Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia
Abstract
:1. Introduction
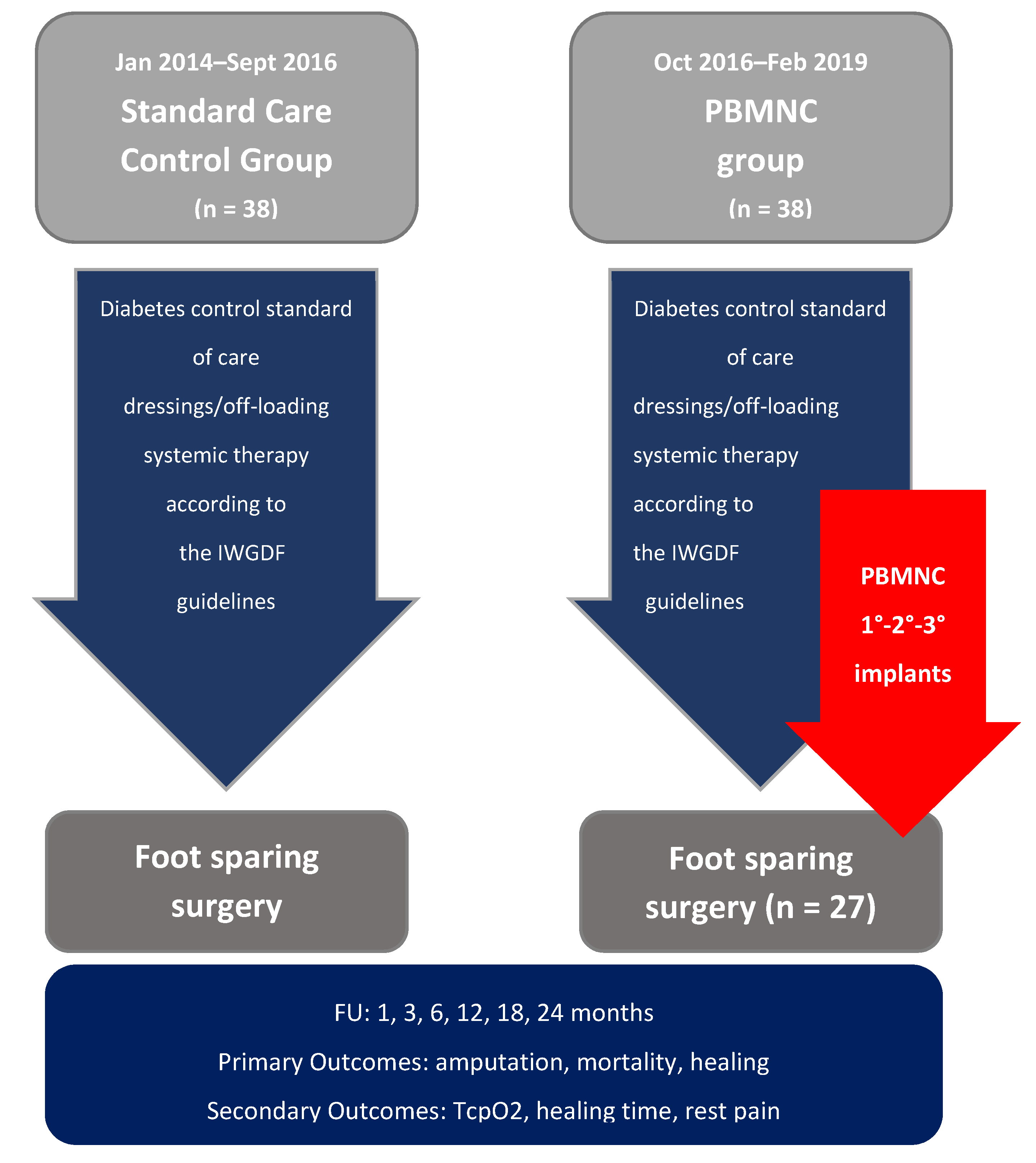
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Features and Baseline Demographics
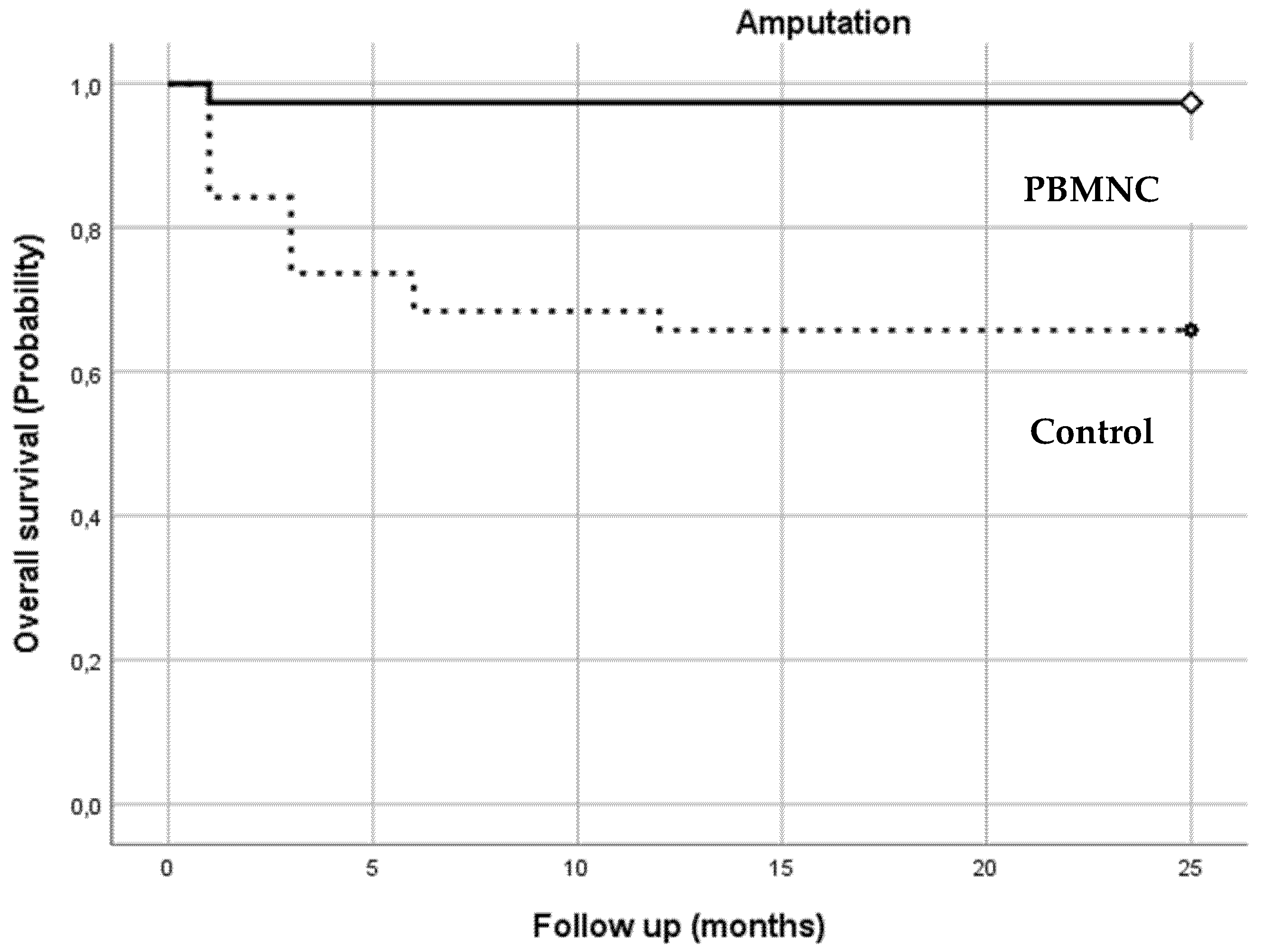
3.2. Clinical Outcome
| Number at Risk | ||||||
| Months | 1 | 3 | 6 | 12 | 18 | 24 |
| PBMNC group | 36 | 34 | 34 | 34 | 34 | 34 |
| Control group | 32 | 26 | 24 | 23 | 23 | 23 |
| Number at Risk | ||||||
| Months | 1 | 3 | 6 | 12 | 18 | 24 |
| PBMNC group | 37 | 34 | 33 | 32 | 30 | 30 |
| Control group | 37 | 33 | 27 | 16 | 11 | 8 |
| Number at Risk | ||||||
| Months | 1 | 3 | 6 | 12 | 18 | 24 |
| PBMNC group | 9 | 25 | 31 | 32 | 33 | 33 |
| Control group | 0 | 0 | 0 | 1 | 1 | 1 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Clinical Trial Registration
References
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Andros, G.; Apelqvist, J.; Bakker, K.; Fiedrichs, S.; Lammer, J.; Lepantalo, M.; Mills, J.L.; Reekers, J.; Shearman, C.P.; et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab. Res. Rev. 2012, 28, 179–217. [Google Scholar] [CrossRef]
- Caetano, A.P.; Conde Vasco, I.; Veloso Gomes, F.; Costa, N.V.; Luz, J.H.; Spaepen, E.; Formiga, A.; Coimbra, É.; Neves, J.; Bilhim, T. Successful Revascularization has a Significant Impact on Limb Salvage Rate and Wound Healing for Patients with Diabetic Foot Ulcers: Single-Centre Retrospective Analysis with a Multidisciplinary Approach. Cardiovasc. Intervent. Radiol. 2020, 43, 1449–1459. [Google Scholar] [CrossRef]
- Meloni, M.; Izzo, V.; Da Ros, V.; Morosetti, D.; Stefanini, M.; Brocco, E.; Giurato, L.; Gandini, R.; Uccioli, L. Characteristics and Outcome for Persons with Diabetic Foot Ulcer and No-Option Critical Limb Ischemia. J. Clin. Med. 2020, 9, 3745. [Google Scholar] [CrossRef] [PubMed]
- Soo, B.P.; Rajbhandari, S.; Egun, A.; Ranasinghe, U.; Lahart, I.M.; Pappachan, J.M. Survival at 10 years following lower extremity amputations in patients with diabetic foot disease. Endocrine 2020, 69, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J.; Minamino, T.; Tateno, K.; Shimizu, N.; Kuwabara, Y.; Sato, Y.; Saito, Y.; Komuro, I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ. Cardiovasc. Interv. 2009, 2, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.P.; Yang, X.F.; Li, S.Z.; Wen, J.C.; Zhang, Y.; Han, Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb. Haemost. 2007, 98, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Liotta, F.; Annunziato, F.; Castellani, S.; Boddi, M.; Alterini, B.; Castellini, G.; Mazzanti, B.; Cosmi, L.; Acquafresca, M.; Bartalesi, F.; et al. Therapeutic Efficacy of Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors in Patients With Critical Limb Ischemia―The SCELTA Trial―. Circ. J. 2018, 82, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Cell Therapy for Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Angiology 2016, 67, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, J.; Martino, M.M.; Lui, K.O. Regulatory T-cells: Potential regulator of tissue repair and regeneration. Front. Immunol. 2018, 9, 585. [Google Scholar] [CrossRef]
- Awad, O. Differential Healing Activities of CD34+ and CD14+ Endothelial Cell Progenitors. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 758–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaltro, G.; Straino, S.; Gambini, E.; Bassetti, B.; Persico, L.; Zoli, S.; Zanobini, M.; Capogrossi, M.C.; Spirito, R.; Quarti, C.; et al. Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy 2015, 17, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb Rescue: A New Autologous-Peripheral Blood Mononuclear Cells Technology in Critical Limb Ischemia and Chronic Ulcers. Tissue Eng. Part C Methods 2015, 21, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Persiani, F.; Paolini, A.; Camilli, D.; Mascellari, L.; Platone, A.; Magenta, A.; Furgiuele, S. Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single-Center Experience. Ann. Vasc. Surg. 2018, 53, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the european society for vascular surgery (ESVS). Russ. J. Cardiol. 2018, 5, 305–368. [Google Scholar]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system: The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The society for vascular surgery lower extremity threatened limb classification system: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsky, B.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; Senneville, É.; Urbančič-Rovan, V.; Van Asten, S. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 45–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Šalounová, D.; Jonszta, T.; Czerný, D.; Krajča, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [Green Version]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br J Anaesth 2008, 101, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L.; Merolla, S.; Gandini, R. Critical limb ischemia: Current challenges and future prospects. Vasc. Health Risk Manag. 2018, 14, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Ferraresi, R.; Mauri, G.; Losurdo, F.; Troisi, N.; Brancaccio, D.; Caravaggi, C.; Neri, L. BAD transmission and SAD distribution: A new scenario for critical limb ischemia. J. Cardiovasc. Surg. 2018, 59, 655–664. [Google Scholar] [CrossRef]
- Fortington, L.V.; Geertzen, J.H.B.; Van Netten, J.J.; Postema, K.; Rommers, G.M.; Dijkstra, P.U. Short and long term mortality rates after a lower limb amputation. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Pagacová, L.; Nemcová, A.; Sixta, B.; Chlupac, J.; Peregrin, J.H.; Syková, E.; et al. Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy 2014, 16, 1733–1738. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A.; Zeller, T.; Miller, L.E.; Jaff, M.R. Disease Burden and Clinical Outcomes Following Initial Diagnosis of Critical Limb Ischemia in the Medicare Population. JACC Cardiovasc. Interv. 2018, 11, 1011–1012. [Google Scholar] [CrossRef]
- Martini, R.; Andreozzi, G.M.; Deri, A.; Cordova, R.; Zulian, P.; Scarpazza, O.; Nalin, F. Amputation rate and mortality in elderly patients with Critical Limb Ischemia not suitable for revascularization. Aging Clin. Exp. Res. 2012, 24, 24–27. [Google Scholar]
- Pannell, M.; Labuz, D.; Celik, M.; Keye, J.; Batra, A.; Siegmund, B.; Machelska, H. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflamm. 2016, 13, 262. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kosaki, A.; Iwasaka, T. Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp. Neurol. 2005, 199, 274–280. [Google Scholar]
- Molavi, B.; Zafarghandi, M.R.; Aminizadeh, E.; Hosseini, S.E.; Mirzayi, H.; Arab, L.; Baharvand, H.; Aghdami, N. Safety and efficacy of repeated bone marrow mononuclear cell therapy in patients with critical limb ischemia in a pilot randomized controlled trial. Arch. Iran. Med. 2016, 19, 388–396. [Google Scholar] [PubMed]
- Kang, W.C.; Oh, P.C.; Lee, K.; Ahn, T.; Byun, K. Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model. Korean J. Physiol. Pharmacol. 2016, 20, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadini, G.P.; Albiero, M.; Bonora, B.M.; Avogaro, A. Angiogenic Abnormalities in Diabetes Mellitus: Mechanistic and Clinical Aspects. J. Clin. Endocrinol. Metab. 2019, 104, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S. Diabetes impairs angiogenesis and induces endothelial cell senescence by up-regulating thrombospondin-CD47-dependent signaling. Int. J. Mol. Sci. 2019, 20, 673. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.K.; Green, L.A.; Motaganahalli, R.L.; Wilson, M.G.; Fajardo, A.; Murphy, M.P. Rationale and design of the MarrowStim PAD Kit for the Treatment of Critical Limb Ischemia in Subjects with Severe Peripheral Arterial Disease (MOBILE) trial investigating autologous bone marrow cell therapy for critical limb ischemia. J. Vasc. Surg. 2017, 65, 1850–1857.e2. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Pan, T.; Fang, Y.; Wei, Z.; Gu, S.; Fang, G.; Liu, Y.; Luo, Y.; Liu, H.; Zhang, T.; et al. Purified CD34+ cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial. EBioMedicine 2018, 35, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Inoue, O.; Usui, S.; Takashima, S.; Nomura, A.; Yamaguchi, K.; Takeda, Y.; Goten, C.; Hamaoka, T.; Ootsuji, H.; Murai, H.; et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem. Biophys. Res. Commun. 2019, 517, 369–375. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Moon, K.-C.; Chung, H.-Y.; Jeong, S.-H.; Dhong, E.-S. Possibility of Injecting Adipose-Derived Stromal Vascular Fraction Cells to Accelerate Microcirculation in Ischemic Diabetic Feet: A Pilot Study. Int. J. Stem Cells 2019, 12, 107–113. [Google Scholar]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. 2018, 14, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zollino, I.; Campioni, D.; Sibilla, M.G.; TessariI, M.; Malagoni, A.M.; Zamboni, P. A phase II randomized clinical trial for the treatment of recalcitrant chronic leg ulcers using centrifuged adipose tissue containing progenitor cells. Cytotherapy 2019, 21, 200–211. [Google Scholar] [CrossRef]


| PBMNC Group | Control Group | Statistical Test | p Value | |
|---|---|---|---|---|
| Age | 77.00 ± 6.72 | 77.58 ± 10.73 | U = 664.500 | p = 0.55 |
| Gender | 26M (68.4%) 12F (31.6%) | 26M (68.4%)12F (31.6%) | X2C = 0.000 | p = 1.000 |
| Type of diabetes | Type 1 = 3 (7.9%) Type 2 = 35 (92.1%) | Type 1 = 1 (2.6%) Type 2 = 37 (97.4%) | X2C = 1.056 | p = 0.304 |
| Duration of diabetes | 16.45 ± 8.96 | 18.63 ± 8.60 | U = 621.000 | p = 0.291 |
| Site of lesion | Forefoot (78.9%); hindfoot (21.1%) | Forefoot (73.7%); hindfoot (26.3%) | X2C = 0.291 | p = 0.589 |
| HbA1c % | 7.48 ± 0.69 (58 mmol/L) | 7.62 ± 0.77 (60 mmol/L) | U = 622.000 | p = 0.389 |
| Rheumatologic disease | 12 (31.6%) | 9 (23.7%) | X2C = 0.592 | p = 0.442 |
| Cardiopathy | 23 (60.5%) | 27 (71.1%) | X2C = 0.935 | p = 0.333 |
| Stroke/TIA | 8 (21.1%) | 17 (44.7%) | X2C = 4.828 | p = 0.028 |
| Retinopathy | 8 (21.1%) | 21 (55.3%) | X2C = 10.077 | p = 0.002 * |
| Neuropathy | 26 (68.4%) | 31 (81.6%) | X2C = 1.754 | p = 0.185 |
| Wound extension (Texas University Classification) | 2C = 9 (23.7%) 3C = 29 (76.3%) | 2C = 5 (13.2%) 3C = 33 (86.8%) | X2C = 1.401 | p = 0.237 |
| WIFi | W1I3Fi0 = 10 (26.3%) W3I3Fi0 = 28 (73.7%) | W1I3Fi0 = 4 (10.5%) W3I3Fi0 = 34 (89.5%) | X2C = 3.152 | p = 0.076 |
| TcpO2 | 11.59 ± 5.2 | 14.05 ± 5 | U = 581.500 | p = 0.196 |
| Renal failure | 21 (55.3%) | 19 (50.0%) | X2C = 0.211 | p = 0.646 |
| Angioplasty Failure | 30 (78.9%) | 21 (55.3%) | X2C = 4.828 | p = 0.028 * |
| Not feasible | 8 (21.1%) | 15 (40.5%) | X2C = 3.348 | p = 0.067 |
| Bypass occlusion | 5 (13.2%) | 4 (10.8%) | X2C = 0.098 | p = 0.754 |
| Tibial/pedal absence | 23 (67.6%) | 29 (76.3%) | X2C = 0.67 | p = 0.412 |
| Calcification | 24 (75.0%) | 34 (89.5%) | X2C = 2.56 | p = 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ambrosone, C.; Ventoruzzo, G.; Liistro, F.; Tacconi, D.; Di Filippi, M.; Attempati, N.; et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. J. Clin. Med. 2021, 10, 2213. https://doi.org/10.3390/jcm10102213
Scatena A, Petruzzi P, Maioli F, Lucaroni F, Ambrosone C, Ventoruzzo G, Liistro F, Tacconi D, Di Filippi M, Attempati N, et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. Journal of Clinical Medicine. 2021; 10(10):2213. https://doi.org/10.3390/jcm10102213
Chicago/Turabian StyleScatena, Alessia, Pasquale Petruzzi, Filippo Maioli, Francesca Lucaroni, Cristina Ambrosone, Giorgio Ventoruzzo, Francesco Liistro, Danilo Tacconi, Marianna Di Filippi, Nico Attempati, and et al. 2021. "Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia" Journal of Clinical Medicine 10, no. 10: 2213. https://doi.org/10.3390/jcm10102213
APA StyleScatena, A., Petruzzi, P., Maioli, F., Lucaroni, F., Ambrosone, C., Ventoruzzo, G., Liistro, F., Tacconi, D., Di Filippi, M., Attempati, N., Palombi, L., Ercolini, L., & Bolognese, L. (2021). Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. Journal of Clinical Medicine, 10(10), 2213. https://doi.org/10.3390/jcm10102213







