HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways
Abstract
1. Introduction
2. Methods and Materials
2.1. Cell Culture
2.2. Transfection and Experimental Protocol
2.3. Subcellular Fractionation
2.4. Immunoblot
2.5. qPCR
2.6. Statistics
2.7. Study Approval
3. Results
3.1. HspB4/αA-Crystallin Overexpression Dampens the Metabolic-Stress-Induced Pro-inflammatory Transition of Müller Glial Cells (MGCs)
3.2. HspB4/αA-Crystallin Effect on the Metabolic-Stress-Induced Activation of Müller Glial Cells Is T148 Phosphorylation Dependent
3.3. Primary Müller Cells Isolated from HspB4/αA-Crystallin Knockout Mice
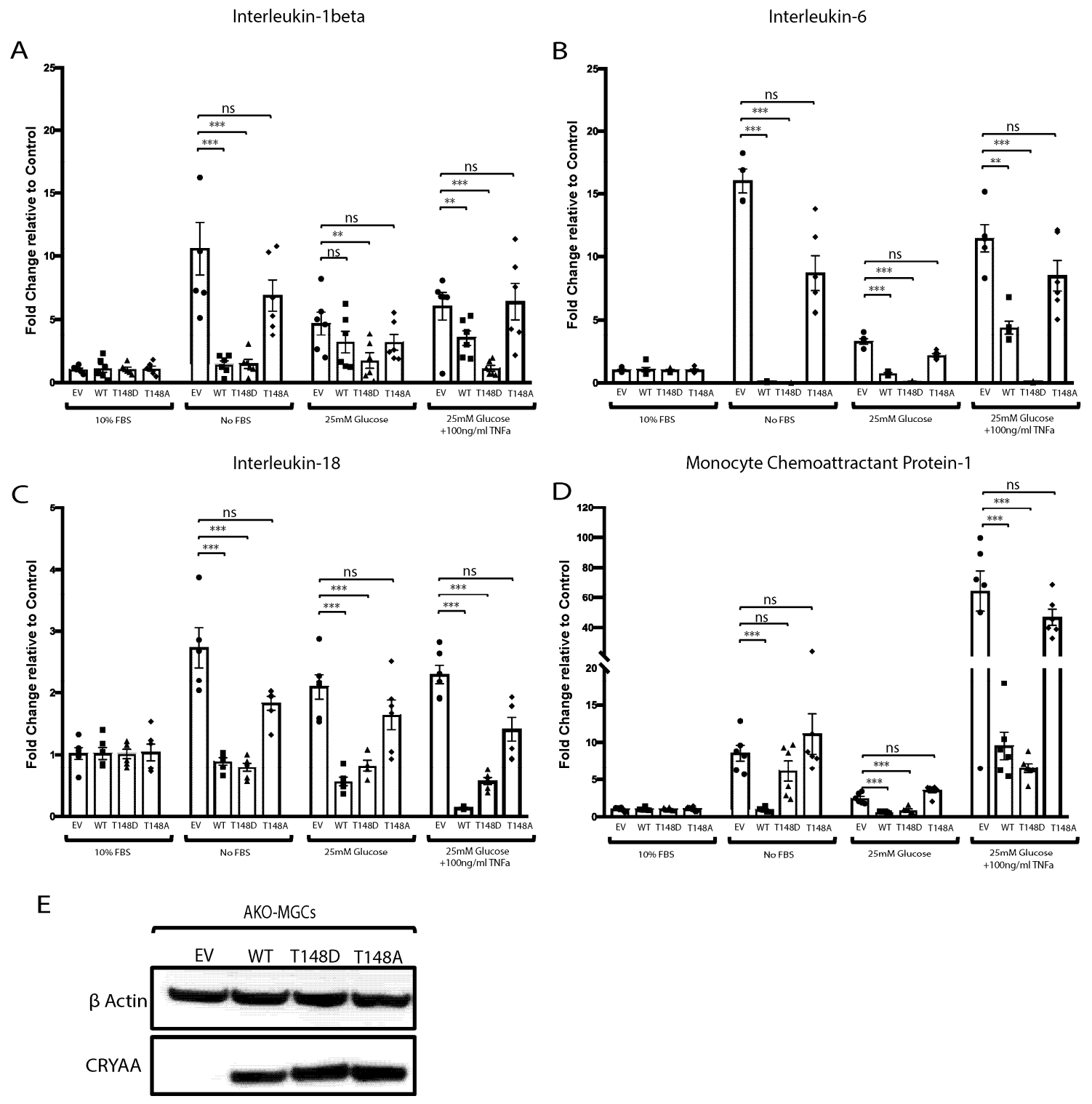
3.4. The Metabolic-Stress-Induced Pro-Inflammatory Response of Primary MGCs (HspB4−/−) Is Dampened by HspB4/αA-Crystallin Expression
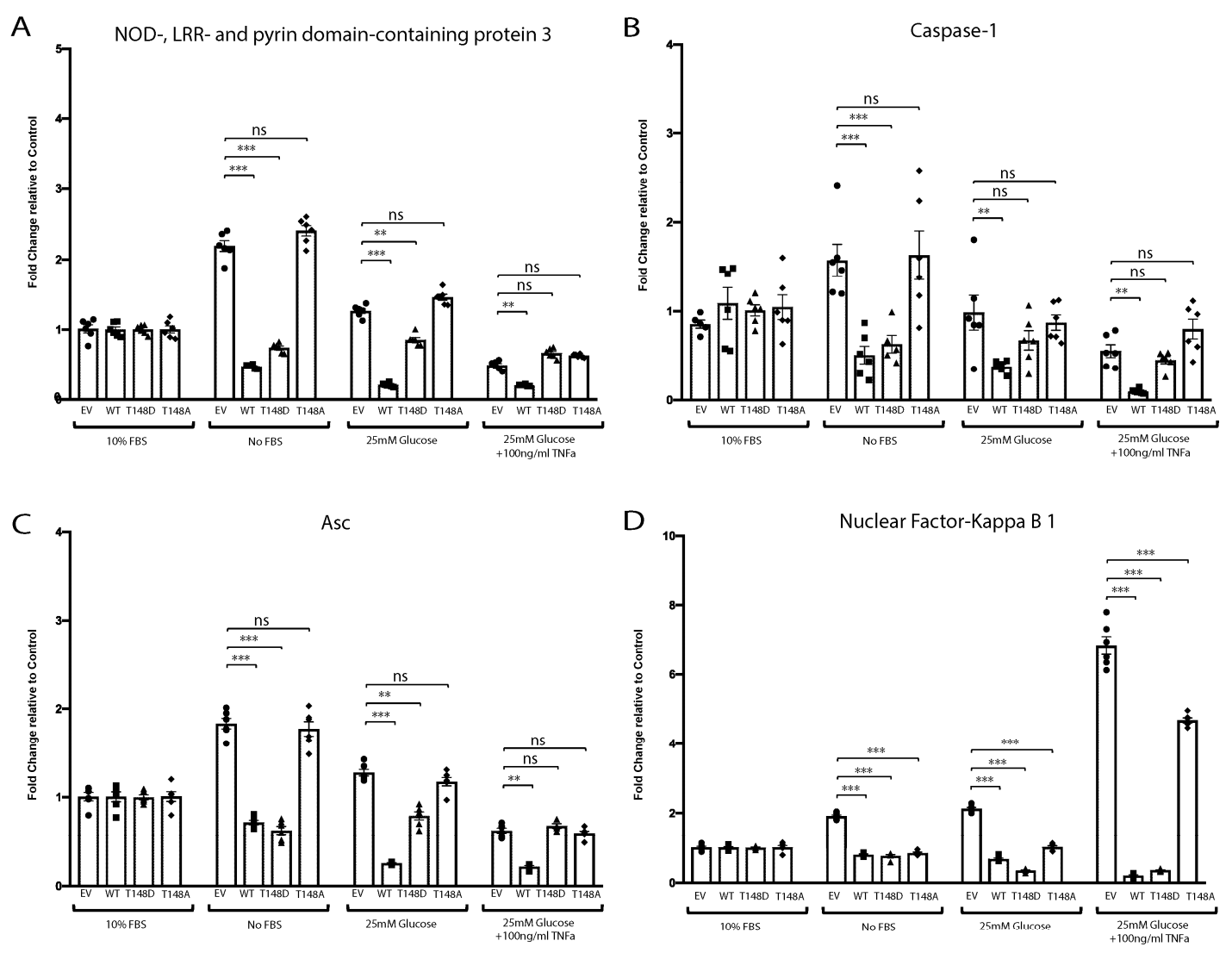
3.5. Phosphorylation of HspB4/αA-Crystallin on Residue 148 Controls the Neuroinflammatory Cascade in Metabolically Stressed Primary MGCs (HspB4−/−)
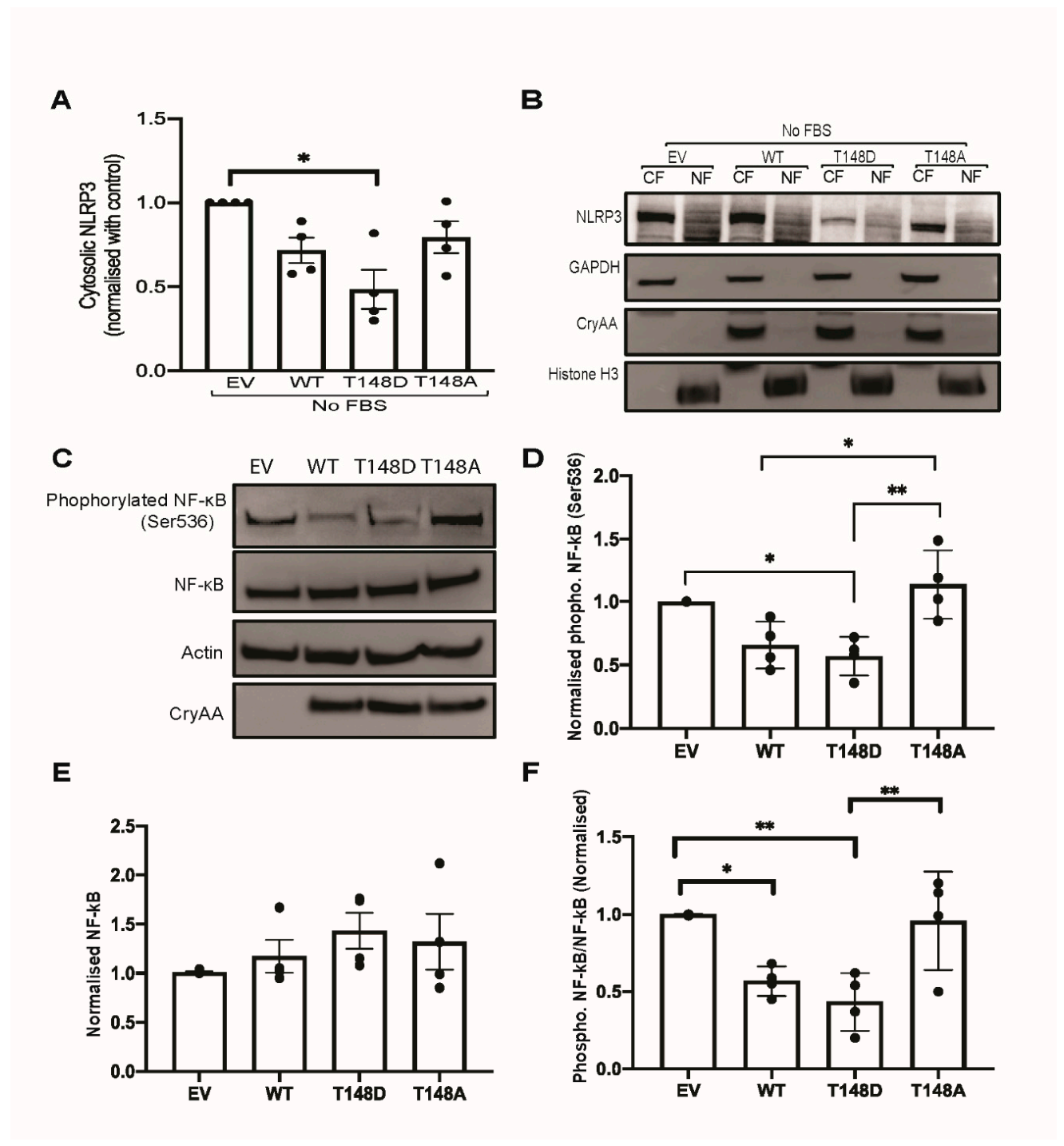
3.6. HspB4/αA-Crystallin Regulates MGCs Inflammatory Response through Stress-Specific Inflammatory Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reichenbach, A.; Bringmann, A. Role of Purines in Muller Glia. J. Ocul. Pharmacol. Ther. 2016, 32, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shen, S.Q.; Jui, J.; Rupp, A.C.; Byrne, L.C.; Hattar, S.; Flannery, J.G.; Corbo, J.C.; Kefalov, V.J. CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Investig. 2015, 125, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.J.; Du, J.; Sloat, S.R.; Contreras, L.; Linton, J.D.; Turner, S.J.; Sadilek, M.; Satrustegui, J.; Hurley, J.B. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. USA 2014, 111, 15579–15584. [Google Scholar] [CrossRef] [PubMed]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sanchez, M.C. A journey into the retina: Muller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Yego, E.C.; Vincent, J.A.; Sarthy, V.; Busik, J.V.; Mohr, S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Muller cells by IL-1beta and IL-6. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhang, J.; Shen, J.; Hu, L.M.; Wu, Y.; Mou, L.; Xu, G.; Li, W.; Xu, G.T. EPO attenuates inflammatory cytokines by Muller cells in diabetic retinopathy. Front. Biosci. 2011, 3, 201–211. [Google Scholar]
- Abu el Asrar, A.M.; Maimone, D.; Morse, P.H.; Gregory, S.; Reder, A.T. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1992, 114, 731–736. [Google Scholar] [CrossRef]
- Blakytny, R.; Carver, J.A.; Harding, J.J.; Kilby, G.W.; Sheil, M.M. A spectroscopic study of glycated bovine alpha-crystallin: Investigation of flexibility of the C-terminal extension, chaperone activity and evidence for diglycation. Biochim. Biophys. Acta 1997, 1343, 299–315. [Google Scholar] [CrossRef]
- Deliyanti, D.; Alrashdi, S.F.; Tan, S.M.; Meyer, C.; Ward, K.W.; de Haan, J.B.; Wilkinson-Berka, J.L. Nrf2 Activation Is a Potential Therapeutic Approach to Attenuate Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 815–825. [Google Scholar] [CrossRef]
- Demircan, N.; Safran, B.G.; Soylu, M.; Ozcan, A.A.; Sizmaz, S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye 2006, 20, 1366–1369. [Google Scholar] [CrossRef]
- Hernandez, C.; Segura, R.M.; Fonollosa, A.; Carrasco, E.; Francisco, G.; Simo, R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet. Med. 2005, 22, 719–722. [Google Scholar] [CrossRef]
- van Noort, J.M.; van Sechel, A.C.; Bajramovic, J.J.; el Ouagmiri, M.; Polman, C.H.; Lassmann, H.; Ravid, R. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature 1995, 375, 798–801. [Google Scholar] [CrossRef]
- Renkawek, K.; Stege, G.J.; Bosman, G.J. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport 1999, 10, 2273–2276. [Google Scholar] [CrossRef]
- Fort, P.E.; Freeman, W.M.; Losiewicz, M.K.; Singh, R.S.; Gardner, T.W. The retinal proteome in experimental diabetic retinopathy: Up-regulation of crystallins and reversal by systemic and periocular insulin. Mol. Cell. Proteom. 2009, 8, 767–779. [Google Scholar] [CrossRef]
- Ruebsam, A.; Dulle, J.E.; Myers, A.M.; Sakrikar, D.; Green, K.M.; Khan, N.W.; Schey, K.; Fort, P.E. A specific phosphorylation regulates the protective role of alphaA-crystallin in diabetes. JCI Insight 2018, 3, e97919. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Nahomi, R.B.; Mueller, N.H.; Raghavan, C.T.; Ammar, D.A.; Petrash, J.M. Therapeutic potential of alpha-crystallin. Biochim. Biophys. Acta 2016, 1860 Pt B, 252–257. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Wang, B.; Raghavan, C.T.; Voss, O.; Doseff, A.I.; Santhoshkumar, P.; Nagaraj, R.H. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J. Biol. Chem. 2013, 288, 13022–13035. [Google Scholar] [CrossRef]
- Masilamoni, J.G.; Jesudason, E.P.; Bharathi, S.N.; Jayakumar, R. The protective effect of alpha-crystallin against acute inflammation in mice. Biochim. Biophys. Acta 2005, 1740, 411–420. [Google Scholar] [CrossRef]
- Masilamoni, J.G.; Vignesh, S.; Kirubagaran, R.; Jesudason, E.P.; Jayakumar, R. The neuroprotective efficacy of alpha-crystallin against acute inflammation in mice. Brain Res. Bull. 2005, 67, 235–241. [Google Scholar] [CrossRef]
- Simirskii, V.N.; Panova, I.G.; Sologub, A.A.; Aleinikova, K.S. Localization of crystallins in Muellerian cells in the grass frog retina. Ontogenez 2003, 34, 365–370. [Google Scholar]
- Zayas-Santiago, A.; Rios, D.S.; Zueva, L.V.; Inyushin, M.Y. Localization of alphaA-Crystallin in Rat Retinal Muller Glial Cells and Photoreceptors. Microsc. Microanal. 2018, 24, 545–552. [Google Scholar] [CrossRef]
- Cherian, M.; Abraham, E.C. Decreased molecular chaperone property of alpha-crystallins due to posttranslational modifications. Biochem. Biophys. Res. Commun. 1995, 208, 675–679. [Google Scholar] [CrossRef]
- Cherian, M.; Smith, J.B.; Jiang, X.Y.; Abraham, E.C. Influence of protein-glutathione mixed disulfide on the chaperone-like function of alpha-crystallin. J. Biol. Chem. 1997, 272, 29099–29103. [Google Scholar] [CrossRef] [PubMed]
- Ciano, M.; Allocca, S.; Ciardulli, M.C.; Della Volpe, L.; Bonatti, S.; D’Agostino, M. Differential phosphorylation-based regulation of alphaB-crystallin chaperone activity for multipass transmembrane proteins. Biochem. Biophys. Res. Commun. 2016, 479, 325–330. [Google Scholar] [CrossRef]
- Kamei, A.; Iwase, H.; Masuda, K. Cleavage of amino acid residue(s) from the N-terminal region of alpha A- and alpha B-crystallins in human crystalline lens during aging. Biochem. Biophys. Res. Commun. 1997, 231, 373–378. [Google Scholar] [CrossRef]
- Morrison, L.E.; Hoover, H.E.; Thuerauf, D.J.; Glembotski, C.C. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ. Res. 2003, 92, 203–211. [Google Scholar] [CrossRef]
- Maddala, R.; Rao, V.P. alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp. Cell Res. 2005, 306, 203–215. [Google Scholar] [CrossRef]
- Heise, E.A.; Marozas, L.M.; Grafton, S.A.; Green, K.M.; Kirwin, S.J.; Fort, P.E. Strain-independent increases of crystallin proteins in the retina of type 1 diabetic rats. PLoS ONE 2013, 8, e82520. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.H.; Choi, M.Y.; Kim, Y.S.; Han, J.M.; Lee, J.H.; Park, C.H.; Kang, S.S.; Choi, W.S.; Cho, G.J. Protein kinase C delta regulates anti-apoptotic alphaB-crystallin in the retina of type 2 diabetes. Neurobiol. Dis. 2007, 28, 293–303. [Google Scholar] [CrossRef]
- Rao, N.A.; Saraswathy, S.; Pararajasegaram, G.; Bhat, S.P. Small heat shock protein alphaA-crystallin prevents photoreceptor degeneration in experimental autoimmune uveitis. PLoS ONE 2012, 7, e33582. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, V.P.; Brodjian, S.J.; Dutt, K.; Kennedy, B.N.; French, R.P.; Crabb, J.W. Establishment and characterization of a retinal Muller cell line. Investig. Ophthalmol. Vis. Sci. 1998, 39, 212–216. [Google Scholar]
- Hicks, D.; Courtois, Y. The growth and behaviour of rat retinal Muller cells in vitro. 1. An improved method for isolation and culture. Exp. Eye Res. 1990, 51, 119–129. [Google Scholar] [CrossRef]
- Brady, J.P.; Garland, D.; Duglas-Tabor, Y.; Robison, W.G., Jr.; Groome, A.; Wawrousek, E.F. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc. Natl. Acad. Sci. USA 1997, 94, 884–889. [Google Scholar] [CrossRef]
- Suzuki, K.; Bose, P.; Leong-Quong, R.Y.; Fujita, D.J.; Riabowol, K. REAP: A two minute cell fractionation method. BMC Res. Notes 2010, 3, 294. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006, 2006, pdb.prot4455. [Google Scholar] [CrossRef]
- Yamagata, K.; Miyashita, A.; Matsufuji, H.; Chino, M. Dietary flavonoid apigenin inhibits high glucose and tumor necrosis factor alpha-induced adhesion molecule expression in human endothelial cells. J. Nutr. Biochem. 2010, 21, 116–124. [Google Scholar] [CrossRef]
- Sun, M.; Yang, J.; Wang, J.; Hao, T.; Jiang, D.; Bao, G.; Liu, G. TNF-alpha is upregulated in T2DM patients with fracture and promotes the apoptosis of osteoblast cells in vitro in the presence of high glucose. Cytokine 2016, 80, 35–42. [Google Scholar] [CrossRef]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar] [CrossRef]
- Ijima, R.; Kaneko, H.; Ye, F.; Nagasaka, Y.; Takayama, K.; Kataoka, K.; Kachi, S.; Iwase, T.; Terasaki, H. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6673–6678. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, Y.; Xie, W.; Shi, X.; Li, F.; Yang, F.; Sun, Y.; Huang, L.; Li, X. Interleukin-1beta Level Is Increased in Vitreous of Patients with Neovascular Age-Related Macular Degeneration (nAMD) and Polypoidal Choroidal Vasculopathy (PCV). PLoS ONE 2015, 10, e0125150. [Google Scholar]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Muller cells and diabetic retinopathy. Vision Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Mohammad, G.; AlSharif, H.M.; Siddiquei, M.M.; Ahmad, A.; Alam, K.; Abu El-Asrar, A.M. Rho-Associated Protein Kinase-1 Mediates the Regulation of Inflammatory Markers in Diabetic Retina and in Retinal Muller Cells. Ann. Clin. Lab. Sci. 2018, 48, 137–145. [Google Scholar]
- Sigurdardottir, S.; Zapadka, T.E.; Lindstrom, S.I.; Liu, H.; Taylor, B.E.; Lee, C.A.; Kern, T.S.; Taylor, P.R. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability. Cell. Immunol. 2019, 341, 103921. [Google Scholar] [CrossRef]
- Zhou, T.; Che, D.; Lan, Y.; Fang, Z.; Xie, J.; Gong, H.; Li, C.; Feng, J.; Hong, H.; Qi, W.; et al. Mesenchymal marker expression is elevated in Muller cells exposed to high glucose and in animal models of diabetic retinopathy. Oncotarget 2017, 8, 4582–4594. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Chen, X. Protective effects of sulforaphane on diabetic retinopathy: Activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C.; McLaughlin, T.; Wang, Y.; Le, Y.Z.; Wang, J.J.; Zhang, S.X. Loss of X-box binding protein 1 in Muller cells augments retinal inflammation in a mouse model of diabetes. Diabetologia 2019, 62, 531–543. [Google Scholar] [CrossRef]
- Hoover, H.E.; Thuerauf, D.J.; Martindale, J.J.; Glembotski, C.C. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J. Biol. Chem. 2000, 275, 23825–23833. [Google Scholar] [CrossRef]
- Piao, C.S.; Kim, S.W.; Kim, J.B.; Lee, J.K. Co-induction of alphaB-crystallin and MAPKAPK-2 in astrocytes in the penumbra after transient focal cerebral ischemia. Exp. Brain Res. 2005, 163, 421–429. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Z.; Reiser, G. Specific phosphorylation of alphaA-crystallin is required for the alphaA-crystallin-induced protection of astrocytes against staurosporine and C2-ceramide toxicity. Neurochem. Int. 2012, 60, 652–658. [Google Scholar] [CrossRef]
- Webster, K.A. Serine phosphorylation and suppression of apoptosis by the small heat shock protein alphaB-crystallin. Circ. Res. 2003, 92, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Potilinski, M.C.; Lorenc, V.; Perisset, S.; Gallo, J.E. Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 2351. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Gawinowicz-Kolks, M.A.; Spector, A. The phosphorylation of the primary gene products of alpha-crystallin. J. Biol. Chem. 1987, 262, 1438–1441. [Google Scholar] [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Novel roles for alpha-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576–604. [Google Scholar] [CrossRef]
- Voorter, C.E.; Mulders, J.W.; Bloemendal, H.; de Jong, W.W. Some aspects of the phosphorylation of alpha-crystallin A. Eur. J. Biochem. 1986, 160, 203–210. [Google Scholar] [CrossRef]
- Harada, C.; Okumura, A.; Namekata, K.; Nakamura, K.; Mitamura, Y.; Ohguro, H.; Harada, T. Role of monocyte chemotactic protein-1 and nuclear factor kappa B in the pathogenesis of proliferative diabetic retinopathy. Diabetes Res. Clin. Pract. 2006, 74, 249–256. [Google Scholar] [CrossRef]
- Meleth, A.D.; Agron, E.; Chan, C.C.; Reed, G.F.; Arora, K.; Byrnes, G.; Csaky, K.G.; Ferris, F.L., 3rd; Chew, E.Y. Serum inflammatory markers in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4295–4301. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, R.K.; Miller, L.J.; Singh, P.K.; Kanwar, M. Muller glia in retinal innate immunity: A perspective on their roles in endophthalmitis. Crit. Rev. Immunol. 2013, 33, 119–135. [Google Scholar] [CrossRef]
- Kumar, A.; Shamsuddin, N. Retinal Muller glia initiate innate response to infectious stimuli via toll-like receptor signaling. PLoS ONE 2012, 7, e29830. [Google Scholar]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Muller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef]
- Vujosevic, S.; Simo, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO68–BIO75. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Parikh, B.H.; Wey, Y.S.; Tun, B.B.; Wong, T.Y.; Luu, C.D.; Agrawal, R.; Ghosh, A.; Mortellaro, A.; et al. The NLRP3 Inflammasome May Contribute to Pathologic Neovascularization in the Advanced Stages of Diabetic Retinopathy. Sci. Rep. 2018, 8, 2847. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| β-actin | Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT | |
| IL-6 | Forward | TAGTCCTTCCTACCCCAATTTCC |
| Reverse | TTGGTCCTTAGCCACTCCTTC | |
| IL-1β | Forward | TTCAGGCAGGCAGTATCACTC |
| Reverse | GAAGGTCCACGGGAAAGACAC | |
| IL-18 | Forward | GACTCTTGCGTCAACTTCAAGG |
| Reverse | CAGGCTGTCTTTTGTCAACGA | |
| CCL2 | Forward | TTAAAAACCTGGATCGGAACCAA |
| Reverse | GCATTAGCTTCAGATTTACGGGT | |
| VEGF | Forward | GGCCTCCGAAACCATGAACTT |
| Reverse | TGGGACCACTTGGCATGGTG | |
| ICAM1 | Forward | GAGCCAATTTCTCATGCCGC |
| Reverse | GCTGGAAGATCGAAAGTCCG | |
| TNFa | Forward | CAGGCGGTGCCTATGTCTC |
| Reverse | CGATCACCCCGAAGTTCAGTAG | |
| Nf-kB1 | Forward | TCCACTGTCTGCCTCTCTCGTC |
| Reverse | GCCTTCAATAGGTCCTTCCTGC | |
| ASC | Forward | CAGCAACACTCCGGTCAG |
| Reverse | AGCTGGCTTTTCGTATATTGTG | |
| Casp1 | Forward | GGAAGCAATTTATCAACTCAGTG |
| Reverse | GCCTTGTCCATAGCAGTAATG | |
| Nlrp3 | Forward | ATTACCCGCCCGAGAAAGG |
| Reverse | TCGCAGCAAAGATCCACACAG | |
| Prdx6 | Forward | CGCCAGAGTTTGCCAAGAG |
| Reverse | TCCGTGGGTGTTTCACCATTG | |
| GLUL | Forward | TGAACAAAGGCATCAAGCAAATG |
| Reverse | CAGTCCAGGGTACGGGTCTT | |
| Abc8a | Forward | CGTGGGCCTTATTGTGCAAGA |
| Reverse | CAGGTCCACATCAGGCAGTG | |
| Rpe65 | Forward | ACCACTAACAGCTCATGTCACA |
| Reverse | ACAGGTGATAGAAAGGCTCAGAT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, M.; Shan, Y.; Myers, A.M.; Fort, P.E. HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways. J. Clin. Med. 2021, 10, 2384. https://doi.org/10.3390/jcm10112384
Nath M, Shan Y, Myers AM, Fort PE. HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways. Journal of Clinical Medicine. 2021; 10(11):2384. https://doi.org/10.3390/jcm10112384
Chicago/Turabian StyleNath, Madhu, Yang Shan, Angela M. Myers, and Patrice Elie Fort. 2021. "HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways" Journal of Clinical Medicine 10, no. 11: 2384. https://doi.org/10.3390/jcm10112384
APA StyleNath, M., Shan, Y., Myers, A. M., & Fort, P. E. (2021). HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways. Journal of Clinical Medicine, 10(11), 2384. https://doi.org/10.3390/jcm10112384






