Findings of Brain Magnetic Resonance Imaging in Girls with Central Precocious Puberty Compared with Girls with Chronic or Recurrent Headache
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Patients and Settings
2.2. Measurements
2.3. Brain MRI
- (1)
- Normal;
- (2)
- Positive:
- (a)
- incidental: non-hypothalamic–pituitary (non-H–P) or H–P lesions (mild intracranial alterations not involving the H–P region), which are not considered associated with CPP or headache;
- (b)
- pathological: lesions known to be the cause of or associated with CPP or headache.
2.4. Statistical Analysis
3. Results
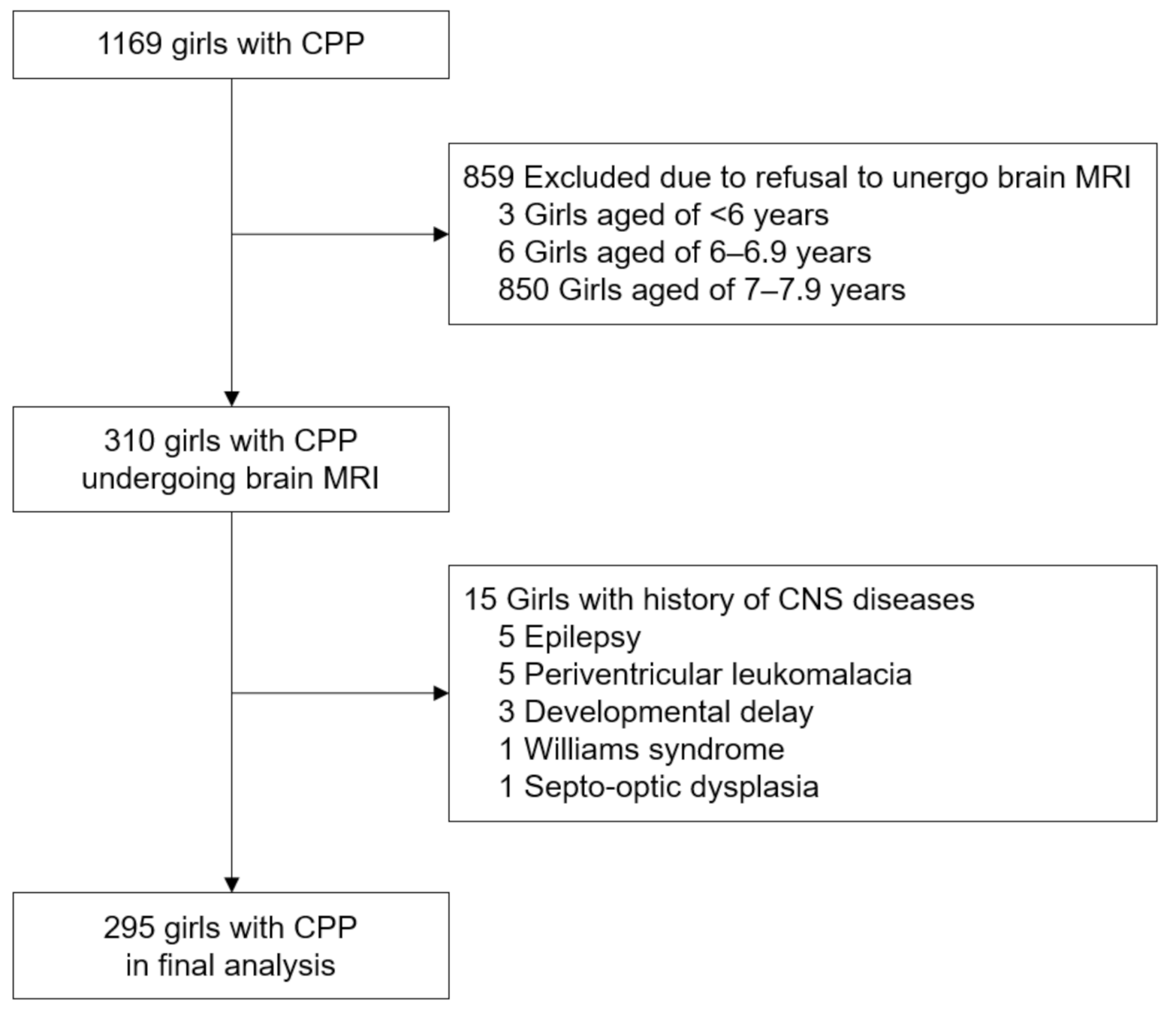
3.1. MRI Findings of Girls with CPP
3.2. Comparison of Brain MRI Findings between Girls with CPP and Girls with Headache
3.3. Brain MRI Findings Based on Age at Pubertal Onset in Girls with CPP
3.4. Follow-Up Brain MRI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemaitilly, W.; Trivin, C.; Adan, L.; Gall, V.; Sainte-Rose, C.; Brauner, R. Central Precocious Puberty: Clinical and Laboratory Features. Clin. Endocrinol. 2001, 54, 289–294. [Google Scholar] [CrossRef]
- Choi, J.-H.; Shin, Y.-L.; Yoo, H.-W. Predictive Factors for Organic Central Precocious Puberty and Utility of Simplified Gonadotropin-Releasing Hormone Tests. Pediatr. Int. 2007, 49, 806–810. [Google Scholar] [CrossRef]
- Cisternino, M.; Arrigo, T.; Pasquino, A.; Tinelli, C.; Antoniazzi, F.; Beduschi, L.; Bindi, G.; Borrelli, P.; De Sanctis, V.; Farello, G.; et al. Etiology and Age Incidence of Precocious Puberty in Girls: A Multicentric Study. J. Pediatr. Endocrinol. Metab. 2000, 13, 695–702. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Corrias, A.; Rizzo, V.; Bertelloni, S.; Urso, L.; Galluzzi, F.; Pasquino, A.; Pozzan, G.; Guarneri, M.; Cisternino, M.; et al. Etiology of Central Precocious Puberty in Males: The Results of the Italian Study Group for Physiopathology of Puberty. J. Pediatr. Endocrinol. Metab. 2000, 13, 687–694. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Argente, J. Central Precocious Puberty, Functional and Tumor-Related. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101262. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, S.S.; Aksglaede, L.; Mouritsen, A.; Sørensen, K.; Main, K.M.; Gideon, P.; Juul, A. Pathological and Incidental Findings on Brain MRI in a Single-Center Study of 229 Consecutive Girls with Early or Precocious Puberty. PLoS ONE 2012, 7, e29829. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.; Goadsby, P.J. MRI in Headache. Expert Rev. Neurother. 2013, 13, 263–273. [Google Scholar] [CrossRef]
- Sperling, M. (Ed.) Pediatric Endocrinology, 4th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2014; ISBN 978-1-4557-4858-7. [Google Scholar]
- Kim, J.H.; Yun, S.; Hwang, S.-S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for Children and Adolescents: Development, Improvement, and Prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Tables for Predicting Adult Height from Skeletal Age: Revised for Use with the Greulich-Pyle Hand Standards. J. Pediatr. 1952, 40, 423–441. [CrossRef]
- Ng, S.; Kumar, Y.; Cody, D.; Smith, C.; Didi, M.; Donaldson, M. Cranial MRI Scans Are Indicated in All Girls with Central Precocious Puberty. Arch. Dis. Child. 2003, 88, 414–418. [Google Scholar] [CrossRef]
- Yoon, J.S.; So, C.H.; Lee, H.S.; Lim, J.S.; Hwang, J.S. Prevalence of Pathological Brain Lesions in Girls with Central Precocious Puberty: Possible Overestimation? J. Korean Med. Sci. 2018, 33, e329. [Google Scholar] [CrossRef] [PubMed]
- Pedicelli, S.; Alessio, P.; Scirè, G.; Cappa, M.; Cianfarani, S. Routine Screening by Brain Magnetic Resonance Imaging Is Not Indicated in Every Girl With Onset of Puberty Between the Ages of 6 and 8 Years. J. Clin. Endocrinol. Metab. 2014, 99, 4455–4461. [Google Scholar] [CrossRef]
- Chiu, C.-F.; Wang, C.-J.; Chen, Y.-P.; Lo, F.-S. Pathological and Incidental Findings in 403 Taiwanese Girls With Central Precocious Puberty at Initial Diagnosis. Front. Endocrinol. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.-S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.-P. The Timing of Normal Puberty and the Age Limits of Sexual Precocity: Variations around the World, Secular Trends, and Changes after Migration. Endocr. Rev. 2003, 24, 668–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.-S. Incidence and Prevalence of Central Precocious Puberty in Korea: An Epidemiologic Study Based on a National Database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Corripio, R.; Labarta, J.I.; Cañete, R.; Castro-Feijóo, L.; Espino, R.; Argente, J. Central Precocious Puberty in Children Living in Spain: Incidence, Prevalence, and Influence of Adoption and Immigration. J. Clin. Endocrinol. Metab. 2010, 95, 4305–4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Isoda, H.; Tanaka, T.; Inagawa, S.; Takeda, H.; Takehara, Y.; Isogai, S.; Sakahara, H. Dynamic Gadolinium-Enhanced MR Imaging of Pituitary Adenomas: Usefulness of Sequential Sagittal and Coronal Plane Images. Eur. J. Radiol. 2001, 39, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.J.; Park, H.K.; Yang, S.; Song, J.H.; Hwang, I.T. Clinical and Radiological Findings of Incidental Rathke’s Cleft Cysts in Children and Adolescents. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Culver, S.A.; Grober, Y.; Ornan, D.A.; Patrie, J.T.; Oldfield, E.H.; Jane, J.A.; Thorner, M.O. A Case for Conservative Management: Characterizing the Natural History of Radiographically Diagnosed Rathke Cleft Cysts. J. Clin. Endocrinol. Metab. 2015, 100, 3943–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, M.; Caracseghi, F.; Gussinyer, M.; Yeste, D.; Albisu, M.; Vázquez, E.; Ortega, A.; Carrascosa, A. Macroorchidism and Panhypopituitarism: Two Different Forms of Presentation of FSH-Secreting Pituitary Adenomas in Adolescence. Horm. Res. Paediatr. 2011, 75, 225–230. [Google Scholar] [CrossRef]
- Vargas, G.; Balcazar-Hernandez, L.-J.; Melgar, V.; Magriña-Mercado, R.-M.; Gonzalez, B.; Baquera, J.; Mercado, M. An FSH and TSH Pituitary Adenoma, Presenting with Precocious Puberty and Central Hyperthyroidism. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Freda, P.U.; Beckers, A.M.; Katznelson, L.; Molitch, M.E.; Montori, V.M.; Post, K.D.; Vance, M.L. Pituitary Incidentaloma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 894–904. [Google Scholar] [CrossRef]
- Sanno, N.; Oyama, K.; Tahara, S.; Teramoto, A.; Kato, Y. A Survey of Pituitary Incidentaloma in Japan. Eur. J. Endocrinol. 2003, 149, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavitaki, N.; Collison, K.; Halliday, J.; Byrne, J.V.; Price, P.; Cudlip, S.; Wass, J.H. What Is the Natural History of Nonoperated Nonfunctioning Pituitary Adenomas? Clin. Endocrinol. 2007, 67, 938–943. [Google Scholar] [CrossRef]
- Feldkamp, J.; Santen, R.; Harms, E.; Aulich, A.; Mödder, U.; Scherbaum, W.A. Incidentally Discovered Pituitary Lesions: High Frequency of Macroadenomas and Hormone-Secreting Adenomas—Results of a Prospective Study. Clin. Endocrinol. 1999, 51, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V.; Lage, A.E.; Kumari, G.; Silvera, V.M.; Cohen, L.E. Clinical Course of Nonfunctional Pituitary Microadenoma in Children: A Single-Center Experience. J. Clin. Endocrinol. Metab. 2019, 104, 5906–5912. [Google Scholar] [CrossRef]
- Jansen, P.R.; Dremmen, M.; van den Berg, A.; Dekkers, I.A.; Blanken, L.M.E.; Muetzel, R.L.; Bolhuis, K.; Mulder, R.M.; Kocevska, D.; Jansen, T.A.; et al. Incidental Findings on Brain Imaging in the General Pediatric Population. N. Engl. J. Med. 2017, 377, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.O.; Piatt, J.H. Section on Neurologic Surgery, American Academy of Pediatrics Incidental Findings on Brain and Spine Imaging in Children. Pediatrics 2015, 135, e1084–e1096. [Google Scholar] [CrossRef] [Green Version]
- Schwedt, T.J.; Guo, Y.; Rothner, A.D. “Benign” Imaging Abnormalities in Children and Adolescents with Headache. Headache 2006, 46, 387–398. [Google Scholar] [CrossRef]
- Chanson, P.; Daujat, F.; Young, J.; Bellucci, A.; Kujas, M.; Doyon, D.; Schaison, G. Normal Pituitary Hypertrophy as a Frequent Cause of Pituitary Incidentaloma: A Follow-up Study. J. Clin. Endocrinol. Metab. 2001, 86, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Chalumeau, M.; Hadjiathanasiou, C.G.; Ng, S.M.; Cassio, A.; Mul, D.; Cisternino, M.; Partsch, C.-J.; Theodoridis, C.; Didi, M.; Cacciari, E.; et al. Selecting Girls with Precocious Puberty for Brain Imaging: Validation of European Evidence-Based Diagnosis Rule. J. Pediatr. 2003, 143, 445–450. [Google Scholar] [CrossRef]
- Cantas-Orsdemir, S.; Garb, J.L.; Allen, H.F. Prevalence of Cranial MRI Findings in Girls with Central Precocious Puberty: A Systematic Review and Meta-Analysis. J. Pediatr. Endocrinol. Metab. 2018, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
| MRI Findings | All Patients (n = 295) |
|---|---|
| Incidental non-hypothalamic-pituitary legion | |
| Neuroglial cyst | 1 (0.3) |
| Callosal lipoma | 1 (0.3) |
| Tornwaldt’s cyst | 1 (1.3) |
| Non-neoplastic cyst | 3 (1.0) |
| Nodule of thalamus | 1 (0.3) |
| Subependymal gray matter | 1 (0.3) |
| Incidental hypothalamic-pituitary legion | |
| Rathke cleft cyst | 7 (2.4) |
| Arachnoid cyst | 6 (2.0) |
| Microadenoma | 5 (1.7) |
| Pituitary hypoplasia | 4 (1.4) |
| Cyst of pituitary pars intermedia | 3 (1.0) |
| Pineal gland cyst | 3 (1.0) |
| Pituitary hyperplasia | 2 (0.7) |
| Pathological lesion | |
| Hypothalamic tuber cinereum hamartoma | 1 (0.3) |
| MRI Findings | All Patients (n = 205) |
|---|---|
| Incidental non-hypothalamic-pituitary legion | |
| Neuroepithelial cyst at the temporal region | 1 (0.5) |
| Empty sella configuration | 1 (0.5) |
| Non-neoplastic cyst | 1 (0.5) |
| Partial agenesis of the corpus callosum | 1 (0.5) |
| Ventricular dilatation | 2 (1.0) |
| Enlarged perivascular space | 1 (0.5) |
| Vascular anomalies | 1 (0.5) |
| Developmental venous anomaly | 4 (2.0) |
| Incidental hypothalamic-pituitary lesion | |
| Arachnoid cyst | 7 (3.4) |
| Microadenoma | 2 (1.0) |
| Rathke cleft cyst | 2 (1.0) |
| Pineal gland cyst | 1 (0.5) |
| Pathological lesion | |
| Moyamoya disease | 1 (0.5) |
| MRI Findings | <6 years (n = 33) | 6–6.9 years (n = 148) | 7–7.9 years (n = 114) | p-Value |
|---|---|---|---|---|
| Normal (n = 256) | 25 (75.8%) | 128 (86.5%) | 103 (90.4%) | NA |
| Positive findings (n = 39) | 8 (24.2%) | 20 (13.5%) | 11 (9.6%) | 0.09 |
| Incidental findings (n = 38) | 7 (21.2%) | 20 (13.5%) | 11 (9.6%) | 0.21 |
| Non-H–P region (n = 7) | 1 (3.0%) | 4 (2.7%) | 3 (2.6%) | >0.99 |
| H–P region (n = 31) | 6 (18.2%) | 16 (10.8%) | 8 (7.0%) | 0.16 |
| Pathological findings (n = 1) | 1 (3.0%) | 0 | 0 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Ahn, M.B.; Cho, W.K.; Cho, K.S.; Jung, M.H.; Suh, B.-K. Findings of Brain Magnetic Resonance Imaging in Girls with Central Precocious Puberty Compared with Girls with Chronic or Recurrent Headache. J. Clin. Med. 2021, 10, 2206. https://doi.org/10.3390/jcm10102206
Kim S-H, Ahn MB, Cho WK, Cho KS, Jung MH, Suh B-K. Findings of Brain Magnetic Resonance Imaging in Girls with Central Precocious Puberty Compared with Girls with Chronic or Recurrent Headache. Journal of Clinical Medicine. 2021; 10(10):2206. https://doi.org/10.3390/jcm10102206
Chicago/Turabian StyleKim, Shin-Hee, Moon Bae Ahn, Won Kyoung Cho, Kyoung Soon Cho, Min Ho Jung, and Byung-Kyu Suh. 2021. "Findings of Brain Magnetic Resonance Imaging in Girls with Central Precocious Puberty Compared with Girls with Chronic or Recurrent Headache" Journal of Clinical Medicine 10, no. 10: 2206. https://doi.org/10.3390/jcm10102206






