Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review
Abstract
1. Introduction
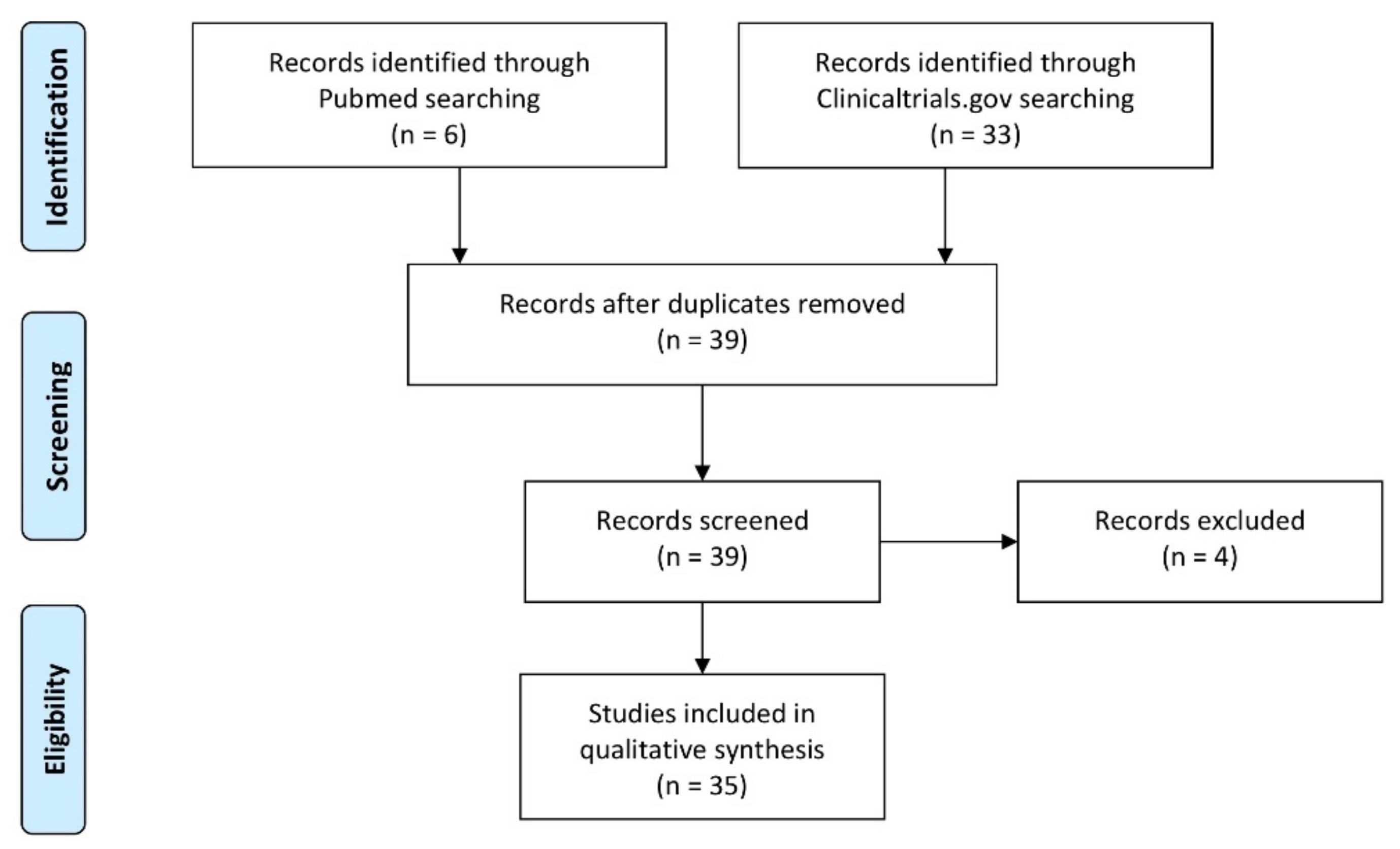
2. Materials and Methods
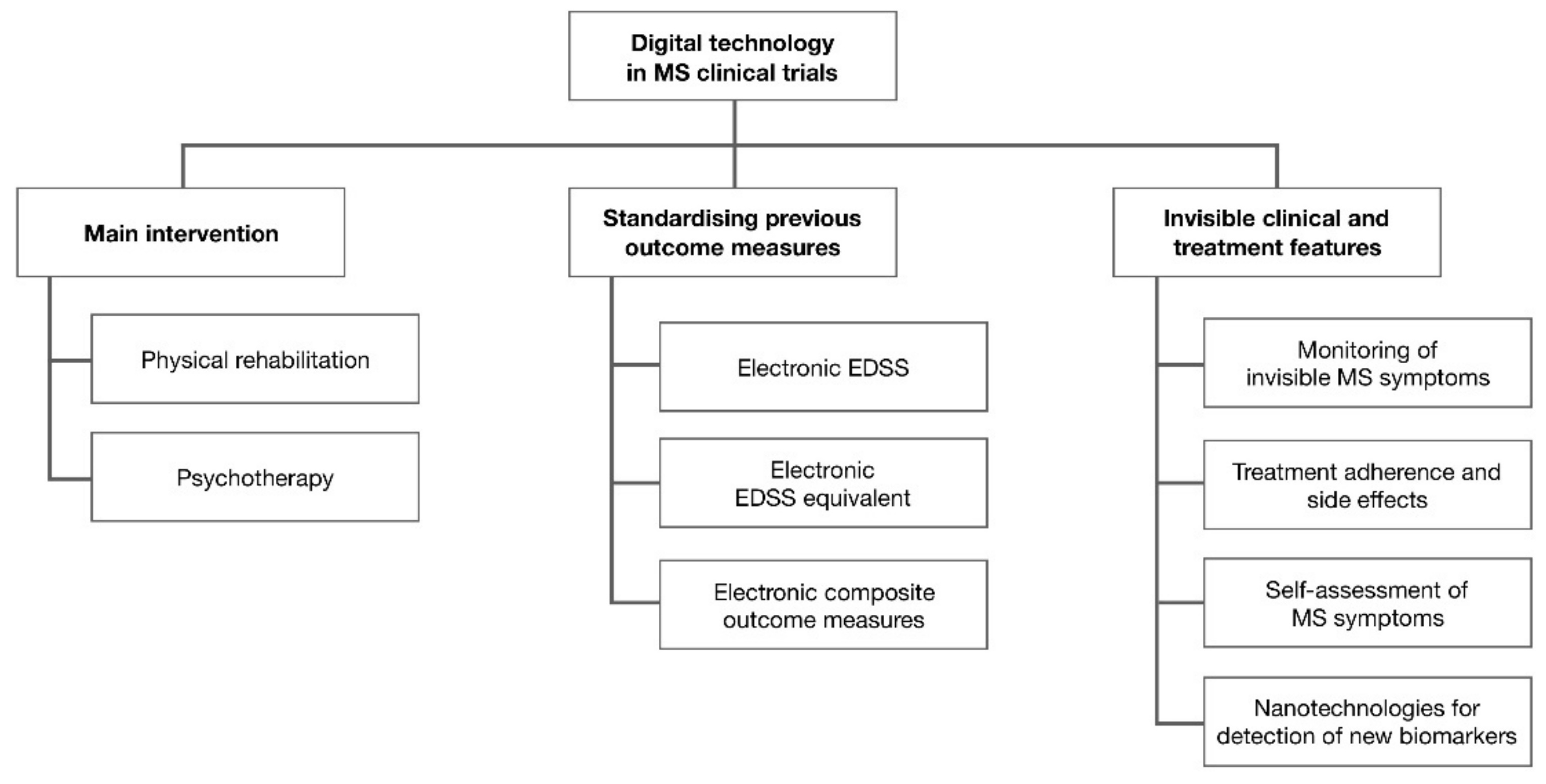
3. Results
3.1. Main Intervention
3.2. Standardising Previous Outcome Measures
3.3. Detection of Invisible Clinical and Treatment Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bill, F. Foundation MG. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2016, 2019, 269–285. [Google Scholar]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Giunti, G.; Fernández, E.G.; Zubiete, E.D.; Romero, O.R. Supply and Demand in mHealth Apps for Persons with Multiple Sclerosis: Systematic Search in App Stores and Scoping Literature Review. JMIR mHealth uHealth 2018, 6, e10512. [Google Scholar] [CrossRef] [PubMed]
- Lorefice, L.; Fenu, G.; Frau, J.; Coghe, G.; Marrosu, M.G.; Cocco, E. The impact of visible and invisible symptoms on employment status, work and social functioning in Multiple Sclerosis. Work 2018, 60, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Marziniak, M.; Brichetto, G.; Feys, P.; Meyding-Lamadé, U.; Vernon, K.; Meuth, S.G. The Use of Digital and Remote Communication Technologies as a Tool for Multiple Sclerosis Management: Narrative Review. JMIR Rehabilit. Assist. Technol. 2018, 5, e5. [Google Scholar] [CrossRef]
- Taylor, C.B.; Ruzek, J.I.; Fitzsimmons-Craft, E.; Sadeh-Sharvit, S.; Topooco, N.; Weissman, R.S.; Eisenberg, D.; Mohr, D.; Graham, A.; Jacobi, C.; et al. Using Digital Technology to Reduce the Prevalence of Mental Health Disorders in Populations: Time for a New Approach. J. Med. Internet Res. 2020, 22, e17493. [Google Scholar] [CrossRef]
- Rossen, S.; Kayser, L.; Vibe-Petersen, J.; Christensen, J.F.; Ried-Larsen, M. Cancer Survivors’ Receptiveness to Digital Technolo-gy-Supported Physical Rehabilitation and the Implications for Design: Qualitative Study. J. Med. Int. Res. 2020, 22, e15335. [Google Scholar]
- Lee, Y.; Raviglione, M.C.; Flahault, A. Use of Digital Technology to Enhance Tuberculosis Control: Scoping Review. J. Med. Internet Res. 2020, 22, e15727. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Classification of Digital Health Interventions v1.A Shared Language to Describe the Uses of Digital Technology for Health; WHO: Rome, Italy, 2018. [Google Scholar]
- Lavorgna, L.; Brigo, F.; Moccia, M.; Leocani, L.; Lanzillo, R.; Clerico, M.; Abbadessa, G.; Schmierer, K.; Solaro, C.; Prosperini, L.; et al. e-Health and multiple sclerosis: An update. Mult. Scler. J. 2018, 24, 1657–1664. [Google Scholar] [CrossRef]
- Haase, R.; Schultheiss, T.; Kempcke, R.; Thomas, K.; Ziemssen, T. Use and Acceptance of Electronic Communication by Patients with Multiple Sclerosis: A Multicenter Questionnaire Study. J. Med. Internet Res. 2012, 14, e135. [Google Scholar] [CrossRef]
- Thomas, S.; Pulman, A.; Thomas, P.; Collard, S.; Jiang, N.; Dogan, H.; Smith, A.D.; Hourihan, S.; Roberts, F.; Kersten, P.; et al. Digitizing a Face-to-Face Group Fatigue Management Program: Exploring the Views of People with Multiple Sclerosis and Health Care Professionals Via Consultation Groups and Interviews. JMIR Form. Res. 2019, 3, e10951. [Google Scholar] [CrossRef]
- Simblett, S.; Matcham, F.; Curtis, H.; Greer, B.; Polhemus, A.; Novák, J.; Ferrao, J.; Gamble, P.; Hotopf, M.; Narayan, V.; et al. Patients’ Measurement Priorities for Remote Measurement Technologies to Aid Chronic Health Conditions: Qualitative Analysis. JMIR mHealth uHealth 2020, 8, e15086. [Google Scholar] [CrossRef]
- Thirumalai, M.; Rimmer, J.H.; Johnson, G.; Wilroy, J.; Young, H.-J.; Mehta, T.; Lai, B. TEAMS (Tele-Exercise and Multiple Sclerosis), a Tailored Telerehabilitation mHealth App: Participant-Centered Development and Usability Study. JMIR mHealth uHealth 2018, 6, e10181. [Google Scholar] [CrossRef]
- Kayser, L.; Karnoe, A.; Furstrand, D.; Batterham, R.; Christensen, K.B.; Elsworth, G.; Osborne, R.H. A Multidimensional Tool Based on the eHealth Literacy Framework: Development and Initial Validity Testing of the eHealth Literacy Questionnaire (eHLQ). J. Med. Internet Res. 2018, 20, e36. [Google Scholar] [CrossRef]
- Zhou, L.; Parmanto, B. Reaching People with Disabilities in Underserved Areas Through Digital Interventions: Systematic Review. J. Med. Internet Res. 2019, 21, e12981. [Google Scholar] [CrossRef]
- Van Kessel, K.; Babbage, D.R.; Reay, N.; Miner-Williams, W.M.; Kersten, P. Mobile Technology Use by People Experiencing Multiple Sclerosis Fatigue: Survey Methodology. JMIR mHealth uHealth 2017, 5, e6. [Google Scholar] [CrossRef]
- Green, B.M.; Van Horn, K.T.; Gupte, K.; Evans, M.; Hayes, S.; Bhowmick, A. Adaptive Engagement and Support Model for People with Chronic Health Conditions: Using Combined Content Analysis to Assess Online Health Communities. J. Med. Internet Res. 2020. [Google Scholar] [CrossRef]
- D’Haeseleer, M.; Eelen, P.; Sadeghi, N.; D’Hooghe, M.B.; Van Schependom, J.; Nagels, G. Feasibility of real-time internet-based tel-econsultation in patients with multiple sclerosis: Interventional pilot study. J. Med. Internet Res. 2020, 22, e18178. [Google Scholar] [CrossRef]
- Midaglia, L.; Mulero, P.; Montalban, X.; Graves, J.; Hauser, S.L.; Julian, L.; Baker, M.; Schadrack, J.; Gossens, C.; Scotland, A.; et al. Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People with Multiple Sclerosis: Nonrandomized Interventional Feasibility Study. J. Med. Internet Res. 2019, 21, e14863. [Google Scholar] [CrossRef]
- Amato, M.P.; Fratiglioni, L.; Groppi, C.; Siracusa, G.; Amaducci, L. Interrater Reliability in Assessing Functional Systems and Dis-ability on the Kurtzke Scale in Multiple Sclerosis. Arch. Neurol. 1988, 45, 746–748. [Google Scholar] [CrossRef]
- Hirst, C.L.; Ingram, G.; Pickersgill, T.P.; Robertson, N.P. Temporal evolution of remission following multiple sclerosis relapse and predictors of outcome. Mult. Scler. J. 2012, 18, 1152–1158. [Google Scholar] [CrossRef]
- Moccia, M.; De Stefano, N.; Barkhof, F. Imaging outcome measures for progressive multiple sclerosis trials. Mult. Scler. J. 2017, 23, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Hohol, M.J.; Orav, E.J.; Weiner, H.L. Disease steps in multiple sclerosis: A longitudinal study comparing Disease Steps and EDSS to evaluate disease progression. Mult. Scler. J. 1999, 5, 349–354. [Google Scholar] [CrossRef]
- Cadavid, D.; Tang, Y.; O’Neill, G. Responsiveness of the Expanded Disability Status Scale (EDSS) to disease progression and therapeutic intervention in progressive forms of multiple sclerosis. Rev. Neurol. 2010, 51, 321–329. [Google Scholar]
- Saccà, F.; Costabile, T.; Carotenuto, A.; Lanzillo, R.; Moccia, M.; Pane, C.; Russo, C.V.; Barbarulo, A.M.; Casertano, S.; Rossi, F.; et al. The EDSS integration with the Brief International Cognitive Assessment for Multiple Sclerosis and orientation tests. Mult. Scler. 2017, 23, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Tur, C.; Moccia, M.; Barkhof, F.; Chataway, J.; Sastre-Garriga, J.; Thompson, A.J.; Ciccarelli, O. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat. Rev. Neurol. 2018, 14, 75–93. [Google Scholar] [CrossRef]
- Jongen, P.J.; Kremer, I.E.; Hristodorova, E.; Evers, S.M.; Kool, A.; Van Noort, E.M.; Hiligsmann, M.; Haase, R.; Lavorgna, L. Adherence to Web-Based Self-Assessments in Long-Term Direct-to-Patient Research: Two-Year Study of Multiple Sclerosis Patients. J. Med. Internet Res. 2017, 19, e249. [Google Scholar] [CrossRef]
- Khurana, V.; Sharma, H.; Afroz, N.; Callan, A.; Medin, J. Patient-reported outcomes in multiple sclerosis: A systematic comparison of available measures. Eur. J. Neurol. 2017, 24, 1099–1107. [Google Scholar] [CrossRef]
- D’Amico, E.; Haase, R.; Ziemssen, T. Review: Patient-reported outcomes in multiple sclerosis care. Mult. Scler. Relat. Disord. 2019, 33, 61–66. [Google Scholar] [CrossRef]
- Ontaneda, D.; Fox, R.J.; Chataway, J. Clinical trials in progressive multiple sclerosis: Lessons learned and future perspectives. Lancet Neurol. 2015, 14, 208–223. [Google Scholar] [CrossRef]
- Graves, J.S.; Montalban, X. Biosensors to monitor MS activity. Mult. Scler. J. 2020, 26, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Inojosa, H.; Schriefer, D.; Ziemssen, T. Clinical outcome measures in multiple sclerosis: A review. Autoimmun. Rev. 2020, 19, 102512. [Google Scholar] [CrossRef] [PubMed]
- Barkhof, F.; Scheltens, P.; Frequin, S.; Nauta, J.; Tas, M.; Valk, J.; Hommes, O. Relapsing-remitting multiple sclerosis: Sequential enhanced MR imaging vs clinical findings in determining disease activity. AJR Am. J. Roentgenol. 1992, 159, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, L.E.; Planchon, S.M.; Bermel, R.A.; Nakamura, K.; Fisher, E.; Feng, J.; Sakaie, K.E.; Ontaneda, D.; Cohen, J.A. Serum neuro-filament light chain concentration in a phase 1/2 trial of autologous mesenchymal stem cell transplantation. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 205521731988719. [Google Scholar]
- Kuhle, J.; Disanto, G.; Lorscheider, J.; Stites, T.; Chen, Y.; Dahlke, F.; Francis, G.; Shrinivasan, A.; Radue, E.-W.; Giovannoni, G.; et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 2015, 84, 1639–1643. [Google Scholar] [CrossRef]
- NCT01918748. Effect of I-TRAVLE Training on Arm Function in MS and Chronic Stroke Patients; US National Library of Medicine: Bethesda, MD, USA, 2013.
- NCT02771652. Effect of an Internet-Based at Home Physical Training Protocol on Quality of Life, Fatigue, Functional Performance, Aerobic Capacity and Muscle Strength in Multiple Sclerosis Patients (ms-intakt); US National Library of Medicine: Bethesda, MD, USA, 2016.
- NCT03458169. LEAP a New Overground Body Weight Support. Robot.: Usability Trial (LEAP); US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT03548974. Internet-Based MOTOmed Exercise to Reduce Spasticity and Improve Physical Function in Persons with Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2018.
- NCT03569618. Digital Cognition in Multiple Sclerosis (DigCog); University of California; US National Library of Medicine: Bethesda, MD, USA, 2020.
- NCT03829267. eFIT: An Internet-based Intervention to Increase Physical Activity in Persons with MS (eFIT); US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT03968172. Interactive Web Platform for EmPOWERment in Early Multiple Sclerosis (POWER@MS1); US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT04147052. iSLEEPms: An Internet-Delivered Intervention for Sleep Disturbance in Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT04212689. Virtual Reality Approach in Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT04244214. Pilot Study for the Evaluation of the More Stamina in Persons with Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2020.
- NCT01829776. Pilot Study of Free from Falls Program in Multiple Sclerosis (FFF); US National Library of Medicine: Bethesda, MD, USA, 2013.
- NCT02369926. Endpoint Calibration for a Phase 2 Study of Lisinopril in Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2015.
- Rhodes, J.K.; Schindler, D.; Rao, S.M.; Venegas, F.; Bruzik, E.T.; Gabel, W.; Williams, J.R.; Phillips, G.A.; Mullen, C.C.; Freiburger, J.L.; et al. Multiple Sclerosis Performance Test: Technical Development and Usability. Adv. Ther. 2019, 36, 1741–1755. [Google Scholar] [CrossRef]
- NCT03369171. Wearable Biosensor to Track and Quantify Limb Dysfunction in Multiple Sclerosis Patients (MYO); US National Library of Medicine: Bethesda, MD, USA, 2017.
- NCT03369470. App Based Dexterity Training in Multiple Sclerosis (AppDext); US National Library of Medicine: Bethesda, MD, USA, 2017.
- NCT03586635. Automated EDSS Score Calculation by Smartphone Application (Easy EDSS); US National Library of Medicine: Bethesda, MD, USA, 2018.
- NCT04281160. Patient Centered Outcomes Analysis for MS Using a Mobile Application; US National Library of Medicine: Bethesda, MD, USA, 2020.
- NCT04288011. Validation of the BeCare Multiple Sclerosis Assessment App; US National Library of Medicine: Bethesda, MD, USA, 2020.
- Kim, E.; Lovera, J.; Schaben, L.; Melara, J.; Bourdette, D.; Whitham, R. Novel method for measurement of fatigue in multiple sclerosis: Real-Time Digital Fatigue Score. J. Rehabil. Res. Dev. 2010, 47, 477–484. [Google Scholar] [CrossRef]
- NCT01206023. Applications of Nanotechnology in Multiple Sclerosis by Respiratory Samples (MS-NANOSE); US National Library of Medicine: Bethesda, MD, USA, 2010.
- NCT01233245. BetaPlus Survey—Observational Study to Assess. Drug Adherence in Patients with Multiple Sclerosis after Conversion to Betaferon® by Using Elements of the BetaPlus Program; US National Library of Medicine: Bethesda, MD, USA, 2010.
- NCT01235455. Portuguese Observational Survey to Assess. Drug Adherence in Patients with Multiple Sclerosis after Conversion to Betaferon by Using Elements of the BetaPlus Program—Nurse Support. Auto-injectors (POR-BetaPlus); US National Library of Medicine: Bethesda, MD, USA, 2010.
- Salimzadeh, Z.; Damanabi, S.; Kalankesh, L.R.; Ferdousi, R. Mobile Applications for Multiple Sclerosis: A Focus on Self-Management. Acta Inform. Med. 2019, 27, 12–18. [Google Scholar] [CrossRef]
- NCT01954823. Use of an Electronic Diary by People with Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2013.
- NCT02583386. Comprehensive Fall Prevention and Detection in Multiple Sclerosis (FFF); US National Library of Medicine: Bethesda, MD, USA, 2015.
- NCT02814487. MSFC versus DAM. A Smartphone Application for Multiple Sclerosis Self-Assessment (DAM); US National Library of Medicine: Bethesda, MD, USA, 2016.
- NCT01419301. Mindfulness Based Stress Reduction in Multiple Sclerosis (MS); US National Library of Medicine: Bethesda, MD, USA, 2011.
- Defer, G.; Le Caignec, F.; Fedrizzi, S.; Montastruc, F.; Chevanne, D.; Parienti, J.-J.; Peyro-Saint-Paul, L. Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip-SEP study): Study protocol for a randomized controlled trial. Trials 2018, 19, 174. [Google Scholar] [CrossRef]
- Limmroth, V.; Hechenbichler, K.; Müller, C.; Schürks, M. Assessment of Medication Adherence Using a Medical App Among Patients with Multiple Sclerosis Treated with Interferon Beta-1b: Pilot Digital Observational Study (PROmyBETAapp). J. Med. Internet Res. 2019, 21, e14373. [Google Scholar] [CrossRef]
- NCT03148938. Digitalization of Neurofunctional Tests via a Mobile Application DAMS for Multiple Sclerosis Patients (MSCopilot); US National Library of Medicine: Bethesda, MD, USA, 2017.
- NCT03523858. A Study to Evaluate Ocrelizumab Treatment in Participants with Progressive Multiple Sclerosis (CONSO-NANCE); US National Library of Medicine: Bethesda, MD, USA, 2018.
- NCT03808142. PROmyBETAappGame: A Study to Learn. More about the Medication Usage & Patient Reported Outcomes via the myBETAapp and to Find Out More about the Usage of Game Principles and Game Design Elements (Gamification) in Medical Care of Patients with Multiple Scle; US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT04116424. VIGIP-SEP2: Evaluation of the Impact of the Training of Patients by a Nurse on the Adverse Drug Reaction Reporting by RRMS Patient via a Mobile Application: Randomized Real-Life Study (VIGIP-SEP2); US National Library of Medicine: Bethesda, MD, USA, 2019.
- NCT04130256. Electronic Pill Bottle Monitoring to Promote Medication Adherence for People with Multiple Sclerosis; US National Library of Medicine: Bethesda, MD, USA, 2019.
- D’Souza, M.; Yaldizli, Ö.; John, R.; Vogt, D.R.; Papadopoulou, A.; Lucassen, E.; Menegola, M.; Andelova, M.; Dahlke, F.; Schnyder, F.; et al. Neurostatus e-Scoring improves consistency of Expanded Disability Status Scale assessments: A proof of concept study. Mult. Scler. J. 2016, 23, 597–603. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.-S.; Maeurer, M.; Dippel, F.-W.; Kohlmann, T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Plow, M.; Golding, M. Using mHealth Technology in a Self-Management Intervention to Promote Physical Activity Among Adults with Chronic Disabling Conditions: Randomized Controlled Trial. JMIR mHealth uHealth 2017, 5, e185. [Google Scholar] [CrossRef]
- Leocani, L.; Diserens, K.; Moccia, M.; Caltagirone, C.; Neurology-Ean, N.S.P.O.T.E.A.O.; Neurorehabilitation Scientific Panel of the European Academy of Neurology—EAN. Disability through COVID-19 pandemic: Neurorehabilitation cannot wait. Eur. J. Neurol. 2020, 27. [Google Scholar] [CrossRef]
- Isernia, S.; Pagliari, C.; Jonsdottir, J.; Castiglioni, C.; Gindri, P.; Gramigna, C.; Palumbo, G.; Salza, M.; Molteni, F.; Baglio, F. Efficiency and Patient-Reported Outcome Measures From Clinic to Home: The Human Empowerment Aging and Disability Program for Digi-tal-Health Rehabilitation. Front. Neurol. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Taylor, M.J.D.; Griffin, M. The use of gaming technology for rehabilitation in people with multiple sclerosis. Mult. Scler. J. 2015, 21, 355–371. [Google Scholar] [CrossRef]
- Mura, G.; Carta, M.G.; Sancassiani, F.; Machado, S.; Prosperini, L. Active exergames to improve cognitive functioning in neuro-logical disabilities: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 450–462. [Google Scholar] [CrossRef]
- Schwartz, I.; Meiner, Z. Robotic-Assisted Gait Training in Neurological Patients: Who May Benefit? Ann. Biomed. Eng. 2015, 43, 1260–1269. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, X.; Wang, J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav. Neurol. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Moccia, M.; Picillo, M.; Erro, R.; Vitale, C.; Longo, K.; Amboni, M.; Santangelo, G.; Palladino, R.; Capo, G.; Orefice, G.; et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson’ s disease. Eur. J. Neurol. 2015, 22, 93–98. [Google Scholar] [CrossRef]
- Moccia, M.; Palladino, R.; Russo, C.; Massarelli, M.; Nardone, A.; Triassi, M.; Lugaresi, A.; Morra, V.B. How many injections did you miss last month? A simple question to predict interferon β-1a adherence in multiple sclerosis. Expert Opin. Drug Deliv. 2015, 12, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, E.; Multanen, J.; Atula, S. Subcutaneous Interferon β-1a Administration by Electronic Auto-Injector is Associated with High Adherence in Patients with Relapsing Remitting Multiple Sclerosis in a Real-Life Study. Neurol. Int. 2017, 9, 8–10. [Google Scholar] [CrossRef]
- Wicks, P.; Massagli, M.; Kulkarni, A.; Dastani, H.; Jacobs, D.; Jongen, P. Use of an Online Community to Develop Patient-Reported Outcome Instruments: The Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). J. Med. Internet Res. 2011, 13, e12. [Google Scholar] [CrossRef]
- Yeh, E.A.; Grover, S.A.; Powell, V.E.; Alper, G.; Banwell, B.L.; Edwards, K.; Gorman, M.; Graves, J.; Lotze, T.E.; Mah, J.K.; et al. Impact of an electronic monitoring device and behavioral feedback on adherence to multiple sclerosis therapies in youth: Results of a randomized trial. Qual. Life Res. 2017, 26, 2333–2349. [Google Scholar] [CrossRef]
- Moccia, M.; Loperto, I.; Lanzillo, R.; Capacchione, A.; Carotenuto, A.; Triassi, M.; Morra, V.B.; Palladino, R. Persistence, adherence, healthcare resource utilisation and costs for interferon Beta in multiple sclerosis: A population-based study in the Campania region (southern Italy). BMC Health Serv. Res. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Hone, T.; Palladino, R.; Filippidis, F.T. Association of searching for health-related information online with self-rated health in the European Union. Eur. J. Public Health 2016, 26, 748–753. [Google Scholar] [CrossRef]
- Moccia, M.; Brigo, F.; Tedeschi, G.; Bonavita, S.; Lavorgna, L. Neurology and the Internet: A review. Neurol. Sci. 2018, 39, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Harrington, R.A.; McClellan, M.B.; Turakhia, M.P.; Eapen, Z.J.; Steinhubl, S.; Mault, J.R.; Majmudar, M.D.; Roessig, L.; Chandross, K.J.; et al. Using Digital Health Technology to Better Generate Evidence and Deliver Evidence-Based Care. J. Am. Coll. Cardiol. 2018, 71, 2680–2690. [Google Scholar] [CrossRef]
- Benedetti, F. Placebo Effects: From the Neurobiological Paradigm to Translational Implications. Neuron 2014, 84, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Bergendorff, R. Digital Devices in Clinical Trials Clinical and Medical Research. IPI Clin. Med. Res. 2018, 10. [Google Scholar]
- FDA. Digital Health Innovation Action Plan; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Aarestrup, F.M.; Albeyatti, A.; Armitage, W.J.; Auffray, C.; Augello, L.; Balling, R.; Benhabiles, N.; Bertolini, G.; Bjaalie, J.G.; Black, M.; et al. Towards a European health research and innovation cloud (HRIC). Genome Med. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Tacchino, A.; Veldkamp, R.; Coninx, K.; Brulmans, J.; Palmaers, S.; Hämäläinen, P.; D’Hooge, M.; Vanzeir, E.; Kalron, A.; Brichetto, G.; et al. Cognitive-motor interference in people with Multiple Sclerosis: Design, development and testing of CMI-APP for dual task assessment and training. JMIR mHealth uHealth 2019, 8. [Google Scholar] [CrossRef]
- Moccia, M. Teleconsultation will replace most face-to-face interactions in the multiple sclerosis clinic—No. Mult. Scler. J. 2021, 27, 176–177. [Google Scholar] [CrossRef]
- Van Dongen, L.; Westerik, B.; van der Hiele, K.; Visser, L.H.; Schoonheim, M.M.; Douw, L.; Twisk, J.W.R.; de Jong, B.A.; Geurts, J.J.G.; Hulst, H.E. Introducing Multiple Screener: An unsupervised digital screening tool for cognitive deficits in MS. Mult. Scler. Relat. Disord. 2020, 38, 101479. [Google Scholar] [CrossRef]
- Ziemssen, T.; Piani-Meier, D.; Bennett, B.; Johnson, C.; Tinsley, K.; Trigg, A.; Hach, T.; Dahlke, F.; Tomic, D.; Tolley, C.; et al. A Physician-Completed Digital Tool for Evaluating Disease Progression (Multiple Sclerosis Progression Discussion Tool): Validation Study. J. Med. Internet Res. 2020, 22, e16932. [Google Scholar] [CrossRef]
- Apolinário-Hagen, J.; Menzel, M.; Hennemann, S.; Salewski, C. Acceptance of Mobile Health Apps for Disease Management Among People with Multiple Sclerosis: Web-Based Survey Study. JMIR Form. Res. 2018, 2, e11977. [Google Scholar] [CrossRef]
- Van Beek, J.J.W.; van Wegen, E.E.H.; Rietberg, M.B.; Nyffeler, T.; Bohlhalter, S.; Kamm, C.P.; Nef, T.; Vanbellingen, T. Feasibility of a Home-Based Tablet App for Dexterity Training in Multiple Sclerosis: Usability Study. JMIR mHealth uHealth 2020, 8, e18204. [Google Scholar] [CrossRef]
- Tacchino, A.; Veldkamp, R.; Coninx, K.; Brulmans, J.; Palmaers, S.; Hämäläinen, P.; D’hooge, M.; Vanzeir, E.; Kalron, A.; Brichetto, G.; et al. Design, Development, and Testing of an App for Dual-Task Assessment and Training Regarding Cognitive-Motor Interference (CMI-APP) in People with Multiple Sclerosis: Multicenter Pilot Study. JMIR mHealth uHealth 2020, 8, e15344. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Morra, V.B.; Bonavita, S.; Tedeschi, G.; Leocani, L.; Lavorgna, L.; on behalf of the Digital Technologies Web and Social Media Study Group of the Italian Society of Neurology. Assessing disability and relapses in multiple sclerosis on tele-neurology. Neurol. Sci. 2020, 41, 1369–1371. [Google Scholar] [CrossRef]
- Moccia, M.; Palladino, R.; Falco, A.; Saccà, F.; Lanzillo, R.; Brescia Morra, V. Google Trends: New evidence for seasonality of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1028–1029. [Google Scholar] [CrossRef]
| Title | MS Phenotype | Other Conditions Included | Age | Study Design | Ref. |
|---|---|---|---|---|---|
| MAIN INTERVENTION | |||||
| Haptic feedback on robot (I-TRAVLE), arm strength and endurance training, 3 times a week for 8 weeks | All MS | Paralytic Stroke | >18 | Interventional Open Label Single Group | [37] |
| Resistance-endurance training at home via internet (e-Training), 10–60 min | All MS | 18–65 | Interventional Randomized Parallel Assignment | [38] | |
| Body-weight support robot (LEAP), usability study, therapist, observer and patient questionnaire | All MS | Spinal Cord Injuries Cerebral Palsy Parkinson Disease Stroke People with impaired lower extremity function | 5–80 | Interventional Open Label Single Group | [39] |
| Home-based movement training (MOTOmed), 12 weeks | All MS | >18 | Interventional Randomized Parallel Assignment | [40] | |
| Processing speed and attention treatment, digital Tablet-based game, 6 weeks | All MS + CIS | Cognitive Decline | 18–71 | Interventional Randomized Parallel Assignment | [41] |
| Internet-based intervention to increase physical activity (eFIT), 12 weeks | All MS | >18 | Interventional Randomized Parallel Assignment | [42] | |
| Web-based behavioral lifestyle intervention (POWER@MS1), 12 months | All MS | 18–65 | Interventional Randomized Parallel Assignment | [43] | |
| Internet-delivered Cognitive Behavioral Intervention for Sleep Disturbance (iSLEEPms), 4 weeks | All MS | >18 | Interventional Randomized Parallel Assignment | [44] | |
| Game Based Virtual Reality Exercises (USE-IT) added to rehabilitation, 4 weeks | All MS | 18–65 | Interventional Randomized Parallel Assignment Open Label | [45] | |
| (MORE STAMINA) Mobile App for Fatigue Management, adherence, 60 days | All MS | Fatigue Chronic Conditions | 18–80 | Observational Prospective | [46] |
| STANDARDASING PREVIOUS OUTCOME MEASURES | |||||
| Fall prevention exercise and education program with electronic diary, 8 weeks | All MS | 18–89 | Interventional Open Label Single Group | [47] | |
| Comparison between Mobile Multiple Sclerosis Functional Composite and classic | Relapsing-remitting MS | 18–65 | Interventional Randomized Crossover Assignment Open Label | [48] | |
| Usability of the Multiple Sclerosis Performance Test Device | All MS + CIS | >18 | Observational Prospective | [49] | |
| (Myo Armband) Wearable Biosensor to Track and Quantify Limb Dysfunction | All MS | 18–65 | Interventional Open Label Single Group | [50] | |
| Change in the Arm Function in Multiple Sclerosis Questionnaire after 4 weeks of Home-Based Dexterity Training via Mobile App | All MS | 18–75 | Interventional Randomized Parallel Assignment | [51] | |
| Automated EDSS Score Calculation Using a Smartphone Application vs paper version | Progressive MS | >18 | Observational Prospective | [52] | |
| Patient Centered Outcomes Analysis using BeCare Mobile App, 6 months | All MS | 18–65 | Observational Prospective | [53] | |
| Validation of the BeCare Multiple Sclerosis Assessment App | All MS | 18–75 | Observational Prospective | [54] | |
| DETECTION OF INVISIBLE CLINICAL AND TREATMENT FEATURES | |||||
| Realtime Digital Fatigue Score as outcome measure | All MS | 18–70 | Interventional Randomized Crossover Assignment | [55] | |
| (NA-NOSE) Artificial olfactory system chemical sensor for the detection and identification of Multiple Sclerosis by Respiratory Samples | All MS | 18–60 | Observational Case-Only Cross-Sectional | [56] | |
| Evaluation of adherence through BetaPlus Program elements (website) | Relapsing-remitting + Secondary Progressive MS | >18 | Observational Prospective | [57] | |
| Portuguese evaluation of adherence through BetaPlus Program elements (website) | Relapsing-remitting MS + Secondary Progressive MS | >18 | Observational Prospective | [58] | |
| Electronic measure of needle disposals (MEMS TrackCaps) as outcome measure | All MS + CIS | 18–70 | Interventional Randomized Parallel Assignment | [59] | |
| Evaluation of adherence through the Use of an Electronic Diary, one year | All MS | 18–70 | Interventional Randomized Parallel Assignment Open Label | [60] | |
| Fall Detection through Electronic Fall Detector, 6 months | All MS | >18 | Interventional Randomized Parallel Assignment Open Label | [61] | |
| Comparing a Smartphone Application with the Composite MSFC Score, 90 days | All MS | >18 | Observational Prospective | [62] | |
| Patient Reported Outcomes Measurement Information System via tablet, 6 weeks | All MS | Fibromyalgia Osteoarthritis Sjögren’s Syndrome Parkinson’s Disease | 18–76 | Interventional Randomized Parallel Assignment | [63] |
| Number of Active and Passive Tests Conducted by patients on smartwatch and smartphone | All MS | 18–55 | Interventional Non-Randomized Parallel Assignment Open Label | [20] | |
| Change in number of reports of adverse drug reactions through app (My eReport France), 6 months | Relapsing-remitting MS | >18 | Interventional Randomized Parallel Assignment Open Label | [64] | |
| Betaferon adherence measured by mobile app (PROmyBETAapp), 6 months | Relapsing-remitting MS | >18 | Observational Prospective | [65] | |
| Digital Assessment Multiple Sclerosis 3 (DAMS-3) vs MSFC | All MS | 18–60 | Interventional Non-Randomized Sequential Assignment Open Label | [66] | |
| Internet collection of Patient Reported Outcomes (SymptoMScreen) as outcome measure, 192 weeks | Progressive MS | 18–65 | Interventional Randomized Single-Group Assignment Open Label | [67] | |
| Ascertaining Medication Usage & Patient Reported Outcomes Via the myBETAapp, 12 weeks | Relapsing-remitting MS | >18 | Observational Prospective | [68] | |
| Evaluation of the Impact of Patients’ Training by Nurse on Adverse Drug Reaction Reporting by Patient Via a Mobile Application (VIGIP-SEP2), 3 months | All MS | >18 | Interventional Randomized Parallel Assignment Open Label | [69] | |
| Promotion and evaluation of adherence via Electronic Pill Bottle, 90 days | Relapsing-remitting MS | >18 | Interventional Randomized Parallel Assignement Open Label | [70] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, M.; Lavorgna, L.; Carotenuto, A.; Petruzzo, M.; Lanzillo, R.; Brescia Morra, V.; Moccia, M. Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review. J. Clin. Med. 2021, 10, 2328. https://doi.org/10.3390/jcm10112328
De Angelis M, Lavorgna L, Carotenuto A, Petruzzo M, Lanzillo R, Brescia Morra V, Moccia M. Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review. Journal of Clinical Medicine. 2021; 10(11):2328. https://doi.org/10.3390/jcm10112328
Chicago/Turabian StyleDe Angelis, Marcello, Luigi Lavorgna, Antonio Carotenuto, Martina Petruzzo, Roberta Lanzillo, Vincenzo Brescia Morra, and Marcello Moccia. 2021. "Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review" Journal of Clinical Medicine 10, no. 11: 2328. https://doi.org/10.3390/jcm10112328
APA StyleDe Angelis, M., Lavorgna, L., Carotenuto, A., Petruzzo, M., Lanzillo, R., Brescia Morra, V., & Moccia, M. (2021). Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review. Journal of Clinical Medicine, 10(11), 2328. https://doi.org/10.3390/jcm10112328







