Paracetamol Intake and Hematologic Malignancies: A Meta-Analysis of Observational Studies
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria and Search Strategy
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
2.5. Subgroup Analysis
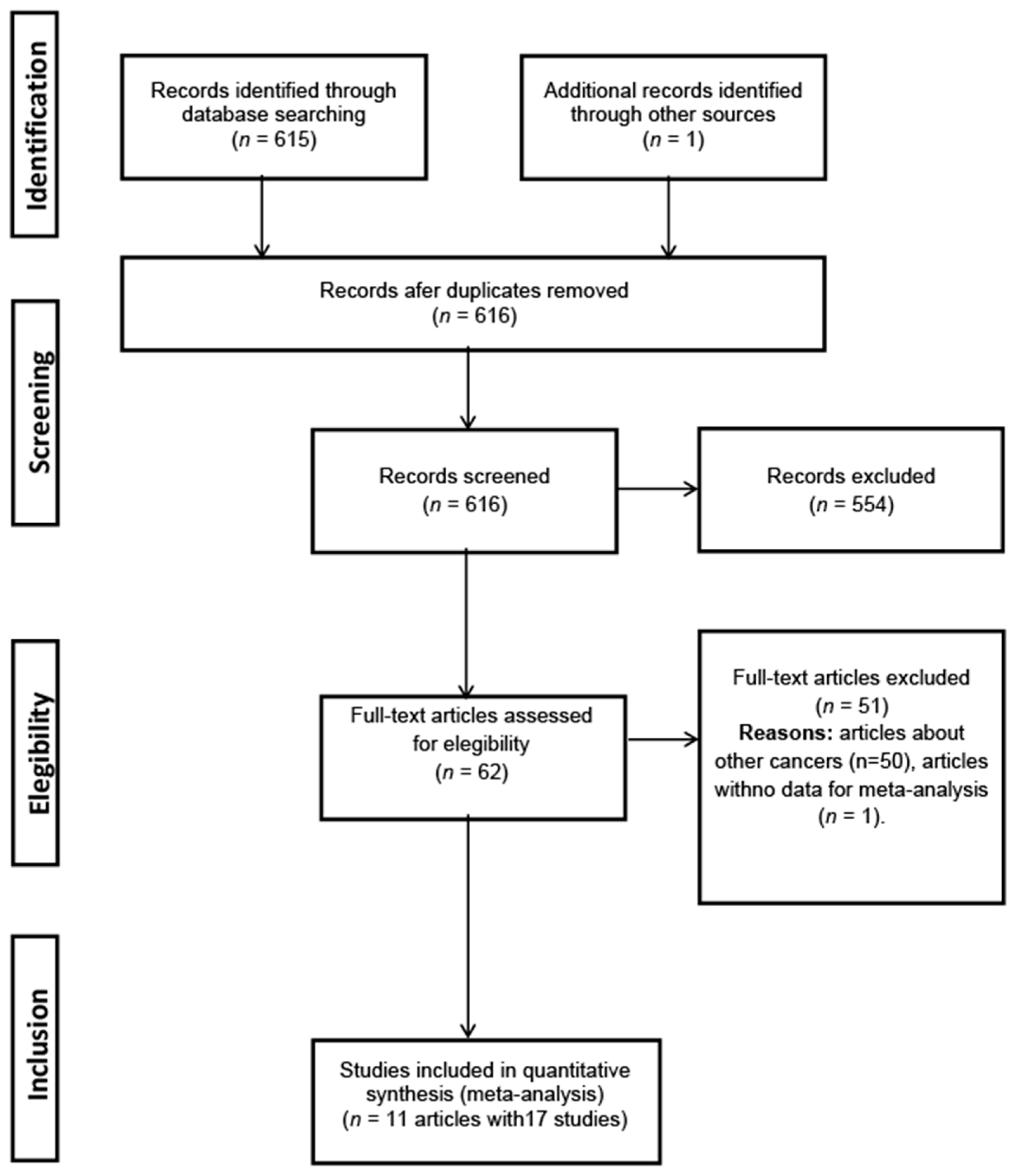
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Agency for Resarch on Cancer. World Fact Sheets. Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 28 May 2021).
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Bs, A.U.F.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Regueira-Méndez, C.; Etminan, M. Breast cancer and use of non-steroidal anti-inflammatory drugs: A meta-analysis. J. Natl. Cancer Inst. 2008, 100, 1439–1447. [Google Scholar] [CrossRef]
- Harris, R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of can-cers of the colon, breast, prostate, and lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F.R. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Catella-Lawson, F.; Reilly, M.P.; Kapoor, S.C.; Cucchiara, A.J.; DeMarco, S.R.N.; Tournier, B.R.N.; Vyas, S.N.; FitzGerald, G.A. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001, 345, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- IARC. Phenacetin. In IARC Monograph Evaluation Carcinogenic Risk to Humans; IARC: Lyon, France, 1980; Volume 24, pp. 135–161. [Google Scholar]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2014, 134, 384–396. [Google Scholar] [CrossRef]
- Jeter, J.M.; Han, J.; Martinez, M.E.; Alberts, D.S.; Qureshi, A.A.; Feskanich, D. Non-steroidal anti-inflammatory drugs, acetaminophen, and risk of skin cancer in the Nurses’ Health Study. Cancer Causes Control 2012, 23, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- IARC. Paracetamol. In IARC Monograph Evaluation Carcinogenic Risk to Humans; IARC: Lyon, France, 1999; Volume 73, pp. 401–449. [Google Scholar]
- Robak, P.; Smolewski, P.; Robak, T. The role of non-steroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leuk. Lymphoma 2008, 49, 1452–1462. [Google Scholar] [CrossRef]
- Weiss, N.S. Use of acetaminophen in relation to the occurrence of cancer: A re-view of epidemiologic studies. Cancer Causes Control 2016, 27, 1411–1418. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Don´t Double Up on Acetaminophen. Available online: https://www.fda.gov/consumers/consumer-updates/dont-double-acetaminophen#:~:text=don%27t%20take%20more%20than,on%20any%20product%20containing%20acetaminophen (accessed on 28 May 2021).
- Duong, M.; Gulmez, S.E.; Salvo, F.; Abouelfath, A.; Lassalle, R.; Droz, C.; Blin, P.; Moore, N. Usage patterns of paracetamol in France. Br. J. Clin. Pharmacol. 2016, 82, 498–503. [Google Scholar] [CrossRef]
- Walker, A.M. Estimation. In Observation and Inference: An Introduction to the Methods of Epidemiology; Walker, A.M., Ed.; Epidemiology Resources Inc.: Chestnut Hill, MA, USA, 1991. [Google Scholar]
- Tomatis, L. Quantification of effect. In Cancer: Causes, Occurrence and Control; Tomatis, L., Ed.; IARC Scientific Publications: Lyon, France, 1980; Volume 288. [Google Scholar]
- Holly, E.A.; Lele, C.; Bracci, P.M.; McGrath, M.S. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Fran-cisco Bay Area, California. Am. J. Epidemiol. 1999, 150, 375–389. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Measures of effect and measures of association. In Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 61. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 May 2021).
- Takkouche, B.; Cadarso-Suárez, C.; Spiegelman, D. Evaluation of Old and New Tests of Heterogeneity in Epidemiologic Meta-Analysis. Am. J. Epidemiol. 1999, 150, 206–215. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Costa-Bouzas, J.; Takkouche, B.; Cadarso-Suárez, C.; Spiegelman, D. HEpiMA: Software for the identification of heterogeneity in meta-analysis. Comput. Methods Programs Biomed. 2001, 64, 101–107. [Google Scholar] [CrossRef]
- Friedman, G.D. Phenylbutazone, musculoskeletal disease, and leukemia. J. Chronic Dis. 1982, 35, 233–243. [Google Scholar] [CrossRef]
- Kato, I.; Koenig, K.L.; Shore, R.E.; Baptiste, M.S.; Lillquist, P.P.; Frizzera, G.; Burke, J.S.; Watanabe, H. Use of anti-inflammatory and non-narcotic analgesic drugs and risk of non-Hodgkin’s lymphoma (NHL) (United States). Cancer Causes Control 2002, 13, 965–974. [Google Scholar] [CrossRef]
- Friis, S.; Nielsen, G.L.; Mellemkjaer, L.; McLaughlin, J.K.; Thulstrup, A.M.; Blot, W.J.; Lipworth, L.; Vilstrup, H.; Olsen, J.H. Cancer risk in persons receiving prescriptions for paracetamol: A Danish cohort study. Int. J. Cancer 2002, 97, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Friis, S.; Mellemkjær, L.; Signorello, L.B.; Johnsen, S.P.; Nielsen, G.L.; McLaughlin, J.K.; Blot, W.J.; Olsen, J.H. A population-based cohort study of mortality among adults prescribed paracetamol in Denmark. J. Clin. Epidemiol. 2003, 56, 796–801. [Google Scholar] [CrossRef]
- Chang, E.T.; Zheng, T.; Weir, E.G.; Borowitz, M.; Mann, R.B.; Spiegelman, D.; Mueller, N.E. Aspirin and the risk of Hodgkin’s lymphoma in a popula-tion-based case-control study. J. Natl. Cancer Inst. 2004, 96, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Weiss, J.R.; Czuczman, M.S.; Menezes, R.J.; Ambrosone, C.B.; Moysich, K.B. Regular use of aspirin or acetaminophen and risk of non-Hodgkin lymphoma. Cancer Causes Control 2005, 16, 301–308. [Google Scholar] [CrossRef]
- Weiss, J.R.; Baker, J.A.; Baer, M.R.; Menezes, R.J.; Nowell, S.; Moysich, K.B. Opposing effects of aspirin and acetaminophen use on risk of adult acute leukemia. Leuk. Res. 2006, 30, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Moysich, K.B.; Bonner, M.R.; Beehler, G.P.; Marshall, J.R.; Menezes, R.J.; Baker, J.A.; Weiss, J.R.; Chanan-Khan, A. Regular analgesic use and risk of multiple myeloma. Leuk. Res. 2007, 31, 547–551. [Google Scholar] [CrossRef]
- Becker, N.; Fortuny, J.; Alvaro, T.; Nieters, A.; Maynadié, M.; Foretova, L.; Staines, A.; Brennan, P.; Boffetta, P.; Cocco, P.L.; et al. Medical history and risk of lymphoma: Results of a European case-control study (EPILYMPH). J. Cancer Res. Clin. Oncol. 2009, 135, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Blair, C.K.; Cerhan, J.R.; Soler, J.T.; Hirsch, B.A.; Roesler, M.A.; Higgins, R.R.; Nguyen, P.L. Nonsteroidal Anti-inflammatory Drug and Acetaminophen Use and Risk of Adult Myeloid Leukemia. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Milano, F.; Brasky, T.M.; White, E. Long-term use of acetamino-phen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of he-matologic malignancies: Results from the prospective Vitamins and Lifestyle (VITAL) study. J. Clin. Oncol. 2011, 29, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Scientific Publications: Lyon, France, 2008. [Google Scholar]
- Rannug, U.; Holme, J.A.; Hongslo, J.K.; Srám, R. International Commission for Protection against Environmental Mutagens and Carcinogens. An evaluation of the genetic toxicity of paracetamol. Mutat. Res. 1995, 327, 179–200. [Google Scholar] [CrossRef]
- Bender, R.P.; Lindsey, R.H., Jr.; Burden, D.A.; Osheroff, N. N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry 2004, 43, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Brado, B.; Haas, R.; Hunstein, W. Etoposide in acute leukemia. Past experience and future perspectives. Cancer 1991, 67, 281–284. [Google Scholar] [CrossRef]
- Bergman, K.; Müller, L.; Teigen, S. The genotoxicity and carcinogenicity of paracetamol: A regulatory (re)view. Mutat. Res. 1996, 349, 263–288. [Google Scholar] [CrossRef]
- Blazka, M.; Wilmer, J.; Holladay, S.; Wilson, R.; Luster, M. Role of Proinflammatory Cytokines in Acetaminophen Hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 133, 43–52. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Institute for Human Data Science. Medicine Use and Spending in the U.S.: A Review of 2017 and Outlook to 2022. 2018. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022 (accessed on 28 May 2021).
- Shen, J.; Gammon, M.D.; Terry, M.B.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M. Genetic polymorphisms in the cyclooxygenase-2 gene, use of nonsteroidal anti-inflammatory drugs, and breast cancer risk. Breast Cancer Res. 2006, 8, R71. [Google Scholar] [CrossRef]
- Greenland, S. Basic methods for sensitivity analysis of biases. Int. J. Epidemiol. 1996, 25, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; McLaughlin, J.K.; Lipworth, L.; Friis, S.; Sørensen, H.T.; Blot, W.J. Confounding by Indication in Epidemiologic Studies of Commonly Used Analgesics. Am. J. Ther. 2002, 9, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Anderson, T.E.; Mitchell, A.A. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone survey. JAMA 2002, 287, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. Measures of disease frequency. In Modern Epidemiology, 1st ed.; Little, Brown & Co: Boston, MA, USA, 1986; Volume 39. [Google Scholar]


| Author | Study Period | Age of Participants (Years) | Paracetamol Intake Assessment | Cancer Ascertainment | Lag Time a | Definition of Any Intake of Paracetamol | Definition of High Intake of Paracetamol |
|---|---|---|---|---|---|---|---|
| Friis et al. [26] | 1989–1995 | 63 (average) | Regional prescription database | Cancer registry | 1 year | Prescribed/Not prescribed | - |
| Lipworth et al. [27] | 1989–1995 | >16 | Regional Pharmacoepidemiologic database | National mortality files | None | SMR based on prescribed/not prescribed | >10 prescriptions |
| Walter et al. [34] | 2000–2002 | 50–76 | Self-administered questionnaire | Cancer registry | Not given | Pooling of OR corresponding to<4 days/week or <4 years, and ≥4 days/week or ≥4 years | ≥4 days/week or ≥4 years |
| Friedman et al. [24] | 1971–1976 | >30 | Hospital charts | Hospital charts | 6 months | Intake/no intake | - |
| Kato et al. [25] | 1995–1998 | 20–79 | Telephone interview | Cancer registry | 1 year | Pooling of OR corresponding to <3 years of use, 3–10 years and >10 years | Consumption for >10 years |
| Chang et al. [28] | 1997–2000 | 15–79 | Telephone interview | Hospital charts and Cancer registry | None | Pooling of OR corresponding to <2 tablets/week and ≥2 tablets/week | ≥2 tablets/week |
| Baker et al. [29] | 1982–1998 | 57 (average) | Self-administered questionnaire | Cancer registry | None | Pooling of OR corresponding to ≤10 tablet-year b and >10 tablet-year | >10 tablet-year |
| Weiss et al. [30] | 1981–1998 | 20–84 | Self-administered questionnaire | Hospital cases | Not given | Pooling of OR corresponding to ≤4 tablet-year and >4 tablet-year | >4 tablet-year |
| Moysich et al. [31] | 1982–1998 | 60 (average) | Self-administered questionnaire | Hospital cases | Not given | Pooling of OR corresponding to <7 times/week and ≥7 times/week | ≥7 times/week |
| Becker et al. [32] | 1998–2004 | <71 | Self-administered questionnaire | Different hospitals | 3 years | Intake/no intake | - |
| Ross et al. [33] | 2005–2009 | 20–79 | Self-administered questionnaire | Regional cancer surveillance system | 2 years | OR corresponding to ≥1use/week for ≥1 year | Pooling of OR corresponding to ≥7 tablets/week and >10 years |
| Author | Year | Country | Sample Size/#Cases | Cancer | RR (95% CI)Any Intake (a) | RR (95% CI)High Intake (b) | Adjustment/Matching |
|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||
| Friis et al. [26] | 2002 | Denmark | 39946/46 | Leukemia | 0.90 (0.50–1.60) | - | Age, sex, other analgesic use |
| Friis et al. [26] | 2002 | Denmark | 39946/25 | Multiple myeloma | 1.60 (0.60–3.20) | - | Age, sex, other analgesic use |
| Friis et al. [26] | 2002 | Denmark | 39946/47 | NHL | 1.20 (0.70–2.00) | - | Age, sex, other analgesic use |
| Friis et al. [26] | 2002 | Denmark | 39946/6 | HL | 1.40 (0.00–8.00) | - | Age, sex, other analgesic use |
| Lipworth et al. [27] | 2003 | Denmark | 49890/286 | All cancers | 2.30 (2.00–2.60) | 1.80 (1.40–2.40) | Age, sex |
| Walter et al. [34] | 2011 | USA | 64839/66 | Leukemia | 1.77 (1.24–2.54) | 2.26 (1.24–4.12) | Age, sex, education, ethnicity, smoking, medical history |
| Walter et al. [34] | 2011 | USA | 64839/235 | Lymphoma | 1.15 (0.85–1.57) | 1.81 (1.12–2.93) | Age, sex, education, ethnicity, smoking, medical history |
| Walter et al. [34] | 2011 | USA | 64839/88 | SLL/CLL | 0.90 (0.53–1.53) | 0.84 (0.31–2.28) | Age, sex, education, ethnicity, smoking, medical history |
| Walter et al. [34] | 2011 | USA | 64839/136 | Multiple myeloma | 1.89 (1.16–3.09) | 2.42 (1.08–5.41) | Age, sex, education, ethnicity, smoking, medical history |
| Case-control studies | |||||||
| Friedman et al. [24] | 1982 | USA | 409/818 | Leukemia | 0.66 (0.35–1.23) | - | Age, sex |
| Kato et al. [25] | 2002 | USA | 376/463 | NHL | 1.17 (0.68–2.01) | 1.39 (0.45–4.26) | Age, sex, education, body mass index, history of hematologic cancer, study year |
| Chang et al. [28] | 2004 | USA | 565/679 | HL | 2.01 (1.62–2.50) | 2.17 (1.58–2.98) | Age, sex, residence, smoking, other analgesic use |
| Baker et al. [29] | 2005 | USA | 628/2512 | NHL | 1.21 (0.90–1.61) | 0.97 (0.49–1.89) | Age, sex, race, study year, smoking, education, income. |
| Weiss et al. [30] | 2006 | USA | 169/676 | Leukemia | 1.53 (1.03–2.26) | 1.30 (0.73–2.34) | Age, sex, race, study year, smoking, education, alcohol consumption |
| Moysich et al. [31] | 2007 | USA | 117/483 | Myeloma | 2.95 (1.72–5.08) | 3.66 (2.02–6.64) | Age, smoking, study year |
| Becker et al. [32] | 2009 | Europe | 2362/2465 | Lymphoma | 2.29 (1.49–3.51) | - | Age, sex, study center. |
| Ross et al. [33] | 2011 | USA | 670/701 | Leukemia | 1.35 (0.98–1.86) | 1.45 (1.04–2.01) | Age, sex, body mass index, other analgesic use |
| Any Intake (b) | Number of Studies | RR (95% CI) Fixed Effects | RR (95% CI) Random Effects | Ri (a) | Q Test p-Value |
|---|---|---|---|---|---|
| All studies | 17 | 1.78 (1.64–1.92) | 1.49 (1.23–1.80) | 0.79 | 0.001 |
| Cohort studies | 9 | 1.90 (1.72–2.09) | 1.43 (1.06–1.92) | 0.85 | 0.001 |
| Case-control studies | 8 | 1.93 (1.74–2.14) | 1.54 (1.19–1.99) | 0.74 | 0.001 |
| Low bias risk | 6 | 1.99 (1.82–2.19) | 1.66 (1.27–2.16) | 0.85 | 0.001 |
| High bias risk | 11 | 1.39 (1.21–1.59) | 1.39 (1.11–1.73) | 0.56 | 0.01 |
| Fully adjusted | 8 | 1.58 (1.40–1.78) | 1.56 (1.24–1.96) | 0.70 | 0.001 |
| Incompletely adjusted | 9 | 1.60 (1.41–1.82) | 1.38 (1.00–1.91) | 0.87 | 0.001 |
| By histologic type: | |||||
| Leukemia | 5 | 1.34 (1.12–1.61) | 1.26 (0.93–1.69) | 0.59 | 0.04 |
| Lymphoma (all) | 7 | 1.54 (1.35–1.76) | 1.46 (1.14–1.89) | 0.67 | 0.001 |
| Non-Hodgkin | 3 | 1.20 (0.96–1.51) | 1.20 (0.96–1.51) | 0.00 | 0.99 |
| Hodgkin | 2 | 2.00 (1.61–2.48) | 2.00 (1.61–2.48) | 0.00 | 0.68 |
| Undetermined | 2 | 1.46 (1.14–1.88) | 1.60 (0.81–3.13) | 0.86 | 0.01 |
| Multiple Myeloma | 3 | 2.13 (1.54–2.94) | 2.13 (1.51–3.01) | 0.12 | 0.32 |
| High Intake (c) | Number of Studies | RR (95% CI) Fixed Effects | RR (95% CI) Random Effects | Ri (a) | Q Test p-Value |
| All studies | 11 | 1.79 (1.55–2.06) | 1.77 (1.45–2.16) | 0.41 | 0.08 |
| Cohort studies | 5 | 1.83 (1.48–2.27) | 1.83 (1.48–2.27) | 0.00 | 0.51 |
| Case-control studies | 6 | 1.76 (1.45–2.12) | 1.71 (1.21–2.41) | 0.65 | 0.02 |
| Low bias risk | 6 | 1.93 (1.62–2.31) | 1.93 (1.62–2.31) | 0.00 | 0.54 |
| High bias risk | 5 | 1.56 (1.24–1.98) | 1.59 (1.03–2.44) | 0.66 | 0.03 |
| Fully adjusted | 8 | 1.93 (1.59–2.33) | 1.84 (1.38–2.47) | 0.52 | 0.04 |
| Incompletely adjusted | 3 | 1.63 (1.32–2.01) | 1.63 (1.32–2.01) | 0.00 | 0.59 |
| By histologic type: | |||||
| Leukemia | 3 | 1.54 (1.19–1.99) | 1.54 (1.19–2.00) | 0.01 | 0.36 |
| Lymphoma (all) | 4 | 1.83 (1.44–2.33) | 1.70 (1.20–2.40) | 0.44 | 0.18 |
| Non-Hodgkin | 2 | 1.07 (0.60–1.89) | 1.07 (0.60–1.89) | 0.00 | 0.58 |
| Multiple Myeloma | 2 | 3.16 (1.96–5.10) | 3.16 (1.96–5.10) | 0.00 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prego-Domínguez, J.; Takkouche, B. Paracetamol Intake and Hematologic Malignancies: A Meta-Analysis of Observational Studies. J. Clin. Med. 2021, 10, 2429. https://doi.org/10.3390/jcm10112429
Prego-Domínguez J, Takkouche B. Paracetamol Intake and Hematologic Malignancies: A Meta-Analysis of Observational Studies. Journal of Clinical Medicine. 2021; 10(11):2429. https://doi.org/10.3390/jcm10112429
Chicago/Turabian StylePrego-Domínguez, Jesús, and Bahi Takkouche. 2021. "Paracetamol Intake and Hematologic Malignancies: A Meta-Analysis of Observational Studies" Journal of Clinical Medicine 10, no. 11: 2429. https://doi.org/10.3390/jcm10112429
APA StylePrego-Domínguez, J., & Takkouche, B. (2021). Paracetamol Intake and Hematologic Malignancies: A Meta-Analysis of Observational Studies. Journal of Clinical Medicine, 10(11), 2429. https://doi.org/10.3390/jcm10112429






