Is Skeletal Muscle Dysfunction a Limiting Factor of Exercise Functional Capacity in Patients with Sickle Cell Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Experimental Design
2.3. Force Measurement
2.4. 6-Minute Walk Test (6-MWT)
2.5. Electromyography
2.6. Statistical Analysis
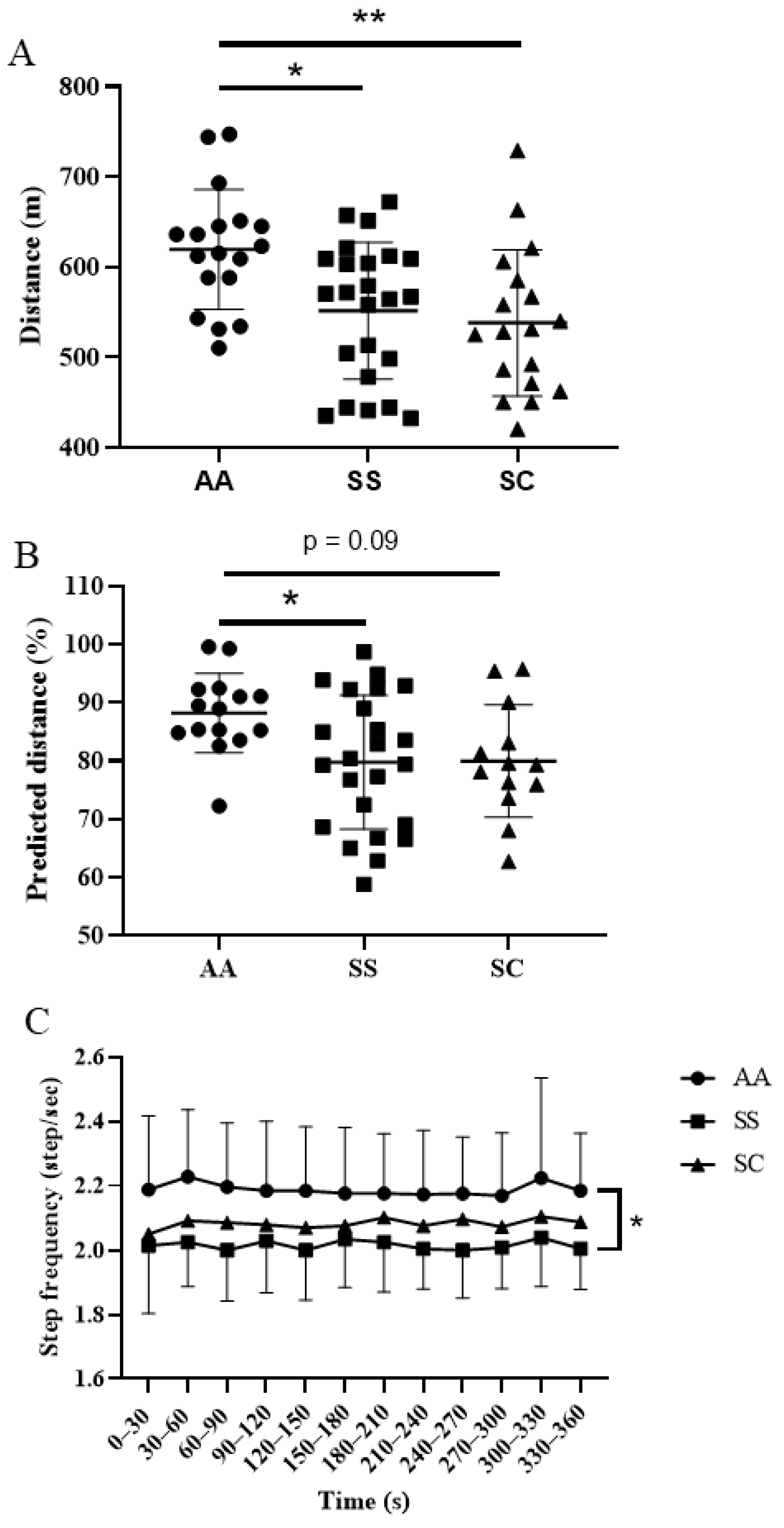
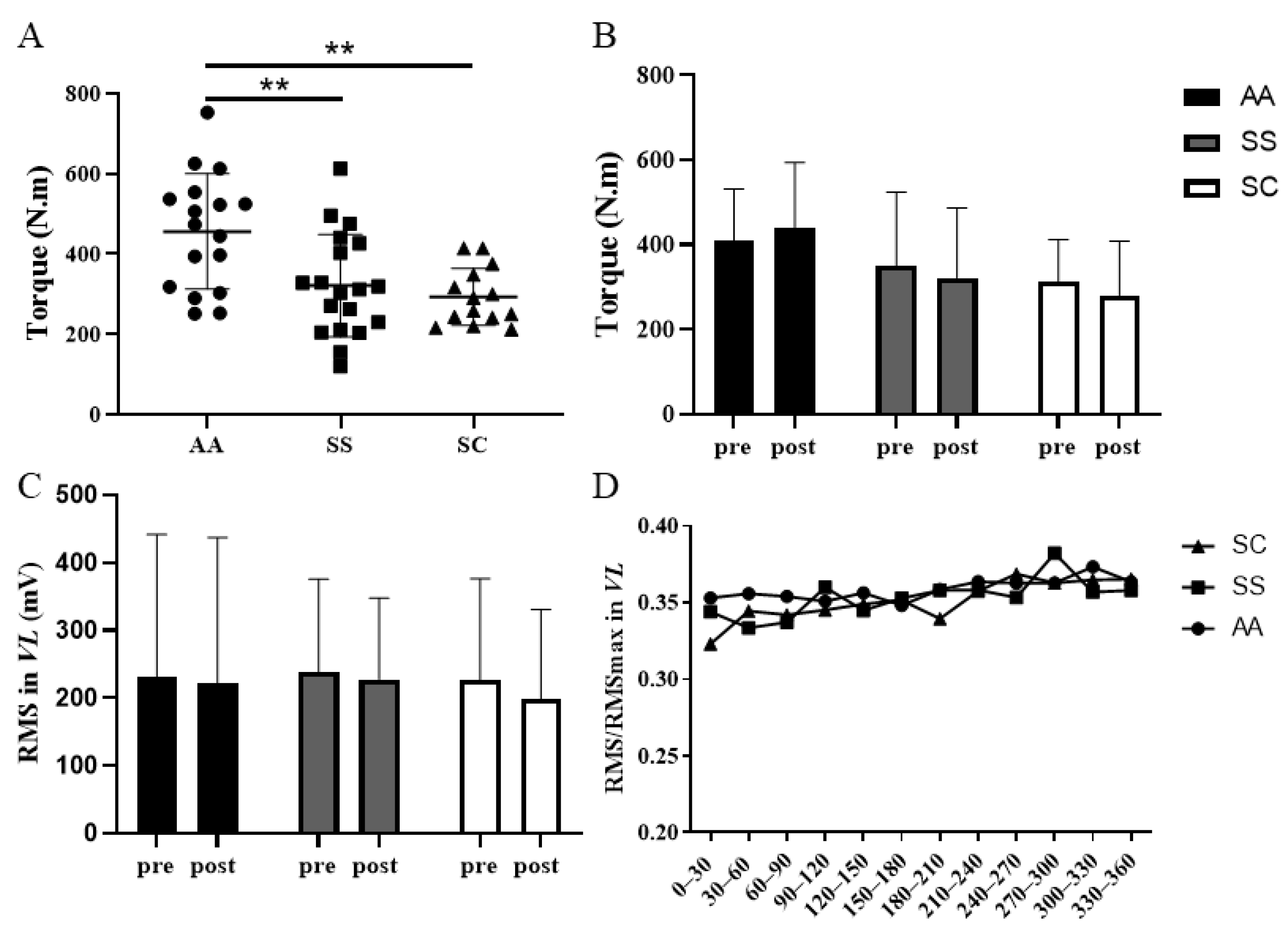
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ron, T. Molecular Medicine; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Howes, R.E.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Bhatt, S.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. The distribution of haemoglobin C and its prevalence in newborns in Africa. Sci. Rep. 2013, 3, 1671. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, A.; Rees, D.C.; Tewari, S.P.; Gibson, J.S. Cation Homeostasis in Red Cells from Patients With Sickle Cell Disease Heterologous for HbS and HbC (HbSC Genotype). EBioMedicine 2015, 2, 1669–1676. [Google Scholar] [CrossRef]
- Nagel, R.L.; Fabry, M.E.; Steinberg, M. The paradox of hemoglobin SC disease. Blood Rev. 2003, 17, 167–178. [Google Scholar] [CrossRef]
- Elmariah, H.; Garrett, M.E.; De Castro, L.M.; Jonassaint, J.C.; Ataga, K.I.; Eckman, J.R.; Ashley-Koch, A.; Telen, M.J. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am. J. Hematol. 2014, 89, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Lionnet, F.; Hammoudi, N.; Stojanovic, K.S.; Avellino, V.; Grateau, G.; Girot, R.; Haymann, J.-P. Hemoglobin sickle cell disease complications: A clinical study of 179 cases. Haematologica 2012, 97, 1136–1141. [Google Scholar] [CrossRef]
- Machado, R.F.; Martyr, S.; Kato, G.J.; Barst, R.J.; Anthi, A.; Robinson, M.R.; Hunter, L.; Coles, W.; Nichols, J.; Hunter, C.; et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br. J. Haematol. 2005, 130, 445–453. [Google Scholar] [CrossRef]
- Anthi, A.; Machado, R.F.; Jison, M.L.; Taveira-DaSilva, A.M.; Rubin, L.J.; Hunter, L.; Hunter, C.J.; Coles, W.; Nichols, J.; Avila, N.A.; et al. Hemodynamic and Functional Assessment of Patients with Sickle Cell Disease and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2007, 175, 1272–1279. [Google Scholar] [CrossRef]
- Alameri, H.F.; Aleem, A.; Kardas, W.; Jehangir, A.; Owais, M.; Al-Momen, A. Dyspnea, pulmonary function and exercise capacity in adult Saudi patients with sickle cell disease. Saudi Med. J. 2008, 29, 707–713. [Google Scholar]
- Ohara, D.; Ruas, G.; Walsh, I.A.P.; Castro, S.S.; Jamami, M. Lung function and six-minute walk test performance in individuals with sickle cell disease. Braz. J. Phys. Ther. 2014, 18, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Marouf, R.; Behbehani, N.; Zubaid, M.; Al Wazzan, H.; El Muzaini, H.; Abdulla, R.; Mojiminiyi, O.A.; Adekile, A.D. Transthoracic Echocardiography and 6-Minute Walk Test in Kuwaiti Sickle Cell Disease Patients. Med. Princ. Pr. 2014, 23, 212–217. [Google Scholar] [CrossRef]
- Melo, H.N.; Stoots, S.J.-M.; Pool, M.A.; Carvalho, V.O.; Almeida, L.O.C.; Aragão, M.L.D.C.; Agyemang, C.; Cipolotti, R. Physical activity level and performance in the six-minute walk test of children and adolescents with sickle cell anemia. Rev. Bras. Hematol. Hemoter. 2017, 39, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Connes, P.; Machado, R.; Hue, O.; Reid, H. Exercise limitation, exercise testing and exercise recommendations in sickle cell anemia. Clin. Hemorheol. Microcirc. 2011, 49, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Marinho, C.D.L.; Maioli, M.C.P.; Soares, A.R.; Bedirian, R.; De Melo, P.L.; Guimarães, F.S.; Ferreira, A.D.S.; Lopes, A.J. Predictive models of six-minute walking distance in adults with sickle cell anemia: Implications for rehabilitation. J. Bodyw. Mov. Ther. 2016, 20, 824–831. [Google Scholar] [CrossRef]
- Brousse, V.; Pondarre, C.; Arnaud, C.; Kamden, A.; De Montalembert, M.; Boutonnat-Faucher, B.; Bourdeau, H.; Charlot, K.; Grévent, D.; Verlhac, S.; et al. One-Fifth of Children with Sickle Cell Anemia Show Exercise-Induced Hemoglobin Desaturation: Rate of Perceived Exertion and Role of Blood Rheology. J. Clin. Med. 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Minniti, C.P.; Nouraie, M.; Arteta, M.; Rana, S.; Onyekwere, O.; Sable, C.; Ensing, G.; Dham, N.; Luchtman-Jones, L.; et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br. J. Haematol. 2009, 147, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Waltz, X.; Romana, M.; Hardy-Dessources, M.-D.; Lamarre, Y.; Divialle-Doumdo, L.; Petras, M.; Tarer, V.; Hierso, R.; Baltyde, K.-C.; Tressières, B.; et al. Hematological and hemorheological Determinants of the Six-Minute Walk Test Performance in Children with Sickle Cell Anemia. PLoS ONE 2013, 8, e77830. [Google Scholar] [CrossRef][Green Version]
- Sachdev, V.; Kato, G.J.; Gibbs, J.S.R.; Barst, R.J.; Machado, R.F.; Nouraie, M.; Hassell, K.L.; Little, J.A.; Schraufnagel, D.E.; Krishnamurti, L.; et al. Echocardiographic Markers of Elevated Pulmonary Pressure and Left Ventricular Diastolic Dysfunction Are Associated with Exercise Intolerance in Adults and Adolescents With Homozygous Sickle Cell Anemia in the US and UK. Circulation 2011, 124, 1452–1460. [Google Scholar] [CrossRef]
- Barst, R.J.; Mubarak, K.K.; Machado, R.F.; Ataga, K.I.; Benza, R.L.; Castro, O.; Naeije, R.; Sood, N.; Swerdlow, P.S.; Hildesheim, M.; et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: Results of the ASSET studies. Br. J. Haematol. 2010, 149, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Gordeuk, V.R.; Minniti, C.P.; Nouraie, M.; Campbell, A.; Rana, S.R.; Luchtman-Jones, L.; Sable, C.; Dham, N.; Ensing, G.; Prchal, J.T.; et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow up in children and adolescents with sickle cell anemia. Haematologica 2010, 96, 33–40. [Google Scholar] [CrossRef]
- Ravelojaona, M.; Féasson, L.; Oyono-Enguéllé, S.; Vincent, L.; Djoubairou, B.; Essoue, C.E.; Messonnier, L.A. Evidence for a Profound Remodeling of Skeletal Muscle and Its Microvasculature in Sickle Cell Anemia. Am. J. Pathol. 2015, 185, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Waltz, X.; Pichon, A.; Lemonne, N.; Mougenel, D.; Lalanne-Mistrih, M.-L.; Lamarre, Y.; Tarer, V.; Tressières, B.; Etienne-Julan, M.; Hardy-Dessources, M.-D.; et al. Normal Muscle Oxygen Consumption and Fatigability in Sickle Cell Patients Despite Reduced Microvascular Oxygenation and Hemorheological Abnormalities. PLoS ONE 2012, 7, e52471. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Antoine-Jonville, S.; Moeckesch, B.; Jumet, S.; Romana, M.; Waltz, X.; Divialle-Doumdo, L.; Hardy-Dessources, M.-D.; Petras, M.; Tressières, B.; et al. Cerebral and muscle microvascular oxygenation in children with sickle cell disease: Influence of hematology, hemorheology and vasomotion. Blood Cells Mol. Dis. 2017, 65, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Eken, M.M.; Richards, R.; Beckerman, H.; van der Krogt, M.; Gerrits, K.; Rietberg, M.; de Groot, V.; Heine, M. Quantifying muscle fatigue during walking in people with multiple sclerosis. Clin. Biomech. 2020, 72, 94–101. [Google Scholar] [CrossRef]
- Rovedder, P.M.E.; Borba, G.C.; Anderle, M.; Flores, J.; Ziegler, B.; Barreto, S.S.M.; Dalcin, P.D.T.R. Peripheral muscle strength is associated with lung function and functional capacity in patients with cystic fibrosis. Physiother. Res. Int. 2019, 24, e1771. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Pépin, J.-L.; Vottero, G.; Mayer, V.; Porsin, B.; Lévy, P.; Wuyam, B. Improvement in Quadriceps Strength and Dyspnea in Daily Tasks After 1 Month of Electrical Stimulation in Severely Deconditioned and Malnourished COPD. Chest 2006, 129, 1540–1548. [Google Scholar] [CrossRef]
- Hendrican, M.C.; McKelvie, R.S.; Smith, T.; McCartney, N.; Pogue, J.; Teo, K.K.; Yusuf, S. Functional capacity in patients with congestive heart failure. J. Card. Fail. 2000, 6, 214–219. [Google Scholar] [CrossRef]
- Nyberg, A.; Törnberg, A.; Wadell, K. Correlation between Limb Muscle Endurance, Strength, and Functional Capacity in People with Chronic Obstructive Pulmonary Disease. Physiother. Can. 2016, 68, 46–53. [Google Scholar] [CrossRef]
- Bachasson, D.; Wuyam, B.; Pepin, J.-L.; Tamisier, R.; Levy, P.; Verges, S. Quadriceps and Respiratory Muscle Fatigue Following High-Intensity Cycling in COPD Patients. PLoS ONE 2013, 8, e83432. [Google Scholar] [CrossRef]
- Melo, H.N.; Stoots, S.J.-M.; Pool, M.A.; Carvalho, V.O.; Aragão, M.L.D.C.; Gurgel, R.Q.; Agyemang, C.; Cipolotti, R. Objectively measured physical activity levels and sedentary time in children and adolescents with sickle cell anemia. PLoS ONE 2018, 13, e0208916. [Google Scholar] [CrossRef]
- Pinto, D.M.R.; Sacramento, M.D.S.D.; Santos, P.H.S.; Silva, W.S.; de Oliveira, E.C.; Gardenghi, G.; Ladeia, A.M.T.; Petto, J. Physical exercise in sickle cell anemia: A systematic review. Hematol. Transfus. Cell Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Merlet, A.N.; Chatel, B.; Hourdé, C.; Ravelojaona, M.; Bendahan, D.; Féasson, L.; Messonnier, L.A. How Sickle Cell Disease Impairs Skeletal Muscle Function: Implications in Daily Life. Med. Sci. Sports Exerc. 2019, 51, 4–11. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.T.; Taylor, J.; Butler, J.; Gandevia, S.C. Depression of Activity in the Corticospinal Pathway during Human Motor Behavior after Strong Voluntary Contractions. J. Neurosci. 2003, 23, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- ATS. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Borg, E.; Borg, G.; Larsson, K.; Letzter, M.; Sundblad, B.-M. An index for breathlessness and leg fatigue. Scand. J. Med. Sci. Sports 2010, 20, 644–650. [Google Scholar] [CrossRef]
- Moheeb, H.; Wali, Y.A.; El-Sayed, M.S. Physical fitness indices and anthropometrics profiles in schoolchildren with sickle cell trait/disease. Am. J. Hematol. 2007, 82, 91–97. [Google Scholar] [CrossRef]
- Burr, J.F.; Bredin, S.S.D.; Faktor, M.D.; Warburton, D.E.R. The 6-Minute Walk Test as a Predictor of Objectively Measured Aerobic Fitness in Healthy Working-Aged Adults. Physician Sportsmed. 2011, 39, 133–139. [Google Scholar] [CrossRef]
- Geiger, R.; Strasak, A.; Treml, B.; Gasser, K.; Kleinsasser, A.; Fischer, V.; Geiger, H.; Loeckinger, A.; Stein, J.I. Six-Minute Walk Test in Children and Adolescents. J. Pediatr. 2007, 150, 395–399.e2. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Huijben, B.; van Schooten, K.; van Dieën, J.; Pijnappels, M. The effect of walking speed on quality of gait in older adults. Gait Posture 2018, 65, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Lin, Y.-C.; Pandy, M.G. Effects of step length and step frequency on lower-limb muscle function in human gait. J. Biomech. 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, R.; Snaterse, M.; Donelan, J.M. Fast and slow processes underlie the selection of both step frequency and walking speed. J. Exp. Biol. 2014, 217, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Hovington, C.L.; Nadeau, S.; Leroux, A. Comparison of Walking Parameters and Cardiorespiratory Changes during the 6-Minute Walk Test in Healthy Sexagenarians and Septuagenarians. Gerontology 2009, 55, 694–701. [Google Scholar] [CrossRef]
- Yandell, M.B.; Zelik, K.E. Preferred Barefoot Step Frequency is Influenced by Factors Beyond Minimizing Metabolic Rate. Sci. Rep. 2016, 6, 23243. [Google Scholar] [CrossRef]
- Ms, J.A.S.; Simonsick, E.M.; Ferrucci, L. The Energetic Pathway to Mobility Loss: An Emerging New Framework for Longitudinal Studies on Aging. J. Am. Geriatr. Soc. 2010, 58, S329–S336. [Google Scholar] [CrossRef]
- Alexander, N.B.; Taffet, G.E.; Horne, F.M.; Eldadah, B.A.; Ferrucci, L.; Nayfield, S.; Studenski, S. Bedside-to-Bench conference: Research agenda for idiopathic fatigue and aging. J. Am. Geriatr. Soc. 2010, 58, 967–975. [Google Scholar] [CrossRef]
- Twomey, R.; Aboodarda, S.J.; Krüger, R.L.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. Neurophysiol. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Barbosa, J.; Bruno, S.; Cruz, N.; De Oliveira, J.; Ruaro, J.; Guerra, R. Perceived fatigability and metabolic and energetic responses to 6-minute walk test in older women. Physiotherapy 2016, 102, 294–299. [Google Scholar] [CrossRef] [PubMed]
- van Beers, E.J.; van der Plas, M.N.; Nur, E.; Bogaard, H.-J.; van Steenwijk, R.P.; Biemond, B.J.; Bresser, P. Exercise tolerance, lung function abnormalities, anemia, and cardiothoracic ratio in sickle cell patients. Am. J. Hematol. 2014, 89, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Liem, R.I.; Nevin, M.A.; Prestridge, A.; Young, L.T.; Thompson, A.A. Functional capacity in children and young adults with sickle cell disease undergoing evaluation for cardiopulmonary disease. Am. J. Hematol. 2009, 84, 645–649. [Google Scholar] [CrossRef]
- Charache, S.; Bleecker, E.R.; Bross, D.S. Effects of blood transfusion on exercise capacity in patients with sickle-cell anemia. Am. J. Med. 1983, 74, 757–764. [Google Scholar] [CrossRef]
- Hoff, J.; Tjønna, A.E.; Steinshamn, S.; Høydal, M.; Richardson, R.S.; Helgerud, J. Maximal Strength Training of the Legs in COPD. Med. Sci. Sports Exerc. 2007, 39, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, T.; Allingham, M.; Prieur, G.; Combret, Y.; Debeaumont, D.; Patout, M.; Cuvelier, A.; Viacroze, C.; Muir, J.-F.; Medrinal, C.; et al. The six-minute stepper test is related to muscle strength but cannot substitute for the one repetition maximum to prescribe strength training in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Merlet, A.N.; Messonnier, L.A.; Coudy-Gandilhon, C.; Bechet, D.; Gellen, B.; Rupp, T.; Galacteros, F.; Bartolucci, P.; Féasson, L. Beneficial effects of endurance exercise training on skeletal muscle microvasculature in sickle cell disease patients. Blood 2019, 134, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Merlet, A.N.; Féasson, L.; Bartolucci, P.; Hourdé, C.; Schwalm, C.; Gellen, B.; Galactéros, F.; Deldicque, L.; Francaux, M.; Messonnier, L.A.; et al. Muscle structural, energetic and functional benefits of endurance exercise training in sickle cell disease. Am. J. Hematol. 2020, 95, 1257–1268. [Google Scholar] [CrossRef]
| AA | SS | SC | |
|---|---|---|---|
| Men | 10 (53%) | 12 (50%) | 6 (33%) |
| Women | 9 (47%) | 12 (50%) | 12 (67%) |
| Age (years) | 32 ± 9 | 27 ± 8 | 27 ± 12 |
| Height (cm) | 172 ± 10 | 172 ± 8 | 167 ± 8 |
| Weight (kg) | 73.1 ± 12.2 | 63.5 ± 9.1 * | 64.1 ± 17.1 |
| Heart rate (bpm) | 74 ± 12 | 73 ± 8 | 77 ± 12 |
| SpO2 (%) | 97 ± 1 | 95 ± 3 | 95 ± 5 |
| Systolic BP (mmHg) | 125 ± 13 | 116 ± 16 | 120 ± 16 |
| Diastolic BP (mmHg) | 86 ± 9 | 75 ± 9 *** | 78 ± 8 * |
| Hematocrit (%) | 43.4 ± 4.4 | 26.2 ± 3.6 $ | 32.6 ± 3.0 # |
| Hemoglobin (g/dL) | 14.3 ± 1.4 | 9.00 ± 1.2 $ | 11.6 ± 1.1 # |
| RBC (1012/L) | 4.95 ± 0.6 | 2.95 ± 0.7 $ | 4.44 ± 0.8 |
| Leucocytes (109/L) | 5.39 ± 1.7 | 7.22 ± 2.3 * | 6.55 ± 2.5 |
| Hydroxyurea (n) | 17 | 1 (6%) | |
| VOC (n/5 years) | 3.9 ± 3.0 | 2.5 ± 5.6 | |
| ACS (n/5 years) | 0.7 ± 0.8 | 0 | |
| Physical activity (Met-min/sem) | 2707 ± 3316 | 5282 ± 5815 | 1589 ± 1148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouraud, E.; Connes, P.; Gauthier-Vasserot, A.; Faes, C.; Merazga, S.; Poutrel, S.; Renoux, C.; Boisson, C.; Joly, P.; Bertrand, Y.; et al. Is Skeletal Muscle Dysfunction a Limiting Factor of Exercise Functional Capacity in Patients with Sickle Cell Disease? J. Clin. Med. 2021, 10, 2250. https://doi.org/10.3390/jcm10112250
Gouraud E, Connes P, Gauthier-Vasserot A, Faes C, Merazga S, Poutrel S, Renoux C, Boisson C, Joly P, Bertrand Y, et al. Is Skeletal Muscle Dysfunction a Limiting Factor of Exercise Functional Capacity in Patients with Sickle Cell Disease? Journal of Clinical Medicine. 2021; 10(11):2250. https://doi.org/10.3390/jcm10112250
Chicago/Turabian StyleGouraud, Etienne, Philippe Connes, Alexandra Gauthier-Vasserot, Camille Faes, Salima Merazga, Solène Poutrel, Céline Renoux, Camille Boisson, Philippe Joly, Yves Bertrand, and et al. 2021. "Is Skeletal Muscle Dysfunction a Limiting Factor of Exercise Functional Capacity in Patients with Sickle Cell Disease?" Journal of Clinical Medicine 10, no. 11: 2250. https://doi.org/10.3390/jcm10112250
APA StyleGouraud, E., Connes, P., Gauthier-Vasserot, A., Faes, C., Merazga, S., Poutrel, S., Renoux, C., Boisson, C., Joly, P., Bertrand, Y., Hot, A., Cannas, G., & Hautier, C. (2021). Is Skeletal Muscle Dysfunction a Limiting Factor of Exercise Functional Capacity in Patients with Sickle Cell Disease? Journal of Clinical Medicine, 10(11), 2250. https://doi.org/10.3390/jcm10112250








