X.Y.; Li, L. Differences in Gastrointestinal Safety Profiles Among Novel Oral Anticoagulants: Evidence from a Network Meta-Analysis. Clin. Epidemiol. 2019, 11, 911–921. [Google Scholar] [CrossRef]
| NOACs | VKA | p Value | |
|---|---|---|---|
| (n = 144) | (n = 237) | ||
| Mean age, years (range) * | 77.9 ± 7.8 (54–95) | 73.3 ± 11.9 (29–95) | <0.001 |
| Male sex (%) | 63 (43.8%) | 122 (51.5%) | 0.071 |
| Mean body mass index * | 23.3 ± 3.8 | 22.1 ± 4.1 | 0.005 |
| History of smoking (%) | 0.187 | ||
| No | 124 (86.1%) | 186 (78.5%) | |
| Ex-smoker | 15 (10.4%) | 38 (16.0%) | |
| Current smoker | 5 (3.5%) | 13 (5.5%) | |
| History of alcohol intake (%) | 0.368 | ||
| No | 117 (81.3%) | 198 (83.5%) | |
| Social | 14 (9.7%) | 26 (11.0%) | |
| Heavy | 13 (9.0%) | 13 (5.5%) | |
| History of major bleeding † (%) | 17 (11.8%) | 26 (11.0%) | 0.903 |
| History of prior gastrointestinal bleeding (%) | 29 (20.1%) | 42 (17.7%) | 0.678 |
| Symptom (%) | 0.061 | ||
| Hematemesis | 25 (17.4%) | 43 (18.1%) | |
| Melena | 60 (41.7%) | 124 (52.3%) | |
| Hematochezia | 59 (41.0%) | 70 (29.5%) | |
| Indication for Anticoagulation (%) | |||
| Atrial fibrillation/flutter | 108 (75.0%) | 117 (49.4%) | <0.001 |
| Pulmonary embolism/DVT | 29 (20.1%) | 40 (16.9%) | 0.329 |
| Prosthetic valve | 1 (0.7%) | 69 (29.1%) | <0.001 |
| Stroke prevention | 6 (4.2%) | 11 (4.6%) | 0.533 |
| Comorbidities (%) | |||
| Congestive heart failure | 49 (34.0%) | 77 (32.5%) | 0.954 |
| Hypertension | 100 (69.4%) | 137 (57.8%) | 0.071 |
| Arrythmia | 108 (75.0%) | 144 (60.8%) | 0.019 |
| Diabetes mellitus | 53 (36.8%) | 74 (31.2%) | 0.362 |
| Dyslipidemia | 31 (21.5%) | 42 (17.7%) | 0.460 |
| Coronary heart disease | 29 (20.1%) | 38 (16.0%) | 0.394 |
| Stroke | 52 (36.1%) | 58 (24.5%) | 0.028 |
| History of transient ischemic attack | 4 (2.8%) | 3 (1.3%) | 0.314 |
| Chronic kidney disease | 14 (9.7%) | 53 (22.4%) | 0.001 |
| Chronic obstructive pulmonary disease | 6 (4.2%) | 5 (2.1%) | 0.273 |
| Chronic hepatitis | 1 (0.7%) | 8 (3.4%) | 0.086 |
| Liver cirrhosis | 13 (9.0%) | 21 (8.9%) | 0.955 |
| Pulmonary embolism/DVT | 26 (18.1%) | 32 (13.5%) | 0.297 |
| Peripheral arterial occlusive disease | 3 (2.1%) | 13 (5.5%) | 0.094 |
| Prosthetic valve | 2 (1.4%) | 74 (31.2%) | <0.001 |
| Concomitant medications (%) | |||
| Aspirin | 13 (9.0%) | 27 (11.4%) | 0.135 |
| Clopidogrel | 12 (8.3%) | 9 (3.8%) | 0.173 |
| NSAIDs | 5 (3.5%) | 18 (7.6%) | 0.080 |
| Steroid | 7 (4.9%) | 15 (6.3%) | 0.474 |
| Proton pump inhibitor | 29 (20.1%) | 35 (14.8%) | 0.233 |
| H2 receptor antagonist | 18 (12.5%) | 10 (4.2%) | 0.004 |
| Examination Modalities (%) | |||
| Esophagogastroduodenoscopy | 43 (21.0%) | 52 (16.0%) | 0.116 |
| Colonoscopy/Sigmoidfibroscopy | 91 (44.4%) | 160 (49.2%) | 0.269 |
| SB enteroscopy | 0 (0.0%) | 3 (0.9%) | 0.294 |
| Capsule endoscopy | 12 (5.9%) | 24 (7.4%) | 0.591 |
| Abdomen pelvis CT | 59 (28.8%) | 86 (26.5%) | 0.440 |
| NOACs (n = 144) | VKA (n = 237) | |
|---|---|---|
| Upper GI findings (%) | 51 (35.4) | 98 (41.4) |
| Esophagus | 8 (5.6) | 13 (5.5) |
| Esophagitis | 2 (1.4) | 1 (0.4) |
| Esophageal ulcer | 1 (0.7) | 1 (0.4) |
| Mallory-Weiss syndrome | 3 (2.1) | 7 (3.0) |
| Esophageal angiodysplasia | 0 (0) | 1 (0.4) |
| Esophageal varix | 2 (1.4) | 3 (1.3) |
| Stomach | 38 (26.4) | 69 (29.1) |
| Gastric varix | 3 (2.1) | 1 (0.4) |
| Gastric antral vascular ectasia | 1 (0.7) | 2 (0.8) |
| Gastric erosion | 2 (1.4) | 3 (1.3) |
| Benign gastric ulcer | 25 (17.4) | 47 (19.8) |
| Gastric cancer | 2 (1.4) | 1 (0.4) |
| Gastric angiodysplasia | 2 (1.4) | 9 (3.8) |
| Gastric dieulafoy | 1 (0.7) | 6 (2.5) |
| Gastric polypectomy Or endoscopic submucosal dissection bleeding | 2 (1.4) | 0 (0) |
| Duodenum | 5 (3.5) | 16 (6.8) |
| Duodenal ulcer | 5 (3.5) | 14 (5.9) |
| Duodenal angiodysplasia | 0 (0) | 1 (0.4) |
| Duodenal dieulafoy lesion | 0 (0) | 1 (0.4) |
| Duodenitis | 0 (0) | 0 (0) |
| Small bowel findings (%) | 6 (4.2) | 16 (6.8) |
| Inflammatory lesion | 2 (1.4) | 9 (3.8) |
| Neoplastic lesion | 0 (0) | 0 (0) |
| Vascular lesion | 4 (2.8) | 6 (2.5) |
| Others | 0 (0) | 1 (0.4) |
| Lower GI findings (%) | 33 (22.9) | 43 (18.1) |
| Vascular lesion | 5 (3.5) | 13 (5.5) |
| Hemorrhoid | 4 (2.8) | 10 (4.2) |
| Ischemic colitis | 1 (0.7) | 3 (1.3) |
| Anatomic lesion | 8 (5.6) | 7 (3.0) |
| Diverticuli without bleeding | 7 (4.9) | 4 (1.7) |
| Diverticuli with current bleeding | 1 (0.7) | 3 (1.3) |
| Inflammatory lesion | 14 (9.7) | 10 (4.2) |
| Rectal ulcer only | 8 (5.6) | 4 (1.7) |
| Rectal ulcer with exposed vessel | 1 (0.7) | 1 (0.4) |
| Colon ulcer | 3 (2.1) | 1 (0.4) |
| Infectious colitis | 1 (0.7) | 1 (0.4) |
| Pseudomembranous colitis | 1 (0.7) | 2 (0.8) |
| Inflammatory bowel disease | 0 (0) | 1 (0.4) |
| Neoplastic lesion | 6 (4.2) | 13 (5.5) |
| Colon polyp | 5 (3.5) | 10 (4.2) |
| Colon cancer | 1 (0.7) | 3 (1.3) |
| Unidentified lesion (%) | 54 (37.5) | 80 (33.8) |
| NOACs (N = 90) | VKA (N = 157) | p Value | |
|---|---|---|---|
| Lesion characteristics (%) | |||
| Vascular lesion | 14 (15.6) | 40 (25.5) | 0.038 |
| Inflammatory lesion | 49 (54.4) | 81 (51.6) | 0.775 |
| Neoplastic lesion | 7 (7.8) | 14 (8.9) | 0.604 |
| Anatomic lesion & Others * | 20 (22.2) | 22 (14.0) | 0.638 |
| Location (%) | |||
| Esophagus | 8 (8.9) | 13 (8.3) | 0.912 |
| Stomach | 38 (42.2) | 69 (43.9) | 0.334 |
| Duodenum | 5 (5.6) | 16 (10.2) | 0.284 |
| Small bowel | 6 (6.7) | 16 (10.2) | 0.090 |
| Colon | 33 (36.7) | 43 (27.4) | 0.460 |
| Clinical Outcomes | NOACs (%) (n = 59) | VKA (%) (n = 123) | Multivariate Logistic Regression Analysis * | |
|---|---|---|---|---|
| Adjusted OR (95% CI) | p Value | |||
| Hemodynamic instability at admission | 26 (17.7%) | 50 (21.6%) | 0.81 (0.47–1.37) | 0.167 |
| Rebleeding | 15 (10.6%) | 46 (20.9%) | 0.42 (0.22–0.79) | 0.007 |
| Need for angiography | 11 (8.1%) | 12 (5.6%) | 1.47 (0.62–3.45) | 0.112 |
| Mortality during Hospital day | 6 (4.1%) | 11 (4.7%) | 0.84 (0.30–2.35) | 0.729 |
| Need for surgery † | 1 (0.7%) | 4 (1.7%) | 0.82 (0.69–0.98) | 0.045 |
| NOACs | Dabigatran (n = 32, 22.2%) | Rivaroxaban (n = 72, 50.0%) | Apixaban (n = 28, 19.5%) | Edoxaban (n = 12, 8.3%) | |
|---|---|---|---|---|---|
| Outcomes | |||||
| Hemodynamic instability at admission | 5 (19.3%) | 13 (50.0%) | 7 (26.9%) | 1 (3.8%) | |
| Need for angiography | 3 (27.3%) | 7 (63.7%) | 1 (9.0%) | 0 (0.0%) | |
| Need for surgery | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | |
| Mortality during Hospital day | 1 (16.7%) | 4 (66.6%) | 1 (16.7%) | 0 (0.0%) | |
| Rebleeding | 2 (13.3%) | 9 (60.0%) | 4 (26.7%) | 0 (0.0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, A.R.; Moon, C.M.; Tae, C.H.; Chun, J.; Bang, K.B.; Lee, Y.J.; Lee, H.S.; Jung, Y.; Park, S.C.; Koo, H.S. Characteristics, Location, and Clinical Outcomes of Gastrointestinal Bleeding in Patients Taking New Oral Anticoagulants Compared to Vitamin K Antagonists. J. Clin. Med. 2021, 10, 2693. https://doi.org/10.3390/jcm10122693
Choe AR, Moon CM, Tae CH, Chun J, Bang KB, Lee YJ, Lee HS, Jung Y, Park SC, Koo HS. Characteristics, Location, and Clinical Outcomes of Gastrointestinal Bleeding in Patients Taking New Oral Anticoagulants Compared to Vitamin K Antagonists. Journal of Clinical Medicine. 2021; 10(12):2693. https://doi.org/10.3390/jcm10122693
Chicago/Turabian StyleChoe, A Reum, Chang Mo Moon, Chung Hyun Tae, Jaeyoung Chun, Ki Bae Bang, Yoo Jin Lee, Hyun Seok Lee, Yunho Jung, Sung Chul Park, and Hoon Sup Koo. 2021. "Characteristics, Location, and Clinical Outcomes of Gastrointestinal Bleeding in Patients Taking New Oral Anticoagulants Compared to Vitamin K Antagonists" Journal of Clinical Medicine 10, no. 12: 2693. https://doi.org/10.3390/jcm10122693
APA StyleChoe, A. R., Moon, C. M., Tae, C. H., Chun, J., Bang, K. B., Lee, Y. J., Lee, H. S., Jung, Y., Park, S. C., & Koo, H. S. (2021). Characteristics, Location, and Clinical Outcomes of Gastrointestinal Bleeding in Patients Taking New Oral Anticoagulants Compared to Vitamin K Antagonists. Journal of Clinical Medicine, 10(12), 2693. https://doi.org/10.3390/jcm10122693






