Reduction of Conduction Velocity in Patients with Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
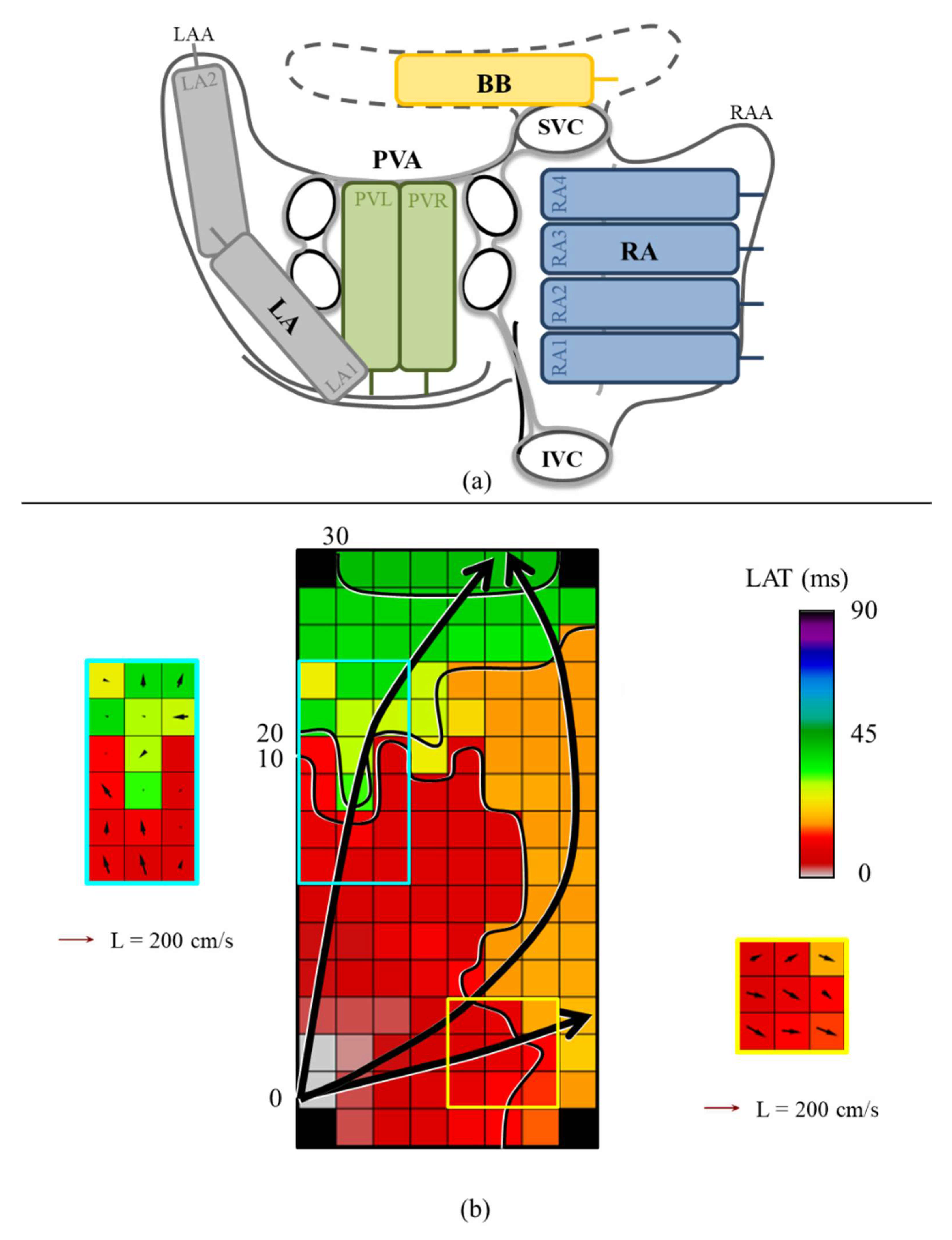
2.2. Mapping Procedure
2.3. Mapping Data Processing
2.4. Calculation of Local Conduction Velocities
2.5. Statistical Analysis
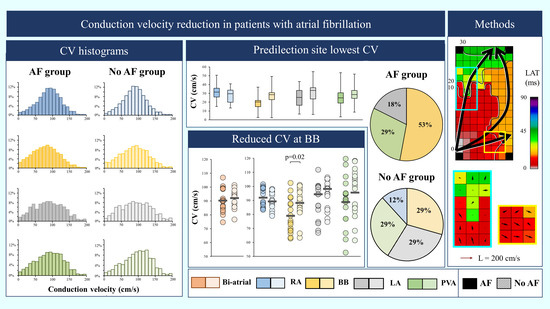
3. Results
3.1. Study Population
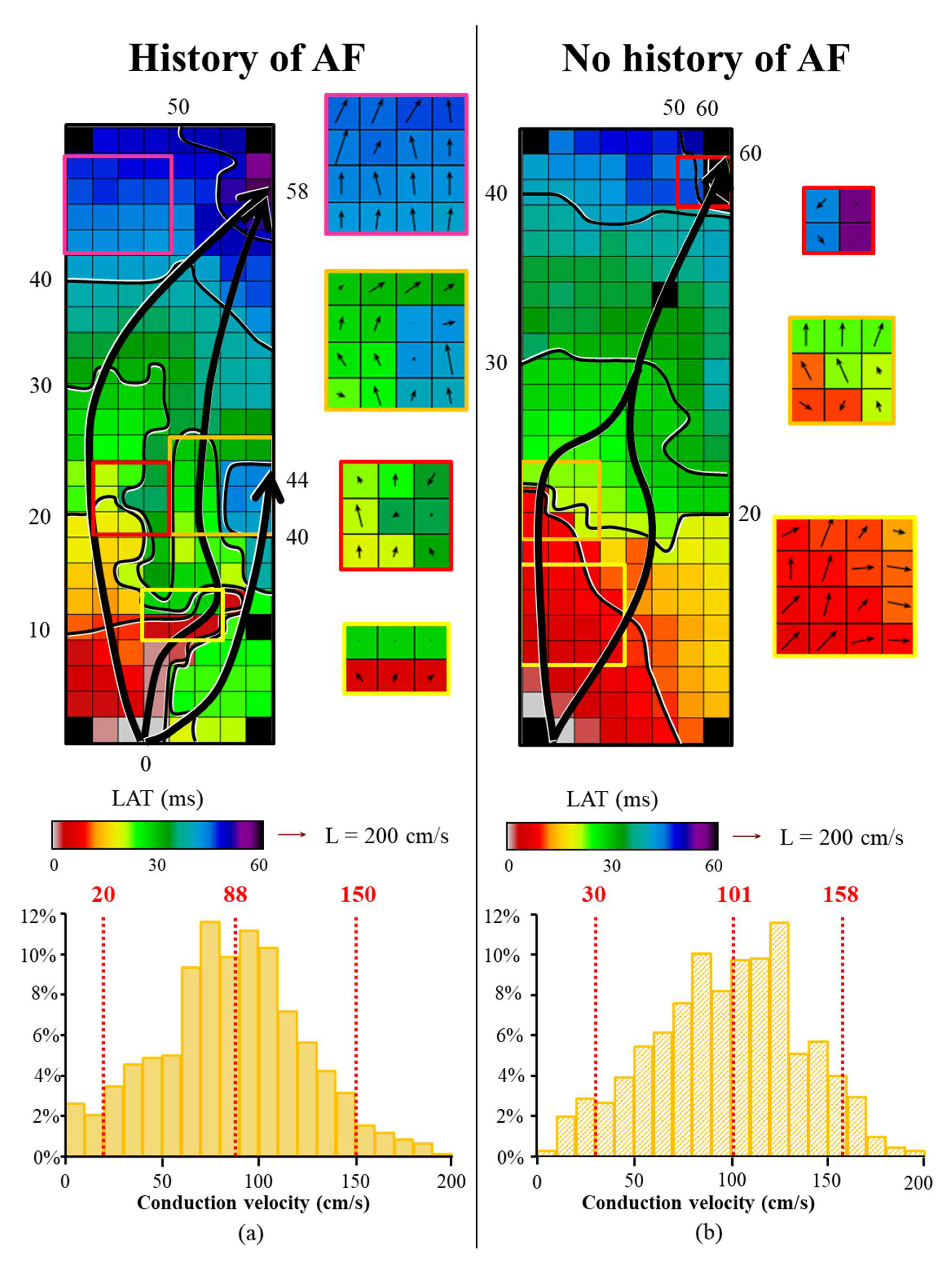
3.2. Mapping Data Characteristics
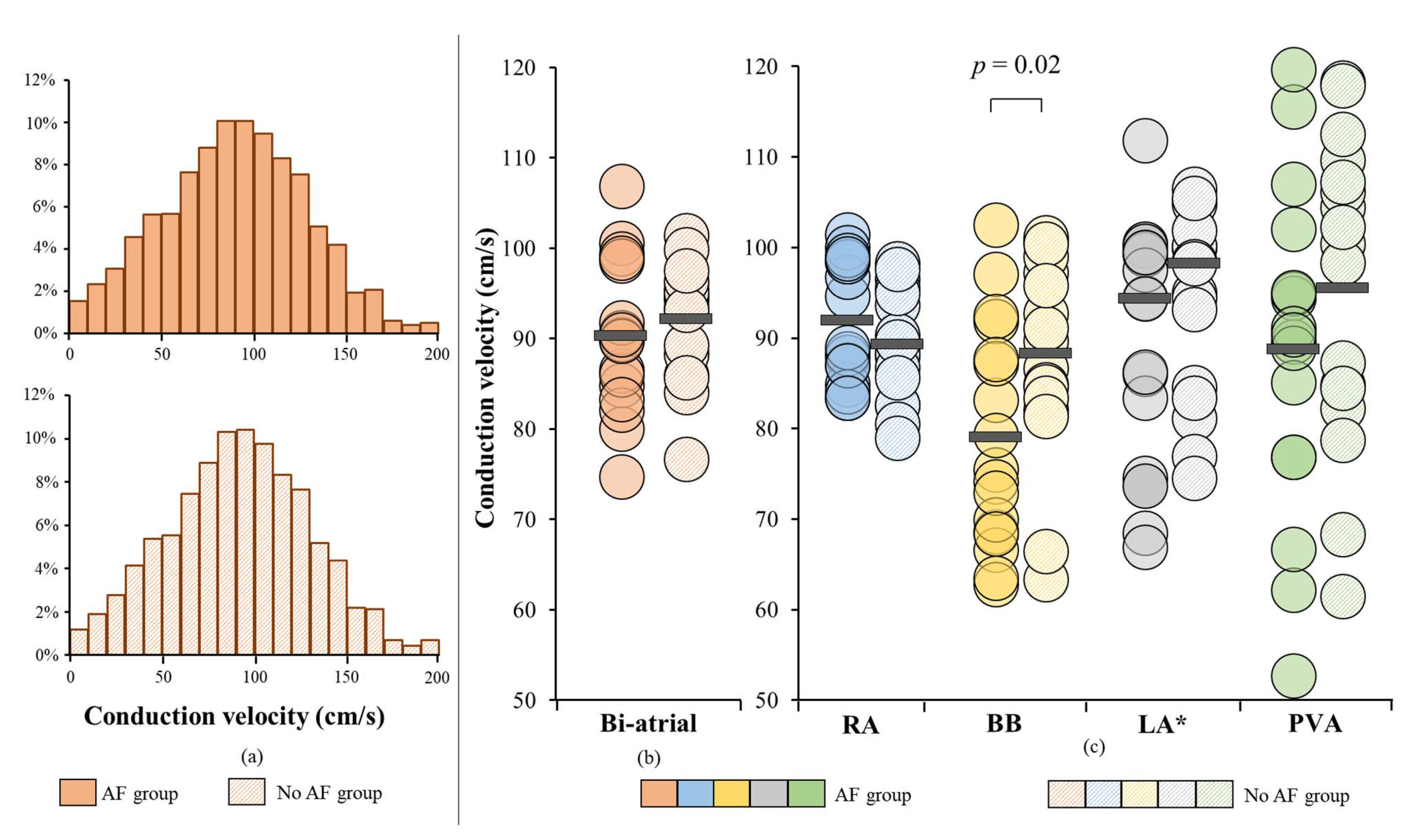
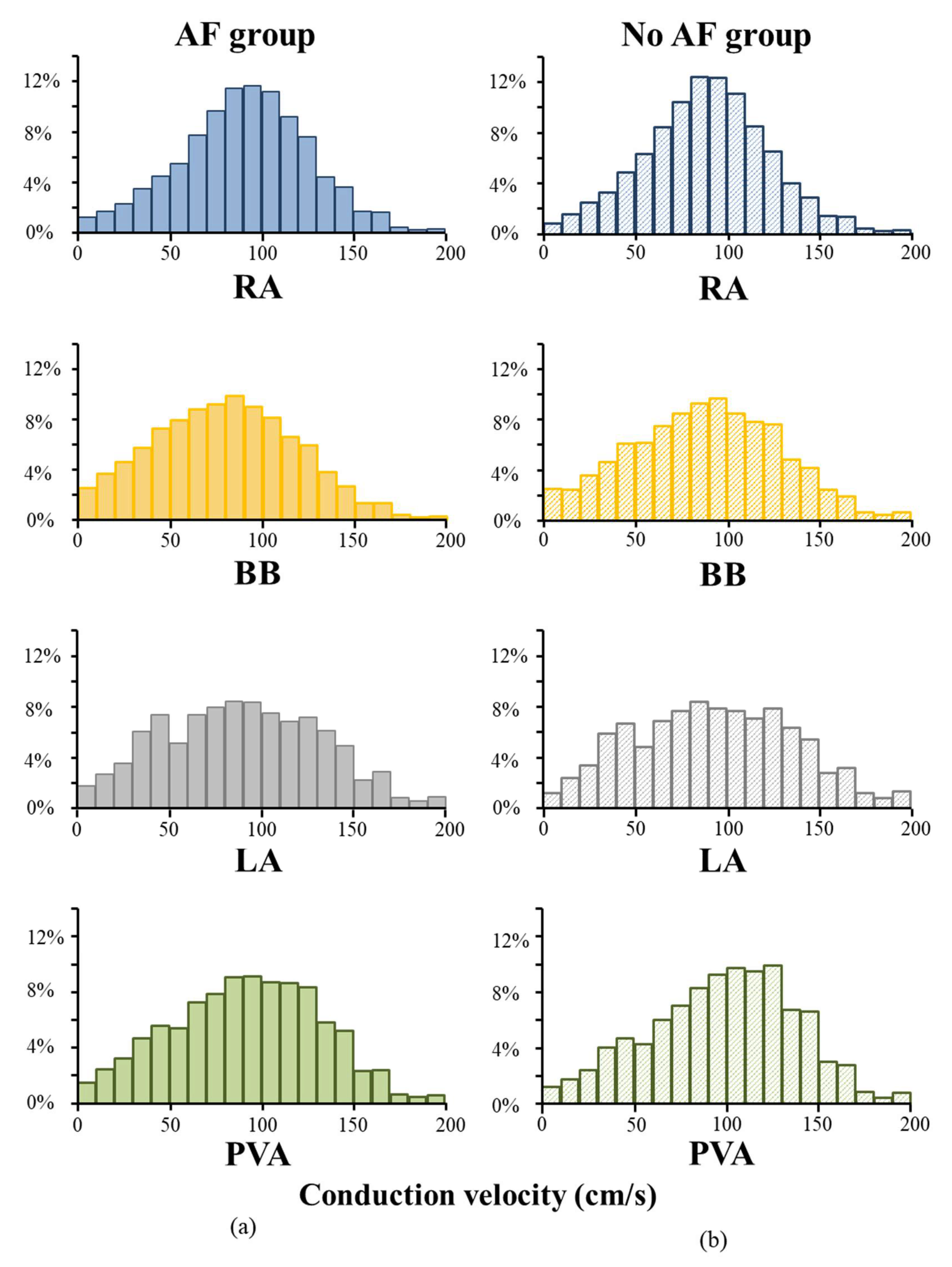
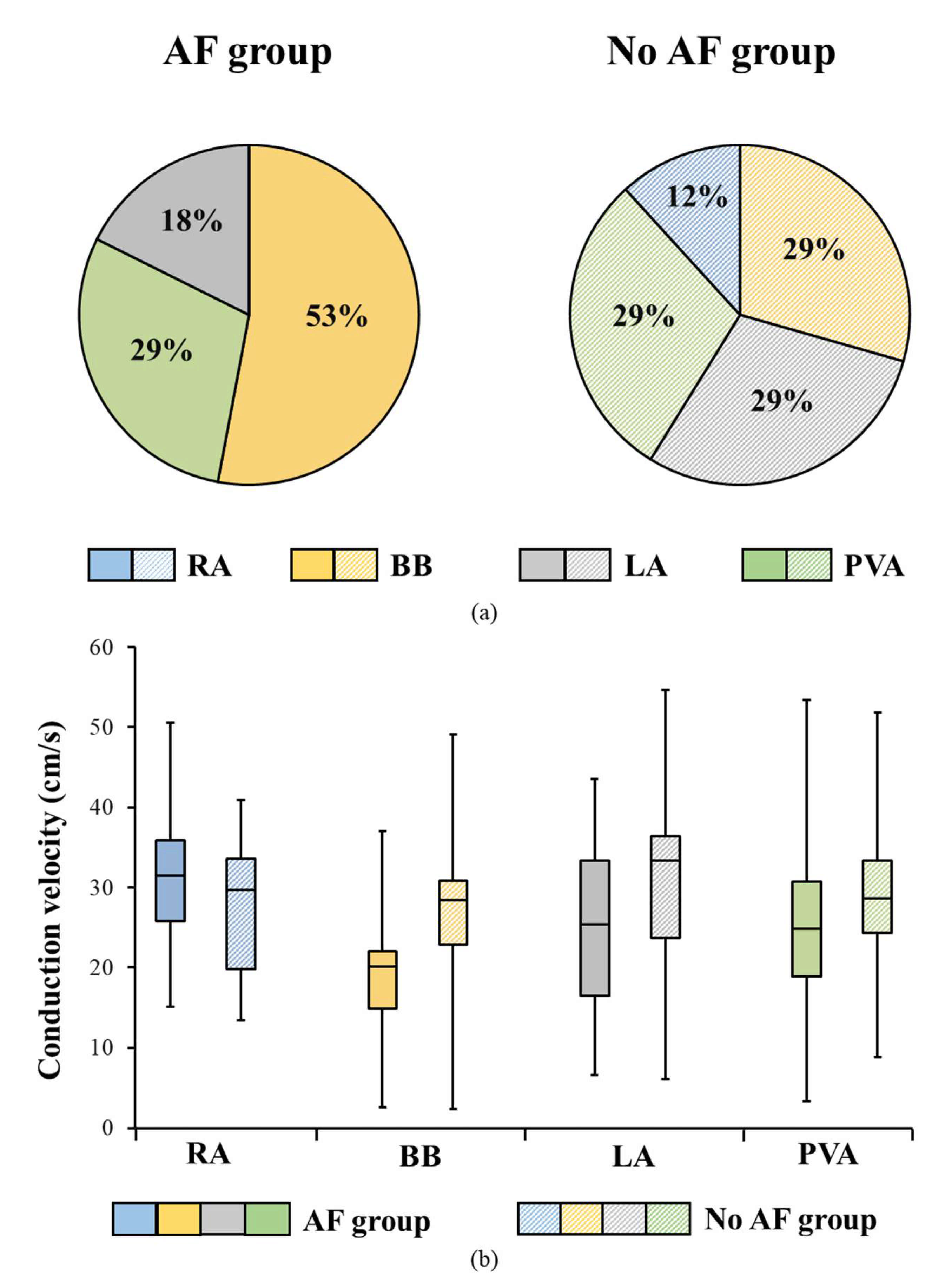
3.3. Conduction Velocity Throughout Both Atria
3.4. AF-related Reduction of Conduction Velocity
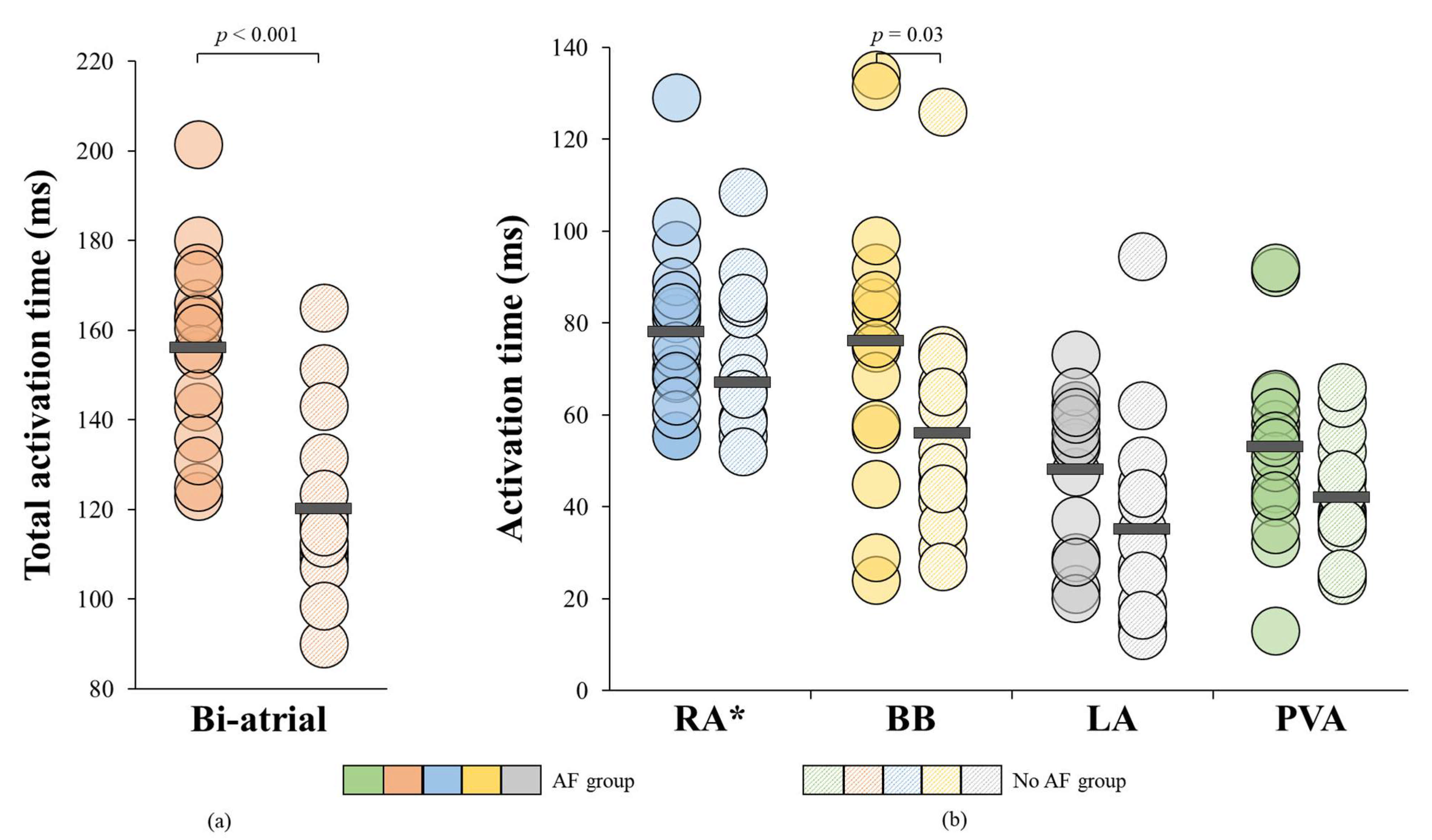
3.5. Relation between Conduction Heterogeneity and Total Activation Time
3.6. Atrial Fibrillation Episodes and Unipolar Voltages
4. Discussion
4.1. Key Findings
4.2. Reduced Conduction Velocity as a Prerequisite for AF Onset
4.3. Bachmann’s Bundle as a Predilection Site for Atrial Fibrillation
4.4. Low Voltages and Atrial Fibrillation
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kleber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Gaeta, S.; Griffith, B.E.; Henriquez, C.S. Muscle thickness and curvature influence atrial conduction velocities. Front. Physiol. 2018, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- King, J.H.; Huang, C.L.; Fraser, J.A. Determinants of myocardial conduction velocity: Implications for arrhythmogenesis. Front. Physiol. 2013, 4, 154. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; Holm, M.; Blomstrom, P.; Johansson, R.; Luhrs, C.; Brandt, J.; Olsson, S.B. Right atrial free wall conduction velocity and degree of anisotropy in patients with stable sinus rhythm studied during open heart surgery. Eur. Heart J. 1998, 19, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, C.P.; Yaksh, A.; Lanters, E.A.; Kik, C.; van der Does, L.J.; Knops, P.; Taverne, Y.J.; van de Woestijne, P.C.; Oei, F.B.; Bekkers, J.A.; et al. Relevance of conduction disorders in bachmann’s bundle during sinus rhythm in humans. Circ. Arrhythmia Electrophysiol. 2016, 9, e003972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xia, Y.; Carlson, J.; Kongstad, O.; Yuan, S. Atrial average conduction velocity in patients with and without paroxysmal atrial fibrillation. Clin. Physiol. Funct. Imaging 2017, 37, 596–601. [Google Scholar] [CrossRef] [PubMed]
- van der Does, W.F.B.; Houck, C.A.; Heida, A.; van Schie, M.S.; van Schaagen, F.R.N.; Taverne, Y.; Bogers, A.; de Groot, N.M.S. Atrial electrophysiological characteristics of aging. J. Cardiovasc. Electrophysiol. 2021, 32, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Schram-Serban, C.; Heida, A.; Roos-Serote, M.C.; Knops, P.; Kik, C.; Brundel, B.; Bogers, A.; de Groot, N.M.S. Heterogeneity in conduction underlies obesity-related atrial fibrillation vulnerability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008161. [Google Scholar] [CrossRef] [PubMed]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef] [PubMed]
- van der Does, L.; Yaksh, A.; Kik, C.; Knops, P.; Lanters, E.A.H.; Teuwen, C.P.; Oei, F.B.S.; van de Woestijne, P.C.; Bekkers, J.A.; Bogers, A.; et al. Quest for the arrhythmogenic substrate of atrial fibrillation in patients undergoing cardiac surgery (quasar study): Rationale and design. J. Cardiovasc. Transl. Res. 2016, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lanters, E.A.; van Marion, D.M.; Kik, C.; Steen, H.; Bogers, A.J.; Allessie, M.A.; Brundel, B.J.; de Groot, N.M. Halt & reverse: Hsf1 activators lower cardiomyocyt damage; towards a novel approach to reverse atrial fibrillation. J. Transl. Med. 2015, 13, 347. [Google Scholar]
- Yaksh, A.; Kik, C.; Knops, P.; Roos-Hesselink, J.W.; Bogers, A.J.; Zijlstra, F.; Allessie, M.; de Groot, N.M. Atrial fibrillation: To map or not to map? Neth. Heart J. 2014, 22, 259–266. [Google Scholar] [CrossRef][Green Version]
- Mouws, E.; van der Does, L.; Kik, C.; Lanters, E.A.H.; Teuwen, C.P.; Knops, P.; Bogers, A.; de Groot, N.M.S. Impact of the arrhythmogenic potential of long lines of conduction slowing at the pulmonary vein area. Heart Rhythm 2019, 16, 511–519. [Google Scholar] [CrossRef]
- Van Schie, M.S.; Heida, A.; Taverne, Y.; Bogers, A.; de Groot, N.M.S. Identification of local atrial conduction heterogeneities using high-density conduction velocity estimation. Europace 2021, euab088. [Google Scholar] [CrossRef]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Ausma, J.; Litjens, N.; Lenders, M.H.; Duimel, H.; Mast, F.; Wouters, L.; Ramaekers, F.; Allessie, M.; Borgers, M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J. Mol. Cell. Cardiol. 2001, 33, 2083–2094. [Google Scholar] [CrossRef]
- Schotten, U.; Duytschaever, M.; Ausma, J.; Eijsbouts, S.; Neuberger, H.R.; Allessie, M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation 2003, 107, 1433–1439. [Google Scholar] [CrossRef]
- Gaspo, R.; Bosch, R.F.; Bou-Abboud, E.; Nattel, S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ. Res. 1997, 81, 1045–1052. [Google Scholar] [CrossRef]
- Yue, L.; Feng, J.; Gaspo, R.; Li, G.R.; Wang, Z.; Nattel, S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997, 81, 512–525. [Google Scholar] [CrossRef]
- Bosch, R.F.; Zeng, X.; Grammer, J.B.; Popovic, K.; Mewis, C.; Kuhlkamp, V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 1999, 44, 121–131. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; Lamorgese, M.; Rossie, S.S.; McCarthy, P.M.; Nerbonne, J.M. Atrial l-type Ca2+ currents and human atrial fibrillation. Circ. Res. 1999, 85, 428–436. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; McCarthy, P.M.; Trimmer, J.S.; Nerbonne, J.M. Outward K+ current densities and kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 1997, 80, 772–781. [Google Scholar] [CrossRef]
- Everett, T.H.; Li, H.; Mangrum, J.M.; McRury, I.D.; Mitchell, M.A.; Redick, J.A.; Haines, D.E. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 2000, 102, 1454–1460. [Google Scholar] [CrossRef]
- Spach, M.S.; Boineau, J.P. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: A major mechanism of structural heart disease arrhythmias. Pacing Clin. Electrophysiol. 1997, 20, 397–413. [Google Scholar] [CrossRef]
- Darby, A.E.; Dimarco, J.P. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012, 125, 945–957. [Google Scholar] [CrossRef]
- Psaty, B.M.; Manolio, T.A.; Kuller, L.H.; Kronmal, R.A.; Cushman, M.; Fried, L.P.; White, R.; Furberg, C.D.; Rautaharju, P.M. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997, 96, 2455–2461. [Google Scholar] [CrossRef]
- Wang, K.; Ho, S.Y.; Gibson, D.G.; Anderson, R.H. Architecture of atrial musculature in humans. Br. Heart J. 1995, 73, 559–565. [Google Scholar] [CrossRef]
- Ho, S.Y.; Sanchez-Quintana, D.; Cabrera, J.A.; Anderson, R.H. Anatomy of the left atrium: Implications for radiofrequency ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1999, 10, 1525–1533. [Google Scholar]
- Ho, S.Y.; Anderson, R.H.; Sanchez-Quintana, D. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovasc. Res. 2002, 54, 325–336. [Google Scholar] [CrossRef]
- Roberts, D.E.; Hersh, L.T.; Scher, A.M. Influence of cardiac fiber orientation on wavefront voltage, conduction velocity, and tissue resistivity in the dog. Circ. Res. 1979, 44, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Spach, M.S.; Miller, W.T., 3rd; Geselowitz, D.B.; Barr, R.C.; Kootsey, J.M.; Johnson, E.A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ. Res. 1981, 48, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, G. The inter-auricular time interval. Am. J. Physiol. 1916, 41, 309–320. [Google Scholar] [CrossRef]
- Agarwal, Y.K.; Aronow, W.S.; Levy, J.A.; Spodick, D.H. Association of interatrial block with development of atrial fibrillation. Am. J. Cardiol. 2003, 91, 882. [Google Scholar] [CrossRef]
- Duytschaever, M.; Danse, P.; Eysbouts, S.; Allessie, M. Is there an optimal pacing site to prevent atrial fibrillation?: An experimental study in the chronically instrumented goat. J. Cardiovasc. Electrophysiol. 2002, 13, 1264–1271. [Google Scholar] [CrossRef]
- Ariyarajah, V.; Fernandes, J.; Kranis, M.; Apiyasawat, S.; Mercado, K.; Spodick, D.H. Prospective evaluation of atrial tachyarrhythmias in patients with interatrial block. Int. J. Cardiol. 2007, 118, 332–337. [Google Scholar] [CrossRef]
- Becker, A.E. How structurally normal are human atria in patients with atrial fibrillation? Heart Rhythm 2004, 1, 627–631. [Google Scholar] [CrossRef]
- Sim, I.; Bishop, M.; O’Neill, M.; Williams, S.E. Left atrial voltage mapping: Defining and targeting the atrial fibrillation substrate. J. Interv. Card. Electrophysiol. 2019, 56, 213–227. [Google Scholar] [CrossRef]
- Van Schie, M.S.; Starreveld, R.; Bogers, A.; de Groot, N.M.S. Sinus rhythm voltage fingerprinting in patients with mitral valve disease using a high-density epicardial mapping approach. Europace 2021, 23, 469–478. [Google Scholar] [CrossRef]
| AF Group (n = 17) | No AF Group (n = 17) | p Value | |
|---|---|---|---|
| Age-Years (mean ± SD) | 73 ± 7 | 74 ± 7 | 0.60 |
| Male Sex-n (%) | 11 (64.7) | 9 (52.9) | 0.69 |
| BMI-kg/m2 (Median (IQR)) | 24.9 (23.0–29.2) | 25.3 (22.9–29.1) | 0.16 |
| Underlying Heart Disease-n (%) | 1.00 | ||
| IHD | 2 (11.8) | 2 (11.8) | |
| (i)VHD | 15 (88.2) | 15 (88.2) | |
| AVD | 2 (11.8) | 3 (17.6) | |
| AVD and CAD | 2 (11.8) | 2 (11.8) | |
| MVD | 9 (52.9) | 7 (41.2) | |
| MVD and CAD | 2 (11.8) | 3 (17.6) | |
| Echocardiography | |||
| LVF-n (%) | 1.00 | ||
| Normal | 11 (64.7) | 12 (70.6) | |
| Mild dysfunction | 3 (17.6) | 2 (11.8) | |
| Moderate dysfunction | 3 (17.6) | 3 (17.6) | |
| Severe dysfunction | 0 (0.0) | 0 (0.0) | |
| Dilated LA >45 mm-n (%) | 11/13 (84.6) | 9/15 (60.0) | 0.41 |
| Medication-n (%) | |||
| Antiarrhythmic Drugs | 0.45 | ||
| Class I | 0 (0.0) | 0 (0.0) | |
| Class II | 11 (64.7) | 7 (41.2) | |
| Class III | 4 (23.5) | 0 (0.0) | |
| Class IV | 1 (5.9) | 1 (5.9) | |
| Digoxin | 5 (29.4) | 0 (0.0) | 0.06 |
| AF Group (n = 17) | No AF Group (n = 17) | p Value | |
|---|---|---|---|
| Variation of CV (Δ P5-P95) | |||
| RA-cm/s (mean ± SD) | 118 ± 9 | 114 ± 8 | 0.14 |
| BB –cm/s (median (IQR)) | 121 (114–133) | 129 (119–137) | 0.31 |
| PVA-cm/s (mean ± SD) | 129 ± 12 | 130 ± 21 | 0.87 |
| LA-cm/s (mean ± SD) | 139 ± 11 | 143 ± 13 | 0.42 |
| AF Group (n = 17) | No AF Group (n = 17) | p Value | |
|---|---|---|---|
| 5th percentiles of unipolar voltages | |||
| Bi-atrial-mV (median (IQR)) | 0.6 (0.6–1.0) | 0.8 (0.6–1.1) | 0.44 |
| RA-mV (median (IQR)) | 1.1 ± 0.6 | 0.9 ± 0.3 | 0.20 |
| BB-mV (mean ± SD) | 0.9 ± 0.6 | 1.5 ± 0.9 | 0.02 |
| PVA-mV (mean ± SD) | 0.6 (0.5–1.4) | 1.4 (0.6–2.7) | 0.08 |
| LA-mV (mean ± SD) | 1.2 ± 0.7 | 1.2 ± 0.7 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heida, A.; van Schie, M.S.; van der Does, W.F.B.; Taverne, Y.J.H.J.; Bogers, A.J.J.C.; de Groot, N.M.S. Reduction of Conduction Velocity in Patients with Atrial Fibrillation. J. Clin. Med. 2021, 10, 2614. https://doi.org/10.3390/jcm10122614
Heida A, van Schie MS, van der Does WFB, Taverne YJHJ, Bogers AJJC, de Groot NMS. Reduction of Conduction Velocity in Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2021; 10(12):2614. https://doi.org/10.3390/jcm10122614
Chicago/Turabian StyleHeida, Annejet, Mathijs S. van Schie, Willemijn F. B. van der Does, Yannick J. H. J. Taverne, Ad J. J. C. Bogers, and Natasja M. S. de Groot. 2021. "Reduction of Conduction Velocity in Patients with Atrial Fibrillation" Journal of Clinical Medicine 10, no. 12: 2614. https://doi.org/10.3390/jcm10122614
APA StyleHeida, A., van Schie, M. S., van der Does, W. F. B., Taverne, Y. J. H. J., Bogers, A. J. J. C., & de Groot, N. M. S. (2021). Reduction of Conduction Velocity in Patients with Atrial Fibrillation. Journal of Clinical Medicine, 10(12), 2614. https://doi.org/10.3390/jcm10122614