Treatment or Prophylaxis against Hepatitis B Virus Infection in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: An Update
Abstract
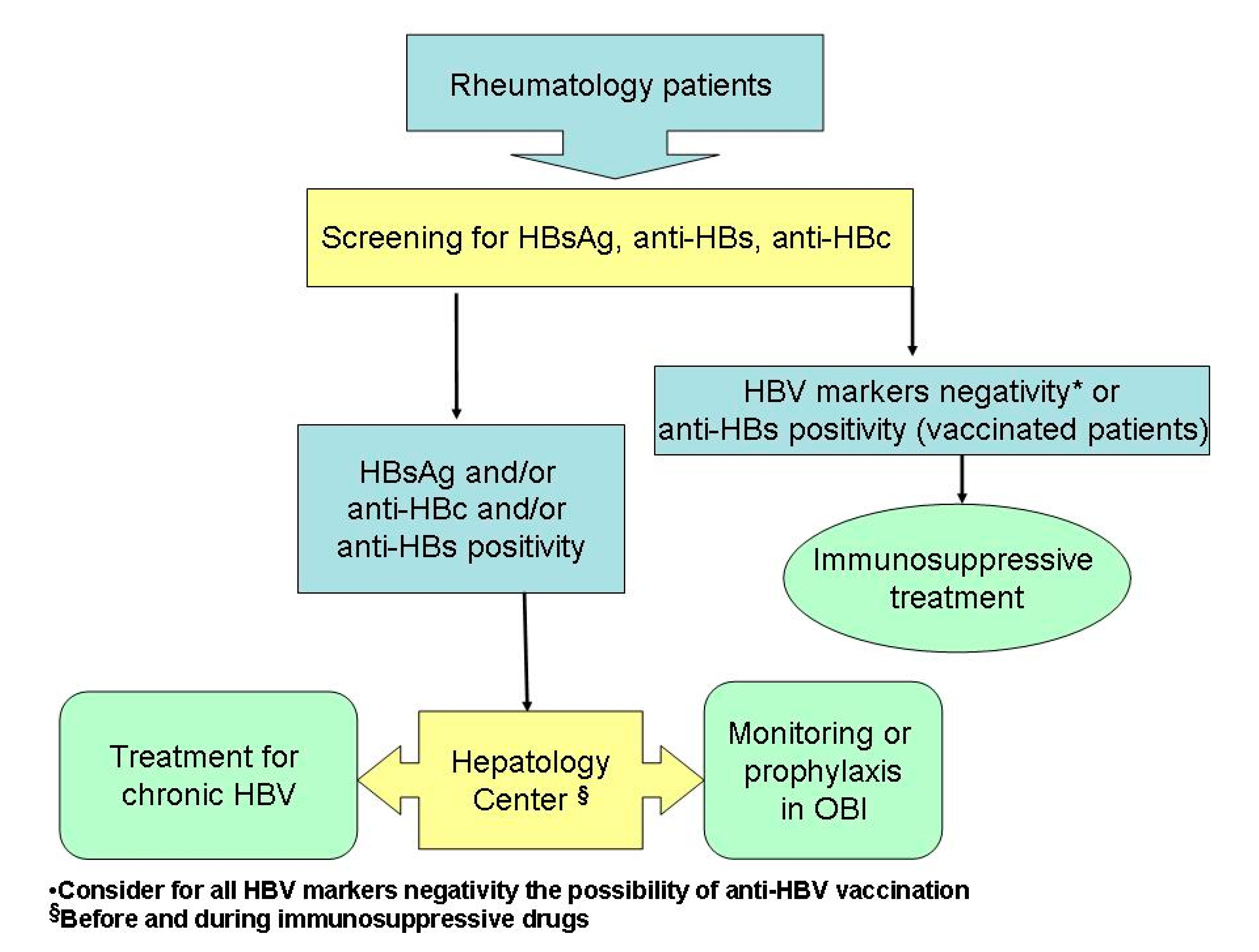
1. Introduction
2. Clinical Epidemiology of HBV Infection and Risk of Reactivation in Patients with Rheumatic Diseases during Immunosuppressive Therapy
3. Occult HBV Infection in Patients Treated with Rheumatologic Drugs
4. Chronic HBV Infection in Patients Treated with Rheumatologic Drugs
Author Contributions
Funding
Conflicts of Interest
References
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Allain, J.-P.; Brunetto, M.R.; Buendia, M.-A.; Chen, D.-S.; Colombo, M.; Craxì, A.; Donato, F.; Ferrari, C.; Gaeta, G.B.; et al. Statements from the Taormina Expert Meeting on Occult Hepatitis B Virus Infection. J. Hepatol. 2008, 49, 652–657. [Google Scholar] [CrossRef] [PubMed]
- WHO | Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Available online: http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/ (accessed on 30 May 2021).
- Wong, R.J.; Jain, M.K.; Therapondos, G.; Niu, B.; Kshirsagar, O.; Thamer, M. Antiviral Therapy Reduces Risk of Cirrhosis in Noncirrhotic HBV Patients Among 4 Urban Safety-Net Health Systems. Am. J. Gastroenterol. 2021. ahead of print. [Google Scholar] [CrossRef]
- Jia, J.; Shang, J.; Tang, H.; Jiang, J.; Ning, Q.; Dou, X.; Zhang, S.; Zhang, M.; Han, T.; Tan, D.; et al. Long-Term Outcomes in Chinese Patients with Chronic Hepatitis B Receiving Nucleoside/Nucleotide Analogue Therapy in Real-World Clinical Practice: 5-Year Results from the EVOLVE Study. Antivir. Ther. 2020, 25, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Chon, Y.E.; Seo, Y.S.; Lee, H.W.; Lee, H.A.; Kim, M.N.; Min, I.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; et al. A Multi-centre Study of Trends in Hepatitis B Virus-related Hepatocellular Carcinoma Risk over Time during Long-term Entecavir Therapy. J. Viral Hepat. 2020, 27, 1352–1358. [Google Scholar] [CrossRef]
- Yang, H.-I.; Yeh, M.-L.; Wong, G.L.; Peng, C.-Y.; Chen, C.-H.; Trinh, H.N.; Cheung, K.-S.; Xie, Q.; Su, T.-H.; Kozuka, R.; et al. Real-World Effectiveness From the Asia Pacific Rim Liver Consortium for HBV Risk Score for the Prediction of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Treated with Oral Antiviral Therapy. J. Infect. Dis 2020, 221, 389–399. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.K.; Park, S.Y.; Tak, W.Y.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Sinn, D.H.; Kim, S.U. The Efficacies of Entecavir and Tenofovir in Terms of Enhancing Prognosis after Curative Treatment of Hepatitis B Virus–Related Hepatocellular Carcinoma. Eur. J. Intern. Med. 2021, S0953620521000777. [Google Scholar] [CrossRef]
- Kim, M.P.; Yang, J.K.; Jun, B.G.; Kim, Y.D.; Cheon, G.J.; Jung, H.J.; Yoo, J.; Kim, S.G.; Kim, Y.S.; Jeong, S.W.; et al. Effect of Antiviral Therapy in Patients with Low HBV DNA Level on Transarterial Chemoembolization for Hepatocellular Carcinoma. J. Viral Hepat. 2021. ahead of print. [Google Scholar] [CrossRef]
- Xia, Z.; He, L.; Xiong, L.; Wen, T. The Comparison of Different Antiviral Therapies on the Prognosis of Hepatitis B Virus-Related Hepatocellular Carcinoma after Curative Treatments: A Network Meta-Analysis. Medicine 2020, 99, e20877. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Salomoni, E.; Arena, U.; Corti, G.; Montalto, P.; Bartalesi, F.; Marra, F.; Laffi, G.; Milani, S.; Zignego, A.L.; et al. Non-Invasive Assessment of Liver Fibrosis in Patients with HBV-Related Chronic Liver Disease Undergoing Antiviral Treatment: A Preliminary Study. Eur. J. Pharmacol. 2017, 806, 105–109. [Google Scholar] [CrossRef]
- Fukuda, W.; Hanyu, T.; Katayama, M.; Mizuki, S.; Okada, A.; Miyata, M.; Handa, Y.; Hayashi, M.; Koyama, Y.; Arii, K.; et al. Risk Stratification and Clinical Course of Hepatitis B Virus Reactivation in Rheumatoid Arthritis Patients with Resolved Infection: Final Report of a Multicenter Prospective Observational Study at Japanese Red Cross Hospital. Arthritis Res. Ther. 2019, 21, 255. [Google Scholar] [CrossRef]
- Mahroum, N.; Watad, A.; Tiosano, S.; Hejly, A.; Mahagna, H.; Waknin, R.; Comaneshter, D.; Cohen, A.D.; Amital, H. Chronic Hepatitis B Viral Infection among RA Patients—A Cross-Sectional Control Study. Clin. Rheumatol. 2019, 38, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Ditto, M.C.; Parisi, S.; Varisco, V.; Talotta, R.; Batticciotto, A.; Antivalle, M.; Gerardi, M.C.; Agosti, M.; Borrelli, R.; Fusaro, E.; et al. Prevalence of Hepatitis B Virus Infection and Risk of Reactivation in Rheumatic Population Undergoing Biological Therapy. Clin. Exp. Rheumatol. 2021, 39, 546–554. [Google Scholar]
- Canzoni, M.; Marignani, M.; Sorgi, M.L.; Begini, P.; Biondo, M.I.; Caporuscio, S.; Colonna, V.; Casa, F.D.; Conigliaro, P.; Marrese, C.; et al. Prevalence of Hepatitis B Virus Markers in Patients with Autoimmune Inflammatory Rheumatic Diseases in Italy. Microorganisms 2020, 8, 1792. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, M.; Atzeni, F.; Milazzo, L.; Quartuccio, L.; Scirè, C.; Gaeta, G.B.; Lapadula, G.; Armignacco, O.; Tavio, M.; Olivieri, I.; et al. Italian Consensus Guidelines for the Management of Hepatitis B Virus Infections in Patients with Rheumatoid Arthritis. Jt. Bone Spine 2017, 84, 525–530. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hashemi, N.; Kim, S.C.; Yang, Y.-H.K.; Yoshida, K.; Tedeschi, S.; Desai, R.; Solomon, D.H. Practice Pattern of Hepatitis B Testing in Rheumatoid Arthritis Patients: A Cross-National Comparison Between the US and Taiwan. Arthritis Care Res. 2018, 70, 30–38. [Google Scholar] [CrossRef]
- Fujita, M.; Sugiyama, M.; Sato, Y.; Nagashima, K.; Takahashi, S.; Mizokami, M.; Hata, A. Hepatitis B Virus Reactivation in Patients with Rheumatoid Arthritis: Analysis of the National Database of Japan. J. Viral Hepat. 2018, 25, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H. Reactivation of Hepatitis B. Hepatology 2009, 49, S156–S165. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lin, J.-Z.; Mo, Y.-Q.; Ma, J.-D.; Li, Q.-H.; Wang, X.-Y.; Yang, Z.-H.; Yan, T.; Zheng, D.-H.; Dai, L. Deleterious Role of Hepatitis B Virus Infection in Therapeutic Response among Patients with Rheumatoid Arthritis in a Clinical Practice Setting: A Case-Control Study. Arthritis Res. Ther. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Winthrop, K.; Takeuchi, T.; Hsieh, T.-Y.; Chen, Y.-M.; Smolen, J.S.; Burmester, G.; Walls, C.; Wu, W.-S.; Dickson, C.; et al. Evaluation of Hepatitis B Virus in Clinical Trials of Baricitinib in Rheumatoid Arthritis. RMD Open 2020, 6, e001095. [Google Scholar] [CrossRef]
- Koutsianas, C.; Thomas, K.; Vassilopoulos, D. Reactivation of Hepatitis B Virus Infection in Rheumatic Diseases: Risk and Management Considerations. Ther. Adv. Musculoskelet Dis. 2020, 12, 1759720X2091264. [Google Scholar] [CrossRef]
- Reddy, K.R.; Beavers, K.L.; Hammond, S.P.; Lim, J.K.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Guideline on the Prevention and Treatment of Hepatitis B Virus Reactivation during Immunosuppressive Drug Therapy. Gastroenterology 2015, 148, 215–219. [Google Scholar] [CrossRef]
- Kim, H.-O. Development of JAK Inhibitors for the Treatment of Immune-Mediated Diseases: Kinase-Targeted Inhibitors and Pseudokinase-Targeted Inhibitors. Arch. Pharm. Res. 2020, 43, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The Anti-Inflammatory and Immunosuppressive Effects of Glucocorticoids, Recent Developments and Mechanistic Insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Padjen, I.; Reihl Crnogaj, M.; Anić, B. Conventional Disease-Modifying Agents in Rheumatoid Arthritis–a Review of Their Current Use and Role in Treatment Algorithms. Reumatologia 2020, 58, 390–400. [Google Scholar] [CrossRef]
- Schwaneck, E.C.; Krone, M.; Kreissl-Kemmer, S.; Weißbrich, B.; Weiss, J.; Tony, H.-P.; Gadeholt, O.; Schmalzing, M.; Geier, A. Management of Anti-HBc-Positive Patients with Rheumatic Diseases Treated with Disease-Modifying Antirheumatic Drugs—a Single-Center Analysis of 2054 Patients. Clin. Rheumatol. 2018, 37, 2963–2970. [Google Scholar] [CrossRef]
- Kuo, M.H.; Tseng, C.-W.; Lee, C.-H.; Tung, C.-H.; Tseng, K.-C.; Lai, N.-S. Moderate Risk of Hepatitis B Virus Reactivation in HBsAg−/HBcAb+ Carriers Receiving Rituximab for Rheumatoid Arthritis. Sci. Rep. 2020, 10, 2456. [Google Scholar] [CrossRef]
- Maloney, D.G.; Smith, B.; Rose, A. Rituximab: Mechanism of Action and Resistance. Semin. Oncol. 2002, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Randall, K.L. Rituximab in Autoimmune Diseases. Aust. Prescr. 2016, 39, 131–134. [Google Scholar] [CrossRef]
- Meroni, P.-L.; Valesini, G. Tumour Necrosis Factor α Antagonists in the Treatment of Rheumatoid Arthritis: An Immunological Perspective. BioDrugs 2014, 28 (Suppl. S1), S5–S13. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Huang, W.; Chen, Y.; Hsieh, T.; Yang, S.; Lan, J.; Chen, D. Reactivation of Hepatitis B Virus Infection Following Rituximab Treatment in HBsAg-negative, HBcAb-positive Rheumatoid Arthritis Patients: A Long-term, Real-world Observation. Int. J. Rheum. Dis. 2019, 1145–1151. [Google Scholar] [CrossRef]
- Varisco, V.; Viganò, M.; Batticciotto, A.; Lampertico, P.; Marchesoni, A.; Gibertini, P.; Pellerito, R.; Rovera, G.; Caporali, R.; Todoerti, M.; et al. Low Risk of Hepatitis B Virus Reactivation in HBsAg-Negative/Anti-HBc–Positive Carriers Receiving Rituximab for Rheumatoid Arthritis: A Retrospective Multicenter Italian Study. J. Rheumatol. 2016, 43, 869–874. [Google Scholar] [CrossRef]
- Carlino, G.; Fornaro, M.; Santo, L.; Bucci, R.; Semeraro, A.; Quarta, L.; D’Onofrio, F.; Marsico, A.; Zuccaro, C.; Falappone, P.C.; et al. Occult HBV Infection May Negatively Impact on Drug Survival in Patients with Rheumatoid Arthritis on Treatment with a First Biologic Drug. An Appraisal from the Biologic Apulian Registry (BIOPURE). Reumatismo 2019, 71, 24–30. [Google Scholar] [CrossRef]
- Xu, C.; Rafique, A.; Potocky, T.; Paccaly, A.; Nolain, P.; Lu, Q.; Iglesias-Rodriguez, M.; St John, G.; Nivens, M.C.; Kanamaluru, V.; et al. Differential Binding of Sarilumab and Tocilizumab to IL-6Rα and Effects of Receptor Occupancy on Clinical Parameters. J. Clin. Pharmacol. 2021, 61, 714–724. [Google Scholar] [CrossRef]
- Narváez, J.; Oton, T.; LLuch, J.; Mora-Limiñana, M.; Nolla, J.M.; Loza, E. Response to Interleukin-6 Receptor Antagonists in Patients with Rheumatoid Arthritis Is Independent of the Number of Prior Used TNF Inhibitors: A Systematic Review and Metaanalysis. Jt. Bone Spine 2021, 88, 105112. [Google Scholar] [CrossRef]
- For the Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG); Brunner, H.I.; Wong, R.; Nys, M.; Kou, T.D.; Dominique, A.; Martini, A.; Lovell, D.J.; Ruperto, N. Abatacept: A Review of the Treatment of Polyarticular-Course Juvenile Idiopathic Arthritis. Paediatr. Drugs 2020, 22, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, R.; Janowska, I.; Smulski, C.R.; Frede, N.; Henneberger, N.; Walter, L.; Schleyer, M.-T.; Hüppe, J.M.; Staniek, J.; Salzer, U.; et al. Abatacept Modulates CD80 and CD86 Expression and Memory Formation in Human B-Cells. J. Autoimmun. 2019, 101, 145–152. [Google Scholar] [CrossRef]
- Ahn, S.S.; Jung, S.M.; Song, J.J.; Park, Y.-B.; Park, J.Y.; Lee, S.-W. Safety of Tocilizumab in Rheumatoid Arthritis Patients with Resolved Hepatitis B Virus Infection: Data from Real-World Experience. Yonsei Med. J. 2018, 59, 452–456. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Eguchi, K.; Nagao, N.; Tsuji, S.; Aramaki, T.; Terada, K.; Iwatsu, S.; Tokimura, I.; Kamo, Y.; Oda, H.; et al. Hepatitis B Virus Reactivation in Patients with Rheumatoid Arthritis: A Single-Center Study. Mod. Rheumatol. 2018, 28, 808–813. [Google Scholar] [CrossRef]
- Fukuda, W.; Hanyu, T.; Katayama, M.; Mizuki, S.; Okada, A.; Miyata, M.; Handa, Y.; Hayashi, M.; Koyama, Y.; Arii, K.; et al. Incidence of Hepatitis B Virus Reactivation in Patients with Resolved Infection on Immunosuppressive Therapy for Rheumatic Disease: A Multicentre, Prospective, Observational Study in Japan. Ann. Rheum. Dis. 2017, 76, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Long, L.; Zou, K. Antiviral Prophylaxis for Preventing Reactivation of Hepatitis B Virus in Rheumatic Patients: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2018, 37, 3201–3214. [Google Scholar] [CrossRef] [PubMed]
- Moghoofei, M.; Mostafaei, S.; Ashraf-Ganjouei, A.; Kavosi, H.; Mahmoudi, M. HBV Reactivation in Rheumatic Diseases Patients under Therapy: A Meta-Analysis. Microb. Pathog. 2018, 114, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bartalesi, F.; Scirè, C.; Requena-Méndez, A.; Abad, M.A.; Buonfrate, D.; Caporali, R.; Conti, F.; Diaz-Gonzalez, F.; Fernández-Espartero, C.; Martinez-Fernandez, C.; et al. Recommendations for Infectious Disease Screening in Migrants to Western Europe with Inflammatory Arthropathies before Starting Biologic Agents. Results from a Multidisciplinary Task Force of Four European Societies (SIR, SER, SIMET, SEMTSI) Facing the Largest Impact of the Flow of Migrants Today. Clin. Exp. Rheumatol. 2017, 35, 752–765. [Google Scholar]
- Chen, M.-H.; Chen, M.-H.; Liu, C.-Y.; Tsai, C.-Y.; Huang, D.-F.; Lin, H.-Y.; Lee, M.-H.; Huang, Y.-H. Hepatitis B Virus Reactivation in Rheumatoid Arthritis Patients Undergoing Biologics Treatment. J. Infect. Dis. 2016, 215, 566–573. [Google Scholar] [CrossRef]
- Chiu, Y.-M.; Lai, M.-S.; Chan, K.A. Assessing Risk of Liver Enzyme Elevation in Patients with Immune-Mediated Diseases and Different Hepatitis B Virus Serostatus Receiving Anti-TNF Agents: A Nested Case-Control Study. Arthritis Res. Ther. 2017, 19, 214. [Google Scholar] [CrossRef][Green Version]
- Silva, L.C.; Ortigosa, L.C.; Benard, G. Anti-TNF-α Agents in the Treatment of Immune-Mediated Inflammatory Diseases: Mechanisms of Action and Pitfalls. Immunotherapy 2010, 2, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Ware, C.F. Tumor Necrosis Factor and Anti-Tumor Necrosis Factor Therapies. J. Rheumatol. Suppl. 2010, 85, 27–39. [Google Scholar] [CrossRef][Green Version]
- Chen, L.-F.; Mo, Y.-Q.; Jing, J.; Ma, J.-D.; Zheng, D.-H.; Dai, L. Short-Course Tocilizumab Increases Risk of Hepatitis B Virus Reactivation in Patients with Rheumatoid Arthritis: A Prospective Clinical Observation. Int J. Rheum. Dis. 2017, 20, 859–869. [Google Scholar] [CrossRef]
- Padovan, M.; Filippini, M.; Tincani, A.; Lanciano, E.; Bruschi, E.; Epis, O.; Garau, P.; Mathieu, A.; Celletti, E.; Giani, L.; et al. Safety of Abatacept in Rheumatoid Arthritis with Serologic Evidence of Past or Present Hepatitis B Virus Infection. Arthritis Care Res. 2016, 68, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, D.; Delicha, E.M.; Settas, L.; Andrianakos, A.; Aslanidis, S.; Boura, P.; Katsounaros, M.; Athanassiou, P.; Tempos, K.; Skarantavos, G.; et al. Safety Profile of Repeated Rituximab Cycles in Unselected Rheumatoid Arthritis Patients: A Long-Term, Prospective Real-Life Study. Clin. Exp. Rheumatol. 2016, 34, 893–900. [Google Scholar] [PubMed]
| Studies, Year | Study Design | Main Inclusion/Exclusion Criteria | No of Patients/Enrollment | Interventions/Treatments | Reactivation of Hepatitis B Virus |
|---|---|---|---|---|---|
| Harigai et al., 2020 [22] | Clinical trials | Patients were excluded if they had: 1) HBsAg+, 2)anti-HBs−/anti-HBc+ (in Japan, patients could enrol if HBV DNA−) or 3) anti-HBs+ and HBV DNA+. | 2890 patients Of whom 215 were HBcAb positive | Baricitinib +/− csDMARDs including methotrexate (MTX), or previous treatment with active comparators including MTX or adalimumab + MTX | 4/215 patients |
| Fukuda et al., 2019 [12] | Multicenter prospective observational study | Patients with negative HBsAg and positive anti-HBs and/or anti-HBc were enrolled, all patients were HBV-DNA negative at entry | 1127 patients | Corticosteroids, bDMARDs, and/or csDMARDs | 57/1127 patients |
| Schwaneck et al., 2018 [28] | Retrospective study | Patients on immunosuppressive therapy were evaluated for HBV screening results at any time during their treatment course | 84 patients were anti-HBc positive and HbsAg negative | Corticosteroids, bDMARDs, and/or csDMARDs | 8/84 patients |
| Kuo et al., 2020 [29] | Retrospective study | Patients who underwent rituximab (RTX) therapy for rheumatoid arthritis (RA) who tested HBsAg negative/anti-HBc positive | 50 patients were anti-HBc positive and HbsAg negative | Rituximab | 4/50 patients |
| Chen et al., 2019 [33] | Retrospective study | Patients who underwent rituximab (RTX) therapy for rheumatoid arthritis (RA) who tested HBsAg negative/anti-HBc positive | 103 patients were HBsAg negative and anti-HBc positive | Rituximab | 5/103 patients |
| Varisco et al., 2016 [34] | Retrospective multicenter study | HBsAg-negative/anti-HBc-positive patients with rheumatoid arthritis (RA) undergoing RTX | 33 patients | Rituximab combined with disease-modifying antirheumatic drugs (DMARD) | 0/33 patients |
| Ahn et al., 2018 [40] | Retrospective study | HBsAg negative and antibody anti-HBc positive | 15 patients | Tocilizumab | None of the patients experienced reactivation of HBV |
| Matsuzaki et al., 2018 [41] | Retrospective study | 1351 patients with RA were screened for HBV | 50/1351 were determined to be HBV carriers and 360 patients had resolved infections | conventional synthetic DMARDs (csDMARDs: bucillamine, cyclosporin, iguratimod, leflunomide, methotrexate, mizoribine, salazosulfapyridine, tacrolimus), bDMARDs (abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab), and glucocorticoids | 6/360 with resolved infections |
| Fukuda et al., 2017 [42] | Multicenter, observational, prospective study over 2 years | 1330 patients being treatment tested for HBsAg, HBsAb, and HBcAb | 1193 rheumatoid arthritis patients, of whom 1123 with resolved HBV infection; 137 rheumatic disease of whom 132 with resolved HBV infection | corticosteroids (≥5 mg of prednisolone or its equivalent dose); immunosuppressive synthetic DMARDs, namely methotrexate, leflunomide, tacrolimus, mizoribine or its equivalent and/or biological DMARDs, namely infliximab, etanercept, adalimumab, tocilizumab, abatacept, golimumab, and certolizumab pegol | Frequency of HBV reactivation was calculated to be 1.93/100 person-years |
| Studies, Year | Study Design | Main Inclusion/Exclusion Criteria | No of Patients/Enrollment | Interventions/Treatments | Reactivation of Hepatitis B Virus |
|---|---|---|---|---|---|
| Chen et al., 2016 [46] | Retrospective study | Patients with rheumatoid arthritis who tested positive for HBsAg and who were not receiving anti-HBV prophylaxis were enrolled. | 36 patients who tested positive for HBsAg received biotechnological treatments without prophylaxis | glucocorticoids, synthetics drugs, and bDMARDS | 30/123 patients |
| Chen et al., 2017 [50] | Prospective observational study | Patients with rheumatoid arthritis with inadequate response to csDMARDs | 5 patients with HBsAg who did not receive prophylaxis | Tocilizumab | 3/5 patients |
| Padovan et al., 2016 [51] | Multicenter retrospective study | Patients with rheumatoid arthritis and chronic HBV infection who were treated with abatacept | 38 inactive carriers received abataceptd without prophylaxis | Abatacept | 0/38 patients |
| Vassilopoulos et al., 2016 [52] | Prospective | Patients with moderate to severe rheumatoid arthritis with inadequate response or intolerance to at least one anti-TNF | 234 patients treated with rituximab | Rituximab | 2 patients developed HBV reactivation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasi, C.; Tiengo, G.; Sadalla, S.; Zignego, A.L. Treatment or Prophylaxis against Hepatitis B Virus Infection in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: An Update. J. Clin. Med. 2021, 10, 2564. https://doi.org/10.3390/jcm10122564
Stasi C, Tiengo G, Sadalla S, Zignego AL. Treatment or Prophylaxis against Hepatitis B Virus Infection in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: An Update. Journal of Clinical Medicine. 2021; 10(12):2564. https://doi.org/10.3390/jcm10122564
Chicago/Turabian StyleStasi, Cristina, Giacomo Tiengo, Sinan Sadalla, and Anna Linda Zignego. 2021. "Treatment or Prophylaxis against Hepatitis B Virus Infection in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: An Update" Journal of Clinical Medicine 10, no. 12: 2564. https://doi.org/10.3390/jcm10122564
APA StyleStasi, C., Tiengo, G., Sadalla, S., & Zignego, A. L. (2021). Treatment or Prophylaxis against Hepatitis B Virus Infection in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: An Update. Journal of Clinical Medicine, 10(12), 2564. https://doi.org/10.3390/jcm10122564







