Treatment of Fetal Arrhythmias
Abstract
1. Introduction
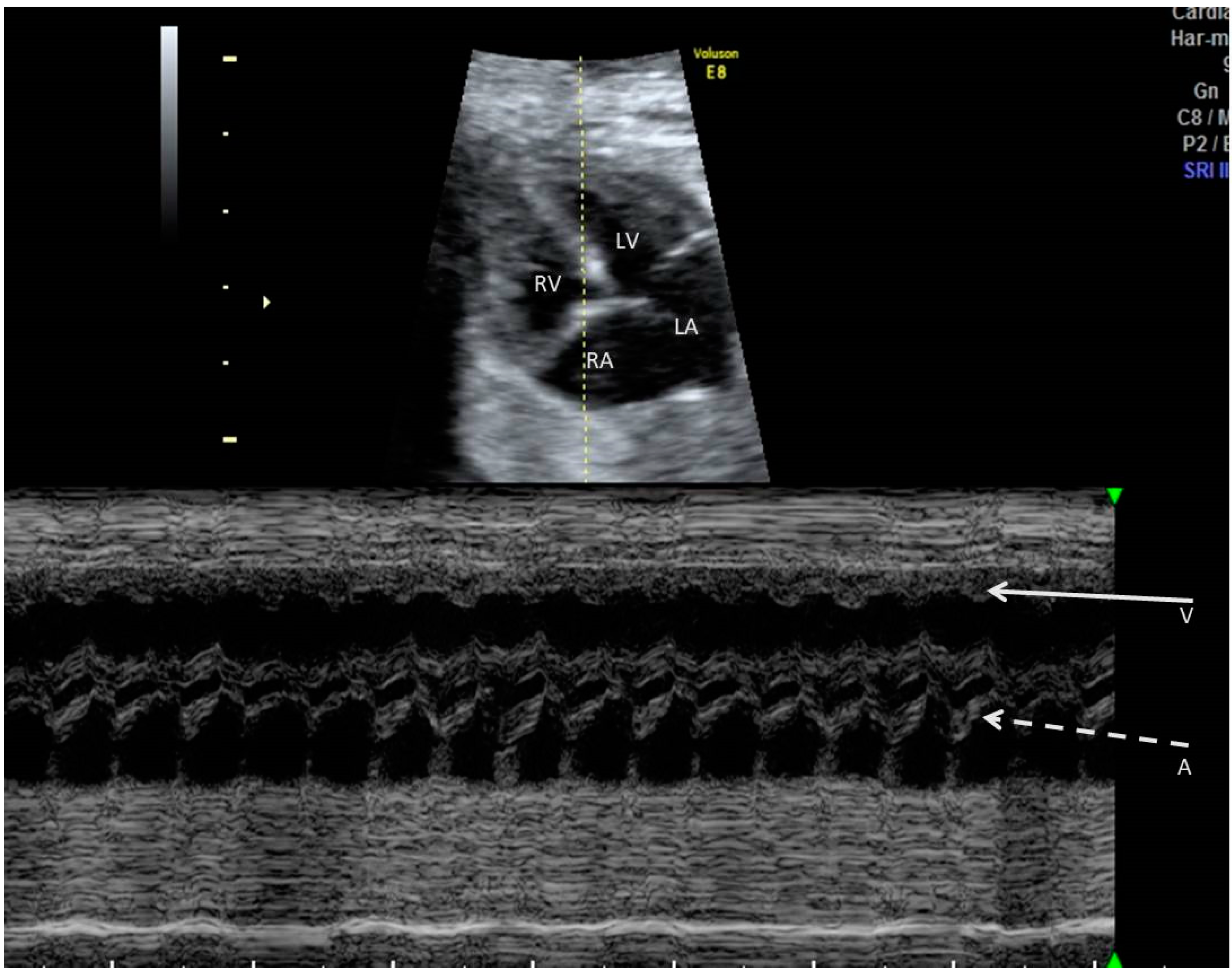
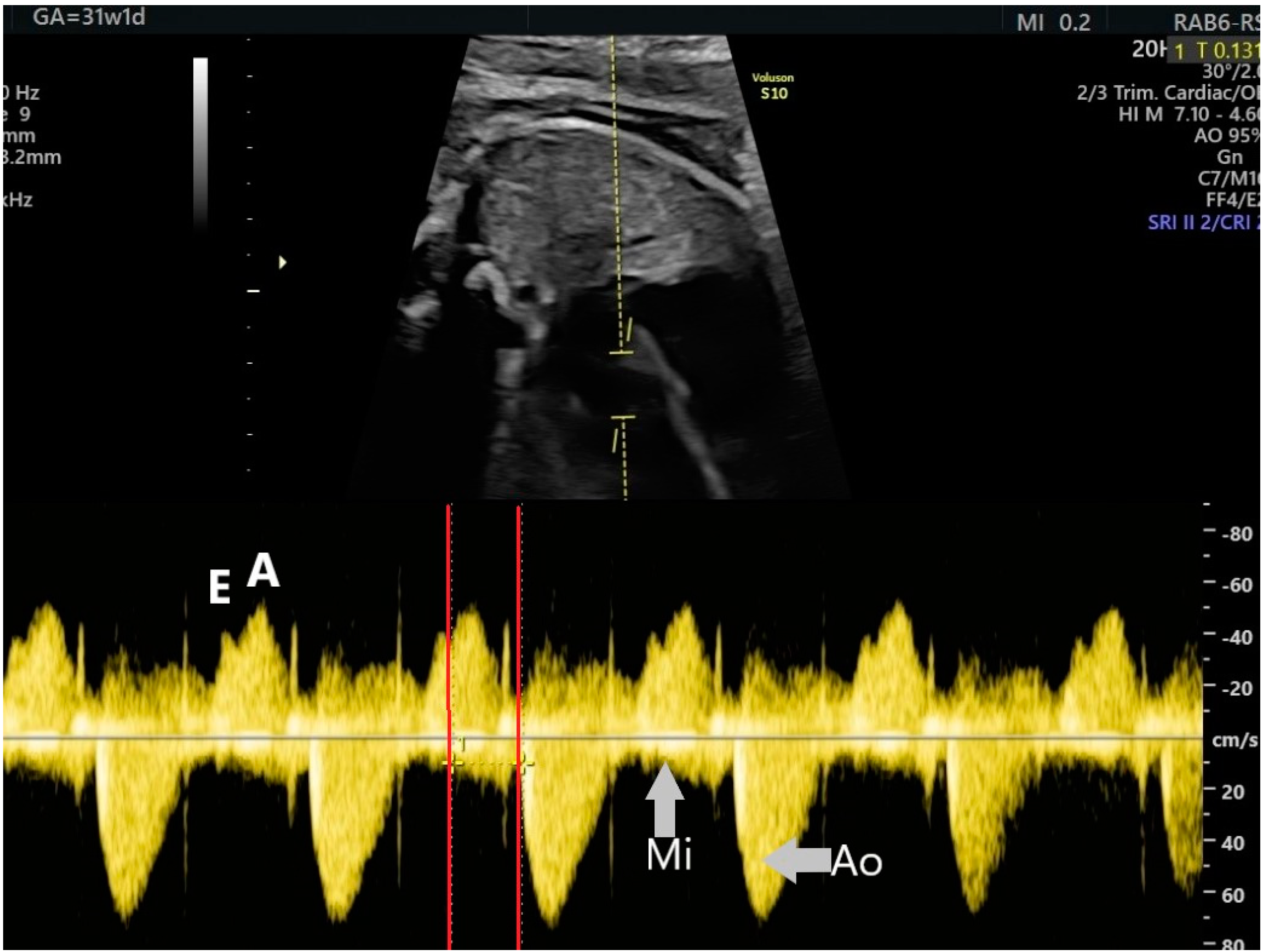
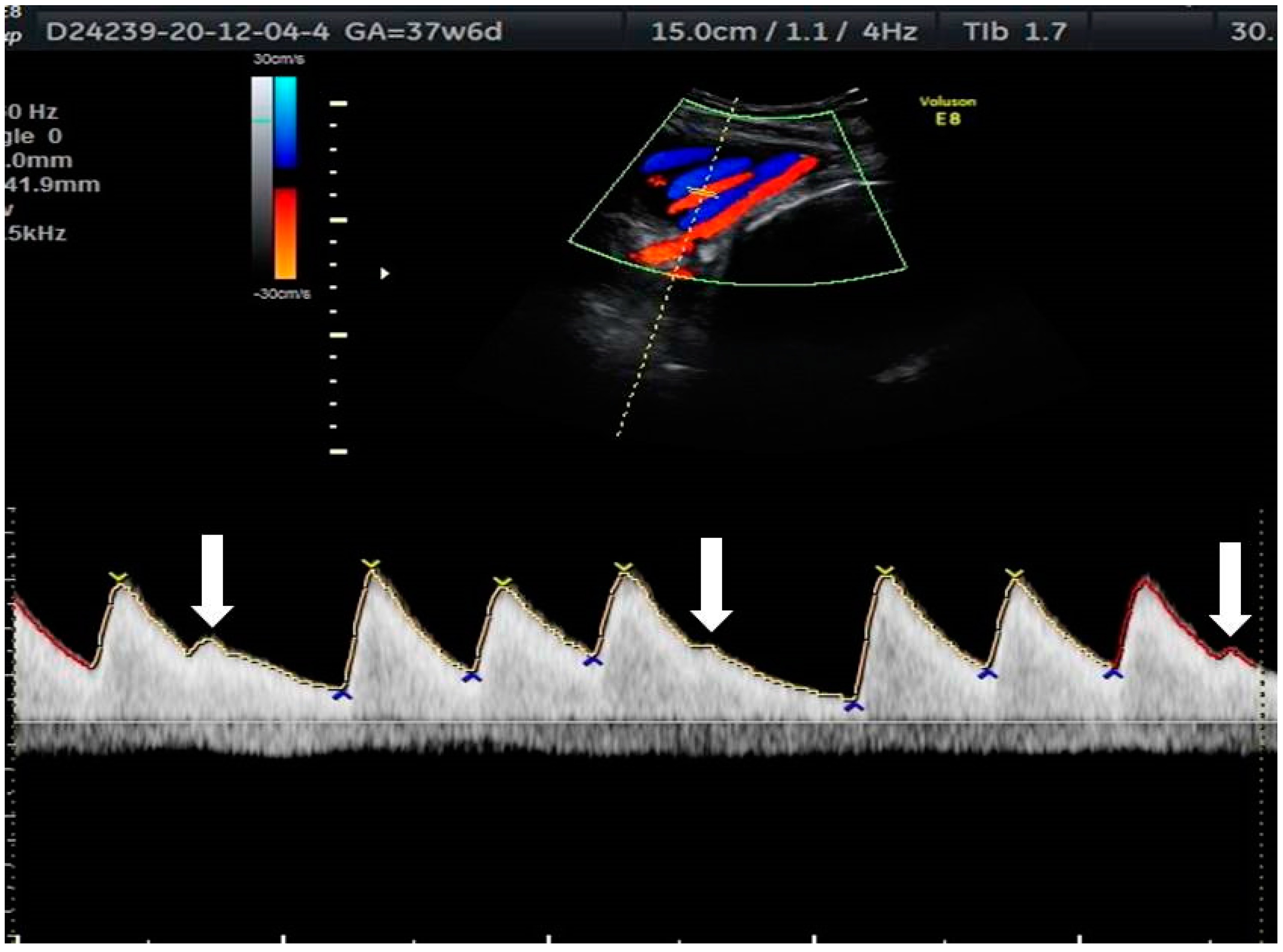
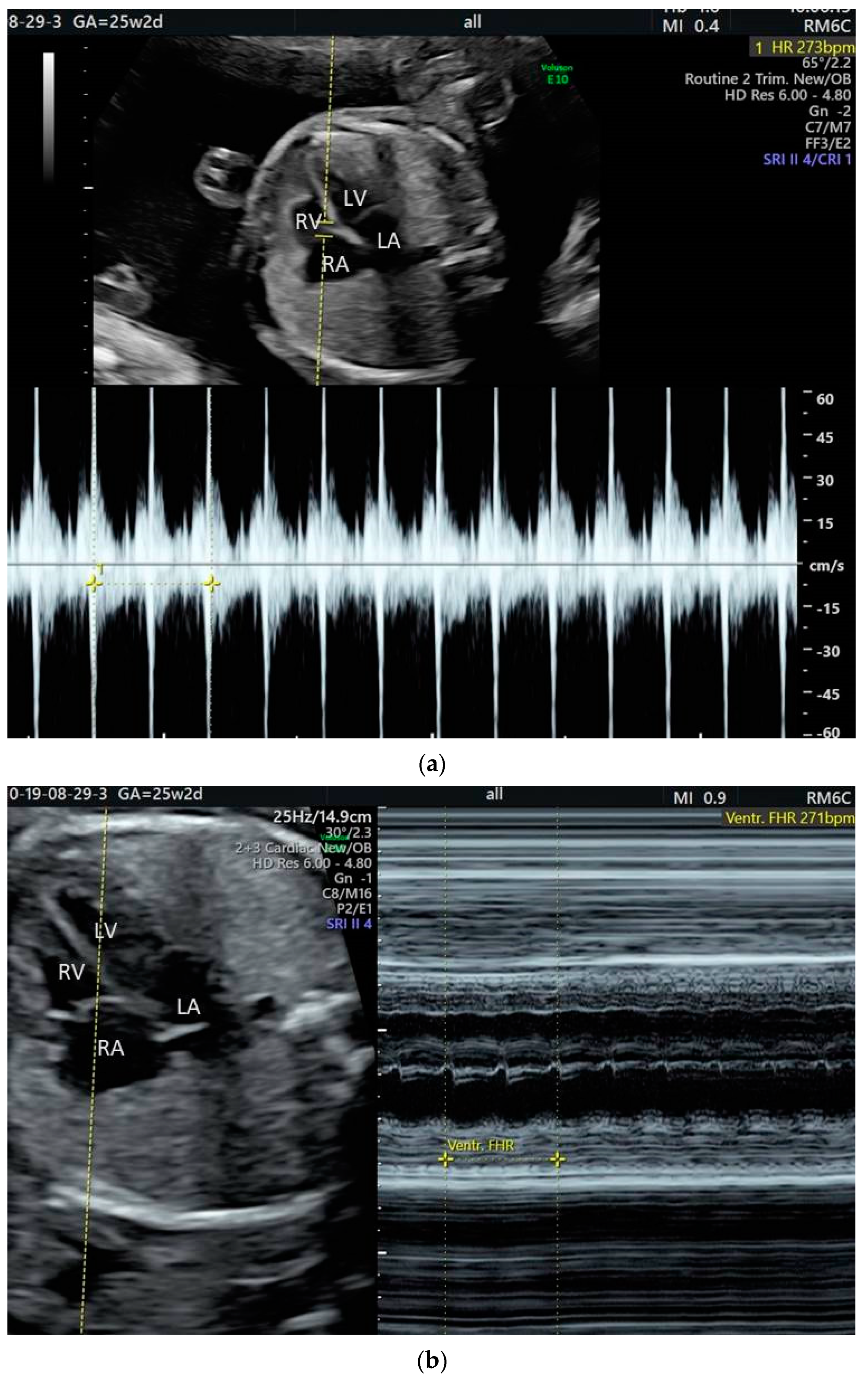
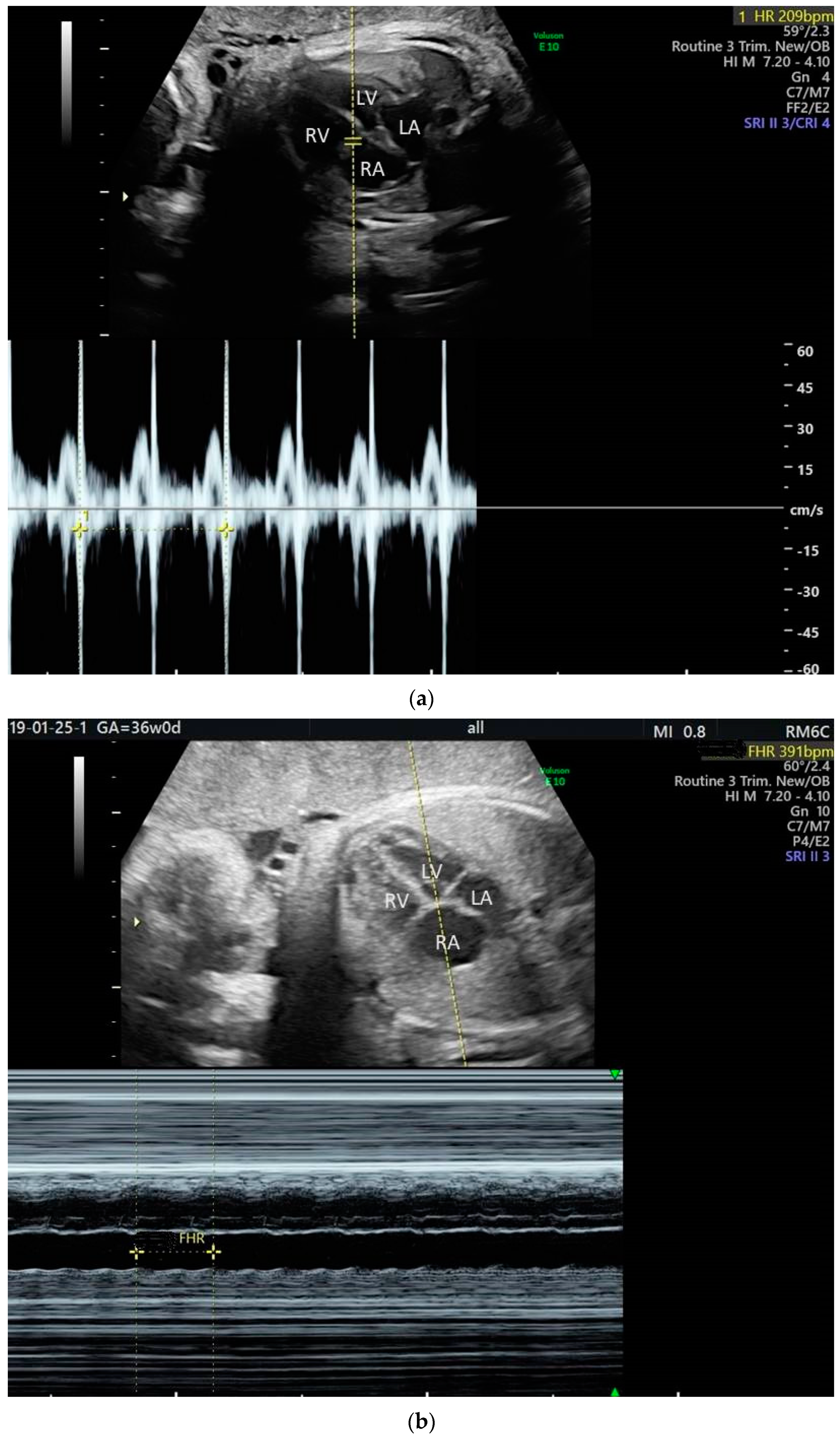
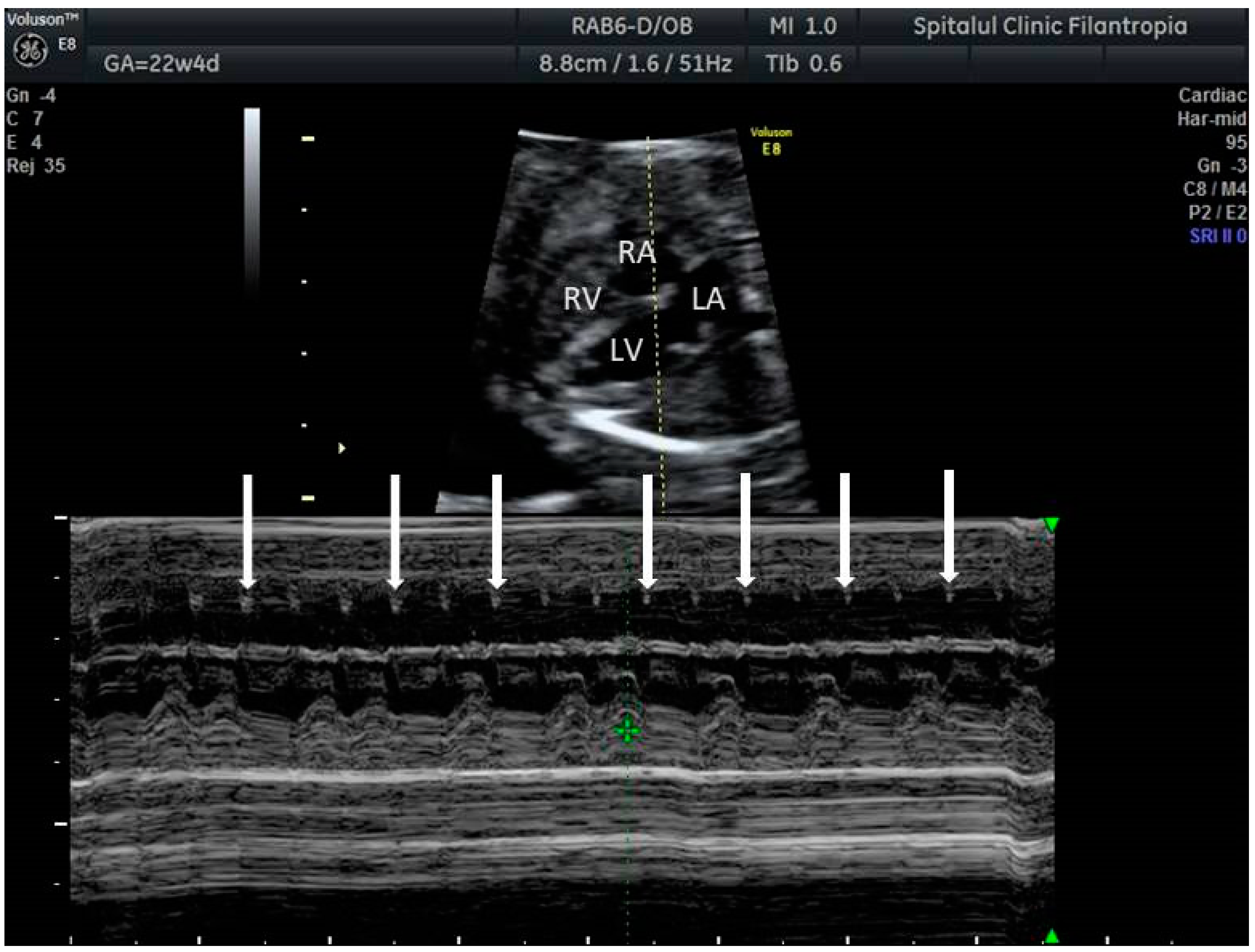
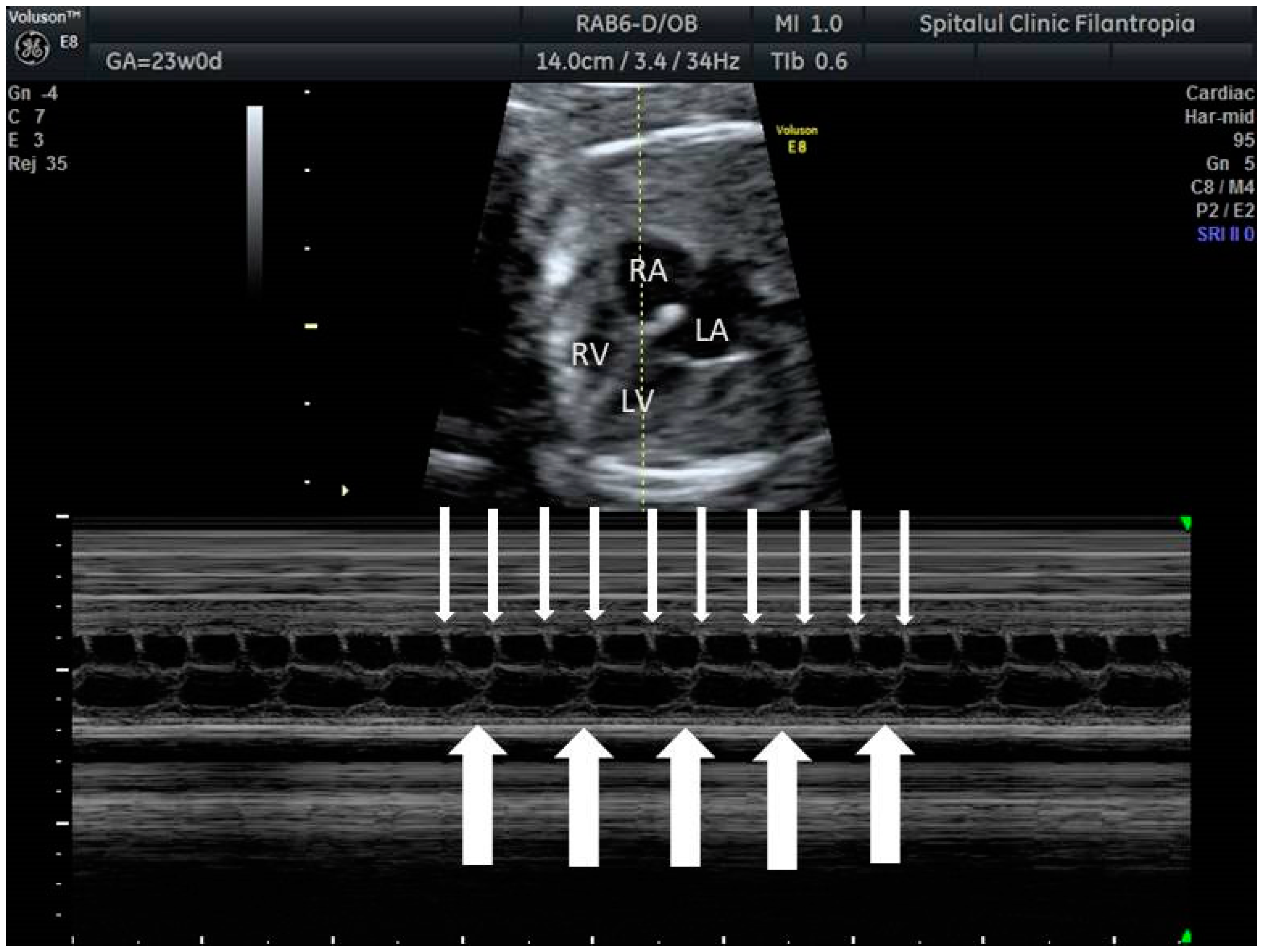
2. The Use of Ultrasound to Assess Fetal Heart Rhythm
3. Irregular Rhythm with Normal HR
4. Tachyarrhythmias
4.1. Sinus Tachycardia
4.2. Supraventricular Tachyarrhythmia
- -
- Short ‘ventriculoatrial’ tachycardia; it usually involves re-entry mechanisms including atrioventricular re-entry (AVRT) using a bypass tract or atrioventricular nodal re-entry (AVNRT) [2];
- -
4.3. Ventricular Tachycardia
5. Bradyarrhythmias
5.1. Sinus Bradycardia
5.2. Long QT Syndrome
5.3. Blocked Ectopic Beats
5.4. Heart Block
6. Discussion
6.1. The Fetal Medicine Specialist Point of View
6.2. The Cardiologist Point of View
Author Contributions
Funding
Conflicts of Interest
Appendix A
| escape atrial beats | heart beat originating in an ectopic focus in the atrial myocardium, after a long sinus pause; the sinus pause can be caused by sinus node exit block, sinus node arrest and so on; the escape rhythm is slower than the sinus rhythm |
| premature atrial complexes/beats (PACs) | early activation of the atrial myocardium by an impulse coming from an ectopic focus within the atrial myocardium, not from the sinus node; the interval between the last sinus beat and the ectopic beat is shorter than the interval between two sinus beats |
| atrial bigeminy/trigeminy | a rhythm in which every other beat (bigeminy) or every third beat (trigeminy) is an ectopic beat (PAC) |
| supraventricular tachycardia (SVT) | an abnormally fast heart rhythm originating above the ventricles; in a broader sense, it includes atrial flutter and atrial fibrillation |
| ectopic atrial tachycardia | sometimes referred to as atrial tachycardia or primary atrial tachycardia, it is a rhythm generated by an ectopic atrial focus, which is faster than the normal heart rate (180 bpm in fetuses) |
| reentry tachycardia | tachyarrhythmia caused by the repetitive propagation of an excitatory wave through a circular path, returning to its site of origin to reactivate that site |
| reciprocating tachycardia | reentry tachycardia |
| paroxysmal tachycardia | also called sporadic, it is a self-limited reentry tachycardias initated by ectopic beats and terminated by functional block |
| paroxysmal SVT | also called sporadic, those intermittent SVTs with abrupt onset and offset and a regular ventricular response, mostly due to reentry, eg AVRT |
| incessant tachycardia | nonparoxysmal tachycardia, most often caused by enhanced/abnormal automaticity or triggered activity |
| sustained tachycardia | arrhythmia present for more than 50% of the examination time |
| intermittent tachycardia | arrhythmia present for less than 50% of the examination time |
| orthodromic tachycardia | SVT in which the reentrant circuit conducts impulses down (antegradely) through the AV node and the His–Purkinje system to the ventricles, and then up (retrogradely) through the accessory pathway (narrow complex tachycardia) |
| antidromic tachycardia | rare SVT in which the reentrant circuit conducts impulses down (antegradely) through the accesory pathway to the ventricles, and then up (retrogradely) through the AV node (wide complex tachycardia) |
| short VA tachycardia | If the RP interval is less than one-half of the RR interval, the tachycardia is considered a short RP (short VA) tachycardia. If the P wave is retrograde, AV reentry with the common slow—fast conduction type is most probable. The slow—fast pattern gives rise to retrograde P waves that is very close or even masked by the QRS complex. If the P wave is morphologically abnormal, atrial (ectopic) tachycardia with abnormal AV nodal conduction is most probable. With specific referral to reentry tachycardia, it is a form of tachycardia in which the conduction from the ventricles back to the atria is by a fast accessory pathway. |
| permanent junctional reciprocating tachycardia, PJRT | orthodromic AVRT with a slowly conducting accessory pathway, resulting in a long VA incessant tachycardia |
| heart block | delayed, intermittent, or absent conduction between the atria and the ventricles |
| first-degree heart block | prolonged PR interval (>0.14 s in fetuses) |
| second-degree heart block | an occasional non-conducted P wave, resulting in a long RR interval Mobitz I (Wenckebach) type: progressive slowing of the conduction of each subsequent impulse in the AV node, until the node fails to conduct one impulse Mobitz II type: unpredictable failure of the His–Purkinje pathway to conduct some of the impulses from the atria to the ventricles; unlike the Mobitz type I, there is no change in the PR interval prior to the non-conducted P wave |
| high-degree block | successive non-conducted P waves |
| complete (third-degree) heart block | also referred to as AV dissociation, absent conduction beteween the atria and the ventricles |
References
- Gembruch, U. Fetal Tachyarrhythmia. In Fetal Cardiology, 3rd ed.; Yagel, S., Silverman, N.H., Gembruch, U., Eds.; Taylor and Francis, LLC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Cuneo, B.F. Fetal Bradycardia. In Fetal Cardiology, 3rd ed.; Yagel, S., Silverman, N.H., Gembruch, U., Eds.; Taylor and Francis, LLC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Srinivasan, S.; Strasburger, J. Overview of fetal arrhythmias. Curr. Opin. Pediatr. 2008, 20, 522–531. [Google Scholar] [CrossRef]
- Abuhamad, A.; Chaoui, R. Fetal Arrhythmias. In A Practical Guide to Fetal Echocardiography Normal and Abnormal Hearts, 3rd ed.; Abuhamad, A., Chaoui, R., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 547–563. [Google Scholar]
- Strasburger, J.F.; Cheulkar, B.; Wichman, H.J. Perinatal arrhythmias: Diagnosis and management. Clin. Perinatol. 2007, 34, 627–652. [Google Scholar] [CrossRef]
- Sharland, G. Fetal Cardiology Simplified: A Practical Manual; TFM Publishing Ltd.: Harley, UK, 2013; Volume 10. [Google Scholar]
- Kleinman, C.S.; Nehgme, R.A. Cardiac Arrhythmias in the Human Fetus. Pediatr. Cardiol. 2004, 25, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Zidere, V.; Vigneswaran, T.V.; Syngelaki, A.; Charakida, M.; Allan, L.D.; Nicolaides, K.H.; Simpson, J.M.; Akolekar, R. Reference Ranges for the Pulsed wave Doppler of the Fetal Cardiac Inflow and Outflow Tracts from 13 to 36 Weeks Gestation. J. Am. Soc. Echocardiogr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Nii, M. Fetal brady- and tachyarrhythmias: New and accepted diagnostic and treatment methods. Semin. Fetal Neonatal Med. 2005, 10, 504–514. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Strasburger, J.F.; Cuneo, B.F.; Wakai, R.T. Diagnosis and treatment of fetal arrhythmia. Am. J. Perinatol. 2014, 7, 617–628. [Google Scholar] [CrossRef]
- Wakai, R.T.; Strasburger, J.F.; Li, Z.; Deal, B.J.; Gotteiner, N.L. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation 2003, 107, 307–312. [Google Scholar] [CrossRef]
- Maeno, Y.; Hirose, A.; Kanbe, T.; Hori, D. Fetal arrhythmia: Prenatal diagnosis and perinatal management. J. Obstet. Gynaecol. Res. 2009, 35, 623–629. [Google Scholar] [CrossRef]
- Bravo-Valenzuela, N.J.; Rocha, L.A.; Machado Nardozza, L.M.; Araujo Júnior, E. Fetal cardiac arrhythmias: Current evidence. Ann. Pediatr. Cardiol. 2018, 11, 148–163. [Google Scholar]
- Cavoretto, P.I.; Seidenari, A.; Amodeo, S.; Della Gatta, A.N.; Nale, R.; Ismail, Y.S.; Candiani, M.; Farina, A. Quantification of Posterior Risk Related to Intrapartum FIGO 2015 Criteria for Cardiotocography in the Second Stage of Labor. Fetal Diagn. Ther. 2021, 48, 149–157. [Google Scholar] [CrossRef]
- Popescu, M.R.; Dudu, A.; Jurcut, C.; Ciobanu, A.M.; Zagrean, A.-M.; Panaitescu, A.M. A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review. Diagnostics 2020, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.N.; Zhou, L.; Huang, X.; Norekyan, V.; Bar-Cohen, Y.; Chmait, R.H.; Loeb, G.E. Design and Testing of a Transcutaneous RF Recharging System for a Fetal Micropacemaker. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 336–346. [Google Scholar] [CrossRef]
- Allan, L.D.; Anderson, R.H.; Sullivan, I.D.; Campbell, S.; Holt, D.W.; Tynan, M. Evaluation of fetal arrhythmias by echocardiography. Br. Heart J. 1983, 50, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.; Fouron, J.C.; Fournier, A.; Van Doesburg, N.; Drblik, S.P.; Proulx, F. Ventriculo-atrial time interval measured on M mode echocardiography: A determining element in diagnosis, treatment, and prognosis of fetal supraventricular tachycardia. Heart 1998, 79, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Fouron, J.C.; Fournier, A.; Proulx, F.; Lamarche, J.; Bigras, J.L.; Boutin, C.; Brassard, M.; Gamache, S. Management of fetal tachyarrhythmia based on superior vena cava/aorta Doppler flow recordings. Heart 2003, 89, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Prefumo, F.; Ciardelli, V.; Sairam, S.; Bhide, A.; Shinebourne, E.A. Evaluation of fetal arrhythmias from simultaneous pulsed wave Doppler in pulmonary artery and vein. Heart 2007, 93, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Deal, B.J.; Strasburger, J.F.; Benson, D.W., Jr. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am. J. Cardiol. 1992, 69, 1028–1032. [Google Scholar] [CrossRef]
- Krapp, M.; Kohl, T.; Simpson, J.M.; Sharland, G.K.; Katalinic, A.; Gembruch, U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart 2003, 89, 913–917. [Google Scholar] [CrossRef]
- Cuneo, B.F. Outcome of fetal cardiac defects. Curr. Opin. Pediatr. 2006, 18, 490–496. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Strasburger, J.F.; Niksch, A.; Ovadia, M.; Wakai, R.T. An expanded phenotype of maternal SSA/SSB antibody-associated fetal cardiac disease. J. Matern. Fetal Neonatal Med. 2009, 3, 233–238. [Google Scholar] [CrossRef]
- Texter, K.M.; Kertesz, N.J.; Friedman, R.A.; Fenrich, A.L., Jr. Atrial Flutter in Infants. J. Am. Coll. Cardiol. 2006, 48, 1040–1046. [Google Scholar] [CrossRef]
- Sahin, G.T.; Lewis, M.; Uzun, O. Association of Fetal Atrial Flutter with Neonatal Atrioventricular Re-entry Tachycardia Involving Accessory Pathway: A Link to be Remembered. Pediatr. Cardiol. 2021, 42, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, P.J.; Gotteiner, N.L.; Deal, B.J.; Johnsrude, C.L.; Strasburger, J.F. Location of Accessory Connection in Infants Presenting with Supraventricular Tachycardia in Utero: Clinical Correlations. Am. J. Perinatol. 2003, 20, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Naheed, Z.J.; Strasburger, J.F.; Deal, B.J.; Benson, D.W., Jr.; Gidding, S.S. Fetal tachycardia: Mechanisms and predictors of hydrops fetalis. J. Am. Coll. Cardiol. 1996, 27, 1736–1740. [Google Scholar] [CrossRef]
- Hornberger, L.K.; Sahn, D.J. Rhythm abnormalities of the fetus. Heart 2007, 93, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- DʼAlto, M.; Russo, M.G.; Paladini, D.; Di Salvo, G.; Romeo, E.; Ricci, C.; Felicetti, M.; Tartaglione, A.; Cardaropoli, D.; Pacileo, G.; et al. The challenge of fetal dysrhythmias: Echocardiographic diagnosis and clinical management. J. Cardiovasc. Med. 2008, 9, 153–160. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Strasburger, J.F. Management Strategy for Fetal Tachycardia. Obstet. Gynecol. 2000, 96, 575–581. [Google Scholar] [CrossRef]
- Furon, J.C. Fetal Arrhythmias: The Saint-Justine hospital experience. Prenat. Diagn. 2004, 24, 1068–1080. [Google Scholar] [CrossRef]
- Miyoshi, T.; Maeno, Y.; Hamasaki, T.; Inamura, N.; Yasukochi, S.; Kawataki, M.; Horigome, H.; Yoda, H.; Taketazu, M.; Nii, M.; et al. Japan Fetal Arrhythmia Group. Antenatal Therapy for Fetal Supraventricular Tachyarrhythmias: Multicenter Trial. J. Am. Coll. Cardiol. 2019, 74, 874–885. [Google Scholar] [CrossRef]
- Kerenyi, T.; Meller, J.; Steinfeld, L.; Gleicher, N.; Brown, E.; Chitkara, U.; Raucher, H. Transplacental cardioversion of intrauterine supraventricular tachycardia with digitalis. Lancet 1980, 316, 393–395. [Google Scholar] [CrossRef]
- Allan, L.D.; Chita, S.K.; Sharland, G.K.; Maxwell, D.; Priestley, K.; Allan, L.D.; Chita, S.K.; Sharland, G.K.; Maxwell, D.; Priestley, K. Flecainide in the treatment of fetal tachycardias. Br. Heart J. 1991, 65, 46–48. [Google Scholar] [CrossRef][Green Version]
- Krapp, M.; Baschat, A.A.; Gembruch, U.; Geipel, A.; Germer, U. Flecainide in the intrauterine treatment of fetal supraventricular tachycardia. Ultrasound Obstet. Gynecol. 2002, 19, 158–164. [Google Scholar] [CrossRef]
- Oudijk, M.A.; Michon, M.M.; Kleinman, C.S.; Kapusta, L.; Stoutenbeek, P.; Visser, G.H.A.; Meijboom, E.J. Sotalol in the treatment of fetal dysrhythmias. Circulation 2000, 101, 2721–2726. [Google Scholar] [CrossRef]
- Sonesson, S.E.; Fouron, J.C.; Wesslen-Eriksson, E.; Jaeggi, E.; Winberg, P. Foetal supraventricular tachycardia treated with sotalol. Acta Paediatr. 1998, 87, 584–587. [Google Scholar] [CrossRef]
- Shah, A.; Moon-Grady, A.; Bhogal, N.; Collins, K.K.; Tacy, T.; Brook, M.; Hornberger, L.K. Effectiveness of Sotalol as First-Line Therapy for Fetal Supraventricular Tachyarrhythmias. Am. J. Cardiol. 2012, 109, 1614–1618. [Google Scholar] [CrossRef]
- Van Der Heijden, L.B.; Oudijk, M.A.; Manten, G.T.R.; Ter Heide, H.; Pistorius, L.; Freund, M.W. Sotalol as first-line treatment for fetal tachycardia and neonatal follow-up. Ultrasound Obstet. Gynecol. 2013, 42, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Sullivan, I.; Tomek, V.; Wolfenden, J.; Škovránek, J.; Yates, R.; Janousek, J.; Dominguez, T.E.; Marek, J. Flecainide versus digoxin for fetal supraventricular tachycardia: Comparison of two drug treatment protocols. Hear. Rhythm. 2016, 13, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Carvalho, J.S.; De Groot, E.; Api, O.; Clur, S.-A.B.; Rammeloo, L.; McCrindle, B.W.; Ryan, G.; Manlhiot, C.; Blom, N.A. Comparison of Transplacental Treatment of Fetal Supraventricular Tachyarrhythmias With Digoxin, Flecainide, and Sotalol. Circulation 2011, 124, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Baskar, S.; Fares, M.; Alahdab, F.; Czosek, R.J.; Murad, M.H.; Prokop, L.J.; Divanovic, A. First-Line Antiarrhythmic Transplacental Treatment for Fetal Tachyarrhythmia: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, 007164. [Google Scholar] [CrossRef]
- Hill, G.D.; Kovach, J.R.; Saudek, D.E.; Singh, A.K.; Wehrheim, K.; Frommelt, M.A. Transplacental treatment of fetal tachycardia: A systematic review and meta-analysis. Prenat. Diagn. 2017, 37, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.T.; Alexander, M.E.; Bezzerides, V.J.; Drogosz, M.; Economy, K.E.; Friedman, K.G.; Pickard, S.S.; Tworetzky, W.; Mah, D.Y. Low mortality in fetal supraventricular tachycardia: Outcomes in a 30-year single-institution experience. J. Cardiovasc. Electrophysiol. 2020, 31, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.L. Fetal supraventricular tachycardias: Diagnosis and management. Semin. Perinatol. 2000, 24, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, J.F.; Cuneo, B.F.; Michon, M.M.; Gotteiner, N.L.; Deal, B.J.; McGregor, S.N.; Oudijk, M.; Meijboom, E.J.; Feinkind, L.; Hussey, M.; et al. Amiodarone Therapy for Drug-Refractory Fetal Tachycardia. Circulation 2004, 109, 375–379. [Google Scholar] [CrossRef]
- Jouannic, J.-M.; Delahaye, S.; Fermont, L.; Le Bidois, J.; Villain, E.; Dumez, Y.; Dommergues, M. Fetal supraventricular tachycardia: A role for amiodarone as second-line therapy? Prenat. Diagn. 2003, 23, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Malhamé, I.; Gandhi, C.; Tarabulsi, G.; Esposito, M.; Lombardi, K.; Chu, A.; Chen, K.K. Maternal monitoring and safety considerations during antiarrhythmic treatment for fetal supraventricular tachycardia. Obstet. Med. 2019, 12, 66–75. [Google Scholar] [CrossRef]
- Zhao, H.; Strasburger, J.F.; Cuneo, B.F.; Wakai, R.T. Fetal Cardiac Repolarization Abnormalities. Am. J. Cardiol. 2006, 98, 491–496. [Google Scholar] [CrossRef]
- Zhao, H.; Cuneo, B.F.; Strasburger, J.F.; Huhta, J.C.; Gotteiner, N.L.; Wakai, R.T. Electrophysiological Characteristics of Fetal Atrioventricular Block. J. Am. Coll. Cardiol. 2008, 51, 77–84. [Google Scholar] [CrossRef]
- Hornberger, L.K.; Collins, K. New Insights Into Fetal Atrioventricular Block Using Fetal Magnetocardiography. J. Am. Coll. Cardiol. 2008, 51, 85–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simpson, J.M.; Maxwell, D.; Rosenthal, E.; Gill, H. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: Case report and review of the literature. Ultrasound Obstet. Gynecol. 2009, 34, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, J.F.; Wakai, R.T. Fetal cardiac arrhythmia detection and in utero therapy. Nat. Rev. Cardiol. 2010, 7, 277–290. [Google Scholar] [CrossRef]
- Vaksmann, G.; Lucidarme, S.; Henriet, E. Fetal ventricular tachycardia: Betablockers should be the first line treatment. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101946. [Google Scholar] [CrossRef]
- Crotti, L.; Tester, D.J.; White, W.M.; Bartos, D.C.; Insolia, R.; Besana, A.; Kunic, J.D.; Will, M.L.; Velasco, E.J.; Bair, J.J.; et al. Long QT Syndrome–Associated Mutations in Intrauterine Fetal Death. JAMA 2013, 309, 1473–1482. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Ovadia, M.; Strasburger, J.F.; Zhao, H.; Petropulos, T.; Schneider, J.; Wakai, R.T. Prenatal diagnosis and In Utero treatment of Torsades de Pointes associated with congenital long QT syndrome. Am. J. Cardiol. 2003, 91, 1395–1398. [Google Scholar] [CrossRef]
- Horigome, H.; Nagashima, M.; Sumitomo, N.; Yoshinaga, M.; Ushinohama, H.; Iwamoto, M.; Shiono, J.; Ichihashi, K.; Hasegawa, S.; Yoshikawa, T.; et al. Clinical Characteristics and Genetic Background of Congenital Long-QT Syndrome Diagnosed in Fetal, Neonatal, and Infantile Life: A nationwide questionnaire survey in Japan. Circ. Arrhythmia Electrophysiol. 2010, 3, 10–17. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Cuneo, B.F.; Etheridge, S.P.; Horigome, H.; Weng, H.-Y.; Benson, D.W. Fetal Heart Rate Predictors of Long QT Syndrome. Circulation 2012, 126, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Eronen, M.; Sirèn, M.-K.; Ekblad, H.; Tikanoja, T.; Julkunen, H.; Paavilainen, T. Short- and Long-Term Outcome of Children With Congenital Complete Heart Block Diagnosed In Utero or as a Newborn. Pediatrics 2000, 106, 86–91. [Google Scholar] [CrossRef]
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Davis, C.; Phoon, C.K.L.; Glickstein, J.S.; Buyon, J.P. Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. Circulation 2008, 117, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, A.; Hammami, A.; Bower, S.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2019, 54, 468–476. [Google Scholar] [CrossRef]
- Vayna, A.M.; Veduta, A.; Duta, S.; Panaitescu, A.M.; Stoica, S.; Buinoiu, N.; Nedelea, F.; Peltecu, G. Diagnosis of Fetal Structural Anomalies at 11 to 14 Weeks. J. Ultrasound Med. 2018, 37, 2063–2073. [Google Scholar] [CrossRef]
- Duta, S.; Veduta, A.; Vayna, A.M.; Panaitescu, A.; Nedelea, F.; Peltecu, G. The outcome of structural heart defects diagnosed in the first trimester of pregnancy. J. Matern. Neonatal Med. 2021, 34, 1389–1394. [Google Scholar] [CrossRef]
- Villain, E.; Marijon, E.; Georgin, S. Is isolated congenital heart block with maternal antibodies a distinct and more severe form of the disease in childhood? Heart Rhythm 2005, 2, S45. [Google Scholar] [CrossRef]
- Baruteau, A.-E.; Fouchard, S.; Behaghel, A.; Mabo, P.; Villain, E.; Thambo, J.-B.; Marcon, F.; Gournay, V.; Rouault, F.; Chantepie, A.; et al. Characteristics and long-term outcome of non-immune isolated atrioventricular block diagnosed in utero or early childhood: A multicentre study. Eur. Heart J. 2012, 33, 622–629. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Friedberg, M.K. Diagnosis and Management of Fetal Bradyarrhythmias. Pacing Clin. Electrophysiol. 2008, 31, S50–S53. [Google Scholar] [CrossRef]
- Carvalho, J.S. Fetal dysrhythmias. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 58, 28–41. [Google Scholar] [CrossRef]
- Ambrosi, A.; Salomonsson, S.; Eliasson, H.; Zeffer, E.; Skog, A.; Dzikaite, V.; Bergman, G.; Fernlund, E.; Tingström, J.; Theander, E.; et al. Development of heart block in children of SSA/SSB-autoantibody-positive women is associated with maternal age and displays a season-of-birth pattern. Ann. Rheum. Dis. 2011, 71, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, B.F.; Zhao, H.; Strasburger, J.F.; Ovadia, M.; Huhta, J.C.; Wakai, R.T. Atrial and Ventricular Rate Response and Patterns of Heart Rate Acceleration during Maternal–Fetal Terbutaline Treatment of Fetal Complete Heart Block. Am. J. Cardiol. 2007, 100, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.M.; Llanos, C.; Izmirly, P.M.; Brock, B.; Byron, J.; Copel, J.; Cummiskey, K.; Dooley, M.A.; Foley, J.; Graves, C.; et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010, 4, 1138–1146. [Google Scholar] [CrossRef]
- Pisoni, C.N.; Brucato, A.; Ruffatti, A.; Espinosa, G.; Cervera, R.; Belmonte-Serrano, M.A.; Sánchez-Román, J.; García-Hernández, F.G.; Tincani, A.; Bertero, M.T.; et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010, 62, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Trucco, S.M.; Jaeggi, E.; Cuneo, B.; Moon-Grady, A.J.; Silverman, E.; Silverman, N.; Hornberger, L.K. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 6, 715–723. [Google Scholar] [CrossRef]
- Hutter, D.; Silverman, E.D.; Jaeggi, E.T. The Benefits of Transplacental Treatment of Isolated Congenital Complete Heart Block Associated with Maternal Anti-Ro / SSA Antibodies: A Review. Scand. J. Immunol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Fouron, J.-C.; Silverman, E.D.; Ryan, G.; Smallhorn, J.; Hornberger, L.K. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 2004, 110, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Radwan, A.A.; Ali, A.K.; Abd-Elkariem, A.Y.; Shazly, S.A. Use of antenatal fluorinated corticosteroids in management of congenital heart block: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100072. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Llanos, C.; Davis, C.; Buyon, J.P. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am. J. Cardiol. 2009, 103, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.; Eudy, A.M.; Kiernan, E.; Williams, M.R.; Bermas, B.; Chakravarty, E.; Sammaritano, L.R.; Chambers, C.D.; Buyon, J. The prevention, screening and treatment of congenital heart block from neonatal lupus:a survey of provider practices. Rheumatology 2018, 57 (Suppl. S5), v9–v17. [Google Scholar] [CrossRef]
- Ciardulli, A.; D’Antonio, F.; Magro-Malosso, E.R.; Manzoli, L.; Anisman, P.; Saccone, G.; Berghella, V. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018, 97, 787–794. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Ambrose, S.E.; Tworetzky, W. Detection and successful treatment of emergent anti-SSA–mediated fetal atrioventricular block. Am. J. Obstet. Gynecol. 2016, 215, 527–528. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Moon-Grady, A.J.; Sonesson, S.-E.; Levasseur, S.; Hornberger, L.; Donofrio, M.T.; Krishnan, A.; Szwast, A.; Howley, L.; Benson, D.W.; et al. Heart sounds at home: Feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J. Perinatol. 2017, 37, 226–230. [Google Scholar] [CrossRef]
- Zhou, L.; Vest, A.N.; Chmait, R.H.; Bar-Cohen, Y.; Pruetz, J.; Silka, M.; Zheng, K.; Peck, R.; Loeb, G.E. A percutaneously implantable fetal pacemaker. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 4459–4463. [Google Scholar]
- Izmirly, P.M.; Costedoat-Chalumeau, N.; Pisoni, C.N.; Khamashta, M.A.; Kim, M.Y.; Saxena, A.; Friedman, D.; Llanos, C.; Piette, J.-C.; Buyon, J. Maternal Use of Hydroxychloroquine Is Associated With a Reduced Risk of Recurrent Anti-SSA/Ro-Antibody–Associated Cardiac Manifestations of Neonatal Lupus. Circulation 2012, 126, 76–82. [Google Scholar] [CrossRef]
- Izmirly, P.; Kim, M.; Friedman, D.M.; Costedoat-Chalumeau, N.; Clancy, R.; Copel, J.A.; Phoon, C.K.; Cuneo, B.F.; Cohen, R.E.; Robins, K.; et al. Hydroxychloroquine to Prevent Recurrent Congenital Heart Block in Fetuses of Anti-SSA/Ro-Positive Mothers. J. Am. Coll. Cardiol. 2020, 76, 292–302. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Levine, J.C.; Alexander, M.E. Fetal Arrhythmias. Available online: https://www.uptodate.com/contents/fetal-arrhythmias (accessed on 1 February 2021).
- Buyon, J.P. Neonatal Lupus, Management and Outcome. Available online: https://www.uptodate.com/contents/neonatal-lupus-management-and-outcomes (accessed on 1 February 2021).
- Dubin, A.M. Clinical Features and Diagnosis of Supraventriculat Tachycardia in Children. Available online: https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-supraventricular-tachycardia-in-children (accessed on 1 February 2021).
| Condition and Drug | Dose | Adverse Reactions |
|---|---|---|
| Supraventricular tachycardia (including atrial flutter) | ||
| Digoxin | LD 1–2 mg per 24 h PO in 3 divided doses MD 375 (500)–750 μg daily POin three equal daily doses | Nausea, severe sinus bradycardia or atrioventricular block, maternal and fetal proarrhythmia |
| Sotalol | 160–480 mg daily in three equal doses PO | Nausea, dizziness, fatigue, bundle branch block, QT prolongation, QRS prolongation, maternal and/or fetal proarrhythmia, neonatal hypoglycemia |
| Flecainide | 250–300 mg daily in three equal doses PO | Visual and central nervous system symptoms, bundle branch block, QT prolongation, QRS prolongation, fetal or neonatal proarrhythmia |
| Amiodarone | LD 1800–2400 mg daily in equal doses given every 6 h for 48 h; MD 200–600 mg daily PO | Nausea, maternal and/or fetal thyroid dysfunction, photosensitivity, thrombocytopenia, bundle branch block, proarrhythmia, torsades de pointes in fetuses with long QT syndrome |
| Procainamide | LD 500–600 mg over 20 min iv; MD 2–6 mg/min iv Initially 1250 mg, followed in 1 h by 750 mg, then 250–1000 mg every 3–6 h PO | Nausea, hypotension, proarrhythmia, platelets abnormalities |
| Ventricular tachycardia | ||
| Magnesium sulfate | LD 2–4 g iv followed by 1–2 g/h iv | Fatigue, central nervous system symptoms, stop for loss of patellar reflex at levels of 3.5–5.0 mmol/L cardiac arrhythmias at high levels |
| Lidocaine | LD 1.0–1.5 mg/kg iv followed by 1–3 mg/min iv | Central nervous system symptoms |
| Propranolol | 40–80 mg every 8 h PO | Fatigue, bradycardia, hypotension |
| Dexamethasone | LD 4–8 mg PO; MD 4 mg daily PO | Maternal effects similar to other corticosteroids, possible negative influence on the development of the fetal brain |
| Sinus node dysfunction in left atrial isomerism |
| Congenital LQTS |
| Sinus node inflammation or fibrosis in viral myocarditis or autoimmune collagen diseases (SSA/Ro [+] or SSA/Ro and SSB/La [+] antibodies) |
| Fetal distress |
| Fetal hypoxia |
| Fetal acidosis |
| Maternal hypotension |
| Maternal hypoglycemia |
| Maternal hypothermia and cardiopulmonary bypass |
| Maternal treatment with beta blockers or sedatives |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veduta, A.; Panaitescu, A.M.; Ciobanu, A.M.; Neculcea, D.; Popescu, M.R.; Peltecu, G.; Cavoretto, P. Treatment of Fetal Arrhythmias. J. Clin. Med. 2021, 10, 2510. https://doi.org/10.3390/jcm10112510
Veduta A, Panaitescu AM, Ciobanu AM, Neculcea D, Popescu MR, Peltecu G, Cavoretto P. Treatment of Fetal Arrhythmias. Journal of Clinical Medicine. 2021; 10(11):2510. https://doi.org/10.3390/jcm10112510
Chicago/Turabian StyleVeduta, Alina, Anca Maria Panaitescu, Anca Marina Ciobanu, Diana Neculcea, Mihaela Roxana Popescu, Gheorghe Peltecu, and Paolo Cavoretto. 2021. "Treatment of Fetal Arrhythmias" Journal of Clinical Medicine 10, no. 11: 2510. https://doi.org/10.3390/jcm10112510
APA StyleVeduta, A., Panaitescu, A. M., Ciobanu, A. M., Neculcea, D., Popescu, M. R., Peltecu, G., & Cavoretto, P. (2021). Treatment of Fetal Arrhythmias. Journal of Clinical Medicine, 10(11), 2510. https://doi.org/10.3390/jcm10112510








