Determining Whether Low Protein Intake (<1.0 g/kg) Is a Risk Factor for Malnutrition in Patients with Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Quality and Quantity of Muscle Mass
2.4. Subjective Global Assessment
2.5. Definition and Assessment of Dietary Intake
2.6. Definition of Malnutrition
2.7. Statistical Analysis
3. Results
3.1. Basic Characteristics and Prevalence of Malnutrition
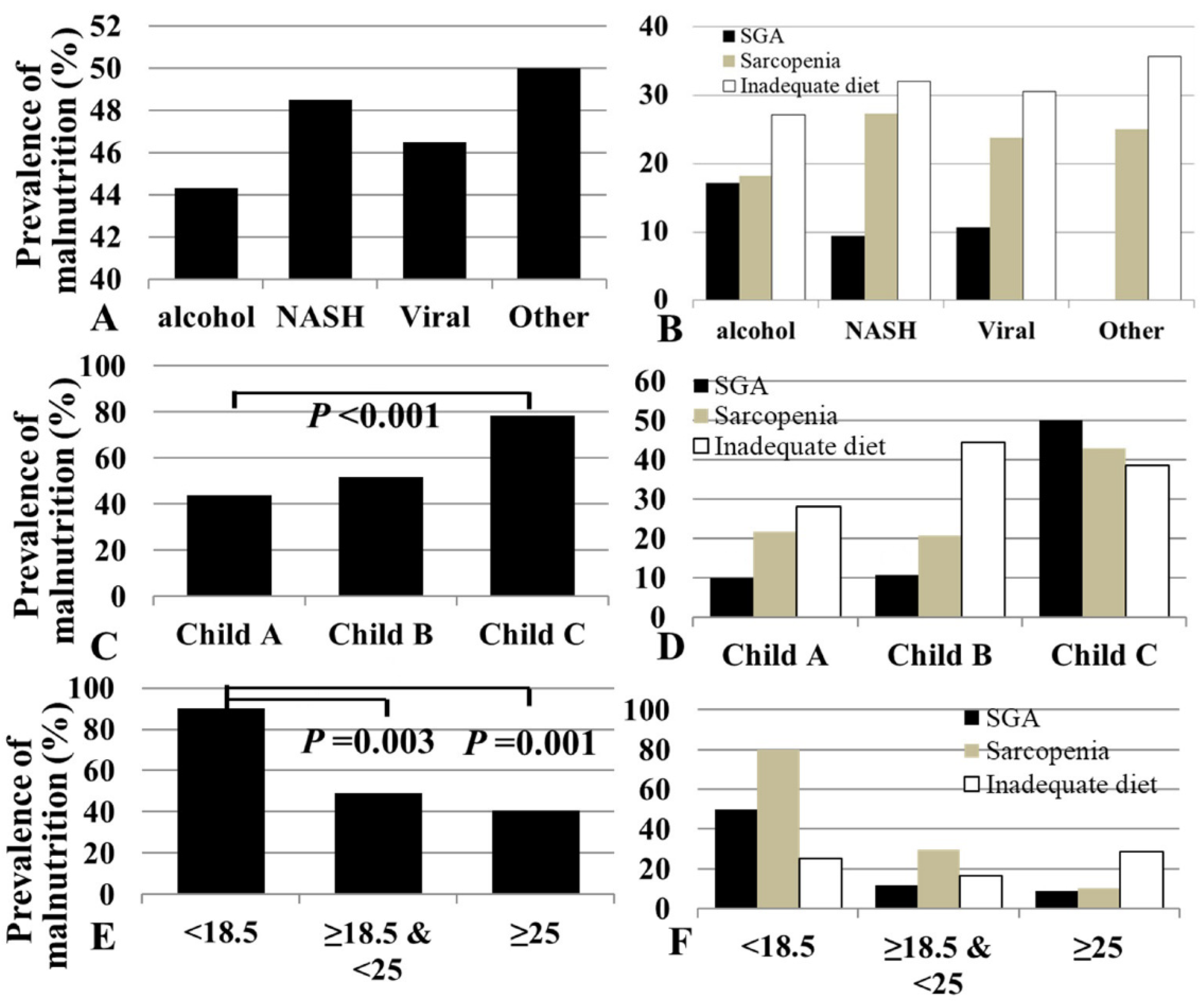
3.2. Prevalence of Malnutrition According to Etiology
3.3. Prevalence of Malnutrition According to Disease Severity
3.4. Prevalence of Malnutrition According to Body Mass Index
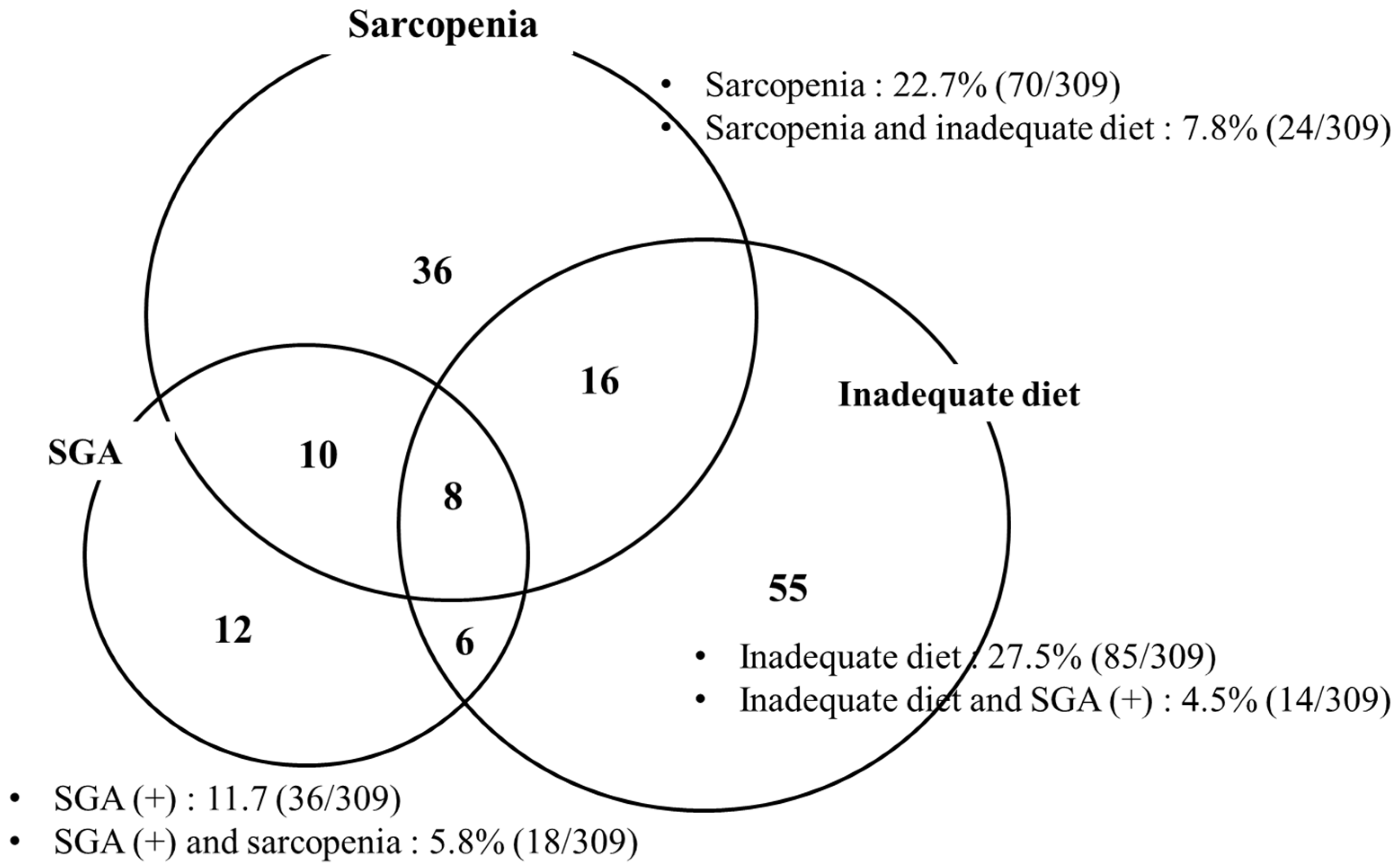
3.5. Prevalence of Malnutrition According to Sarcopenia, SGA, and Dietary Intake
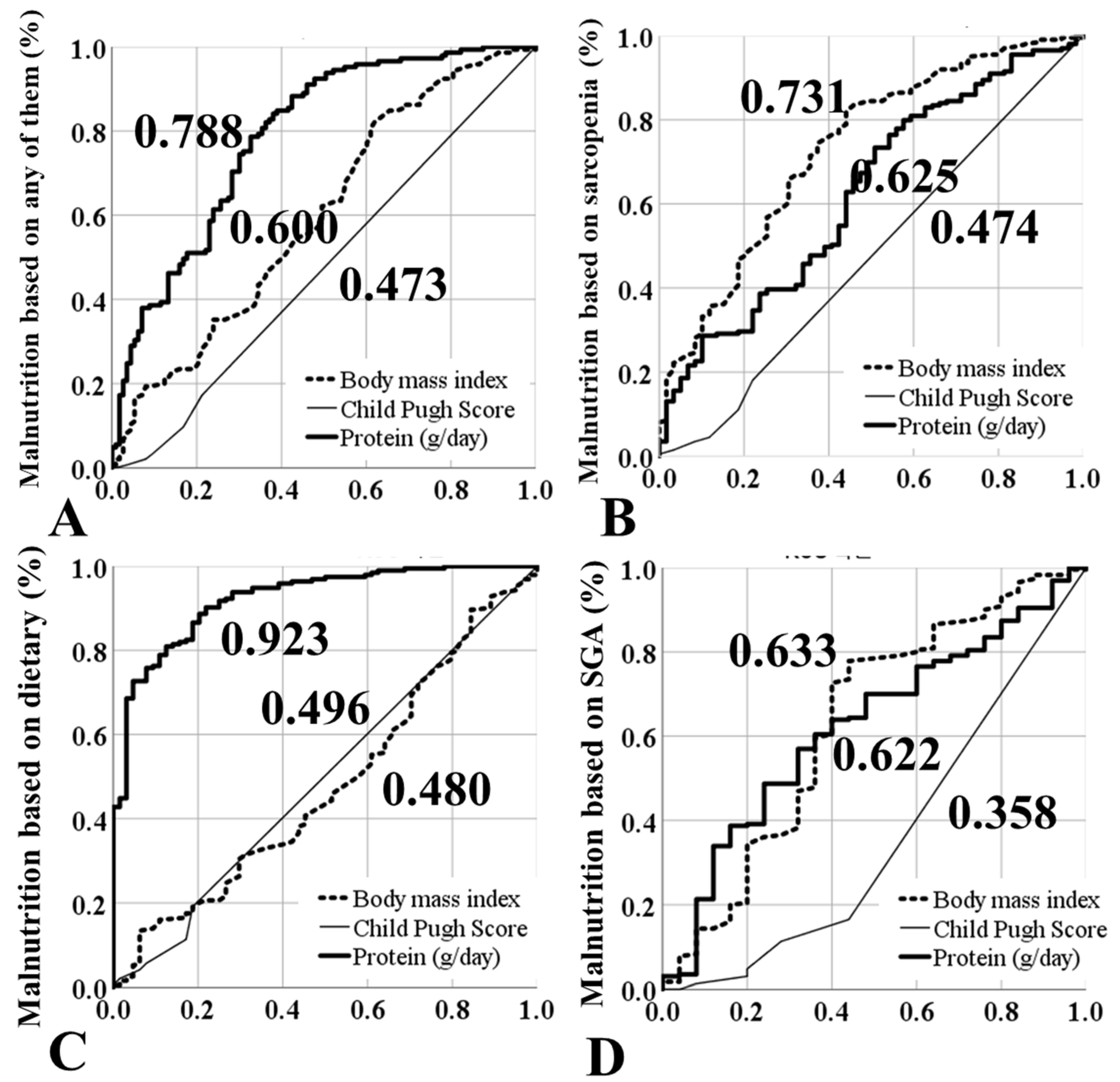
3.6. Risk Factors for Malnutrition (Multivariate Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunsar, F.; Raimondo, M.L.; Jones, S.; Terreni, N.; Wong, C.; Patch, D.; Sabin, C.; Burroughs, A.K. Nutritional status and prognosis in cirrhotic patients. Aliment. Pharmacol. Ther. 2006, 24, 563–572. [Google Scholar] [CrossRef]
- Alberino, F.; Gatta, A.; Amodio, P.; Merkel, C.; Di Pascoli, L.; Boffo, G.; Caregaro, L. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001, 17, 445–450. [Google Scholar] [CrossRef]
- Huisman, E.J.; Trip, E.J.; Siersema, P.D.; Van Hoek, B.; Van Erpecum, K.J. Protein energy malnutrition predicts complications in liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Lucidi, C.; Giannelli, V.; Giusto, M.; Riggio, O.; Falcone, M.; Ridola, L.; Attili, A.F.; Venditti, M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin. Gastroenterol. Hepatol. 2010, 8, 979–985.e1. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J. Parenter. Enteral Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Figueiredo, F.; Dickson, E.R.; Pasha, T.M.; Porayko, M.K.; Therneau, T.M.; Malinchoc, M.; DiCecco, S.R.; Francisco-Ziller, N.M.; Kašparová, P.; Charlton, M.R. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transplant. 2000, 6, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.A.; Bassani, L.; Nunes, F.F.; Aydos, M.E.D.; Alves, A.V.; Marroni, C.A. Nutritional assessment in patients with cirrhosis. Arq. Gastroenterol. 2012, 49, 19–27. [Google Scholar] [CrossRef]
- Nutritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J. Hepatol. 1994, 21, 317–325.
- Eghtesad, S.; Poustchi, H.; Malekzadeh, R. Malnutrition in liver cirrhosis: The influence of protein and sodium. Middle East J. Dig. Dis. 2013, 5, 65–75. [Google Scholar]
- Baum, J.I.; Kim, I.-Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Juakiem, W.; Torres, D.M.; Harrison, S.A. Nutrition in cirrhosis and chronic liver disease. Clin. Liver Dis. 2014, 18, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Døssing, H.; Larsson, B.; Stilling, B.; Jensen, M.G. Long-term oral refeeding of patients with cirrhosis of the liver. Br. J. Nutr. 1995, 74, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Stilling, B.; Wikman, B. Nutritional assessment and adequacy of dietary intake in hospitalized patients with alcoholic liver cirrhosis. Br. J. Nutr. 1993, 69, 665–679. [Google Scholar] [CrossRef]
- Gottschall, C.B.; Pereira, T.G.; Rabito, E.I.; Álvares-Da-Silva, M.R. Nutritional Status and Dietary Intake in Non-Cirrhotic Adult Chronic Hepatitis C Patients. Arq. Gastroenterol. 2015, 52, 204–209. [Google Scholar] [CrossRef][Green Version]
- Carvalho, L.; Parise, E.R. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arq. Gastroenterol. 2006, 43, 269–274. [Google Scholar] [CrossRef]
- Hayashi, F.; Momoki, C.; Yuikawa, M.; Simotani, Y.; Kawamura, E.; Hagihara, A.; Fujii, H.; Kobayashi, S.; Iwai, S.; Morikawa, H.; et al. Nutritional status in relation to lifestyle in patients with compensated viral cirrhosis. World J. Gastroenterol. 2012, 18, 5759–5770. [Google Scholar] [CrossRef]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Hsu, P.-S.; Krairit, O.; Lee, J.S.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. 1), 5–41. [Google Scholar] [PubMed]
- Harris, J.A.; Benedict, F.G. Biometric Studies of Basal Metabolism in Man; Publication No. 279; Carnegie Institute of Washington: Washington, DC, USA, 1919; pp. 223–250. [Google Scholar]
- Alvares-da-Silva, M.R.; da Silveira, T.R. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005, 21, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Caly, W.R.; Strauss, E.; Carrilho, F.J.; Laudanna, A.A. Different degrees of malnutrition and immunological alterations according to the aetiology of cirrhosis: A prospective and sequential study. Nutr. J. 2003, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, B.C.; Srivastava, S. Malnutrition in cirrhosis increases morbidity and mortality. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar] [CrossRef]
- Tandon, P.; Raman, M.; Mourtzakis, M.; Merli, M. A Practical Approach to Nutritional Screening and Assessment in Cirrhosis. Hepatology 2017, 65, 1044–1057. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef]
- Pirlich, M.; Schütz, T.; Spachos, T.; Ertl, S.; Weiß, M.-L.; Lochs, H.; Plauth, M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000, 32, 1208–1215. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total LC (n = 309) | Alcoholic LC (n = 88) | NASH-LC (n = 33) | Viral LC (n = 172) | Other LC (n = 16) | p |
|---|---|---|---|---|---|---|
| Age (yr.) | 58.7 ± 9.26 | 56.7 ± 8.94 | 63.4 ± 9.31 | 58.5 ± 9.34 | 59.3 ± 6.78 | 0.005 |
| Sex (%) | ||||||
| Male | 191 (61.8) | 82 (93.2) | 12 (36.4) | 93 (54.1) | 4 (25.0) | <0.001 |
| Female | 118 (38.2) | 6 (6.8) | 21 (63.6) | 79 (45.9) | 12 (75.0) | |
| Height (cm) | 164.5 ± 8.63 | 168.3 ± 6.66 | 161.4 ± 8.33 | 164.0 ± 8.84 | 156.5 ± 7.32 | <0.001 |
| Weight (kg) | 66.6 ± 12.12 | 68.0 ± 12.80 | 63.7 ± 10.53 | 66.9 ± 12.25 | 61.5 ± 8.28 | 0.119 |
| BMI (%) | 0.303 | |||||
| <18.5 (kg/m2) | 10 (3.3) | 4 (4.8) | 1 (3.0) | 5 (2.9) | 0 (0) | |
| 18.5–25 (kg/m2) | 165 (54.5) | 52 (61.9) | 18 (54.6) | 85 (50.0) | 10 (62.5) | |
| ≥25 (kg/m2) | 128 (42.2) | 28 (33.3) | 14 (42.4) | 80 (47.1) | 6 (37.5) | |
| ASM (kg) | 20.1 ± 4.79 | 22.4 ± 4.19 | 18.2 ± 4.72 | 19.7 ± 4.77 | 16.3 ± 2.37 | <0.001 |
| Percent body fat (%) | 28.0 ± 9.12 | 21.8 ± 8.61 | 31.1 ± 8.37 | 29.8 ± 8.17 | 34.4 ± 6.59 | <0.001 |
| FFMI (kg/m2) | 17.5 ± 2.41 | 18.6 ± 2.37 | 16.7 ± 2.31 | 17.2 ± 2.36 | 16.4 ± 1.59 | <0.001 |
| Handgrip strength (kg) | 30.0 ± 9.89 | 33.6 ± 7.75 | 23.9 ± 8.03 | 29.7 ± 10.68 | 25.1 ± 7.30 | <0.001 |
| Sarcopenia (%) | 70 (22.7) | 16 (18.2) | 9 (27.3) | 41 (23.8) | 4 (25.0) | 0.339 |
| Child–Pugh (%) | 0.005 | |||||
| A | 266 (86.1) | 66 (75.0) | 30 (90.9) | 155 (90.1) | 15 (93.8) | |
| B | 29 (9.4) | 14 (15.9) | 2 (6.1) | 12 (7.0) | 1 (6.2) | |
| C | 14 (4.5) | 8 (9.1) | 1 (3.0) | 5 (2.9) | 0 (0) | |
| MELD score | 8.6 ± 2.72 | 9.8 ± 3.78 | 8.2 ± 2.82 | 8.2 ± 1.84 | 7.28 ± 1.05 | <0.001 |
| SGA (%) | 0.186 | |||||
| A | 267 (86.4) | 72 (82.8) | 29 (90.6) | 151 (89.3) | 15 (100.0) | |
| B | 34 (11.0) | 14 (16.1) | 3 (9.4) | 17 (10.1) | 0 (0) | |
| C | 2 (0.7) | 1 (1.1) | 0 (0.0) | 1 (0.6) | 0 (0) | |
| Dietary intake | ||||||
| Total energy (kcal) | 2090 ± 910 | 2294 ± 940 | 2069 ± 962 | 2014 ± 895 | 1821 ± 632 | 0.114 |
| Inadequate (%) | 85 (27.5) | 22 (27.2) | 9 (32.1) | 49 (30.6) | 5 (35.7) | 0.894 |
| Carbohydrate (g) | 310 ± 126 | 316 ± 131 | 310 ± 130 | 310 ± 125 | 281 ± 99 | 0.843 |
| Protein (g) | 86 ± 46 | 100 ± 51 | 87 ± 48 | 80 ± 42 | 74 ± 39 | 0.024 |
| Lipid (g) | 57 ± 35 | 66 ± 38 | 57 ± 37 | 53 ± 32 | 48 ± 29 | 0.038 |
| Carbohydrate (g/kg) | 4.8 ± 1.88 | 4.8 ± 2.07 | 4.8 ± 2.07 | 4.7 ± 1.76 | 5.0 ± 1.79 | 0.869 |
| Protein (g/kg) | 1.3 ± 0.68 | 1.5 ± 0.79 | 1.3 ± 0.70 | 1.2 ± 0.60 | 1.3 ± 0.64 | 0.030 |
| Lipid (g/kg) | 0.8 ± 0.45 | 1.0 ± 0.67 | 0.8 ± 0.50 | 0.8 ± 0.46 | 0.8 ± 0.45 | 0.044 |
| Cholesterol | 417 ± 277 | 417 ± 316 | 438 ± 246 | 402 ± 231 | 520 ± 480 | 0.448 |
| Vitamin A (µg) | 984 ± 562 | 954 ± 596 | 1023 ± 575 | 965 ± 477 | 1254 ± 986 | 0.264 |
| Vitamin C (mg) | 145 ± 94 | 151 ± 113 | 130 ± 74 | 141 ± 81 | 187 ± 124 | 0.243 |
| Vitamin D (µg) | 5.0 ± 4.47 | 4.7 ± 3.77 | 6.4 ± 7.15 | 4.7 ± 3.56 | 7.4 ± 7.74 | 0.040 |
| Vitamin E (mg) | 18.2 ± 10.46 | 17.2 ± 9.78 | 20.1 ± 11.03 | 17.7 ± 9.54 | 24.5 ± 17.85 | 0.058 |
| Thiamin (mg) | 1.6 ± 0.84 | 1.5 ± 0.79 | 1.9 ± 1.10 | 1.6 ± 0.77 | 1.9 ± 1.14 | 0.113 |
| Riboflavin (mg) | 1.4 ± 0.78 | 1.4 ± 0.77 | 1.6 ± 0.88 | 1.4 ± 0.66 | 1.9 ± 1.38 | 0.088 |
| Calcium (mg) | 599 ± 356 | 584 ± 404 | 649 ± 261 | 579 ± 305 | 788 ± 608 | 0.146 |
| Iron (mg) | 12.9 ± 6.51 | 12.0 ± 6.65 | 14.4 ± 6.70 | 12.7 ± 5.79 | 15.7 ± 10.62 | 0.125 |
| Malnutrition (%) | ||||||
| by ESPEN CPG | 36 (11.6) | 11 (13.3) | 2 (6.1) | 18 (10.7) | 2 (12.5) | 0.731 |
| by EASL CPG | 143 (46.3) | 39 (44.3) | 16 (48.5) | 80 (46.5) | 8 (50.0) | 0.962 |
| Child–Pugh Classification | Body Mass Index (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | A (n = 266) | B (n = 29) | C (n = 14) | p | <18.5 (n = 10) | 18.5–25 (n = 165) | ≥25 (n = 128) | p |
| BMI (%, kg/m2) | 0.145 | |||||||
| <18.5 | 8 (3.1) | 0 (0.0) | 2 (14.3) | |||||
| 18.5–25 | 144 (55.4) | 15 (51.7) | 6 (42.8) | |||||
| ≥25 | 108 (41.5) | 14 (48.3) | 6 (42.8) | |||||
| Child–Pugh classification (%) | 0.145 | |||||||
| A | 8 (80.0) | 144 (87.3) | 108 (79.5) | |||||
| B | 0 (0.0) | 15 (9.1) | 14 (13.7) | |||||
| C | 2 (20.0) | 6 (3.3) | 6 (6.8) | |||||
| ASM (kg) | 20 ± 4.7 | 21 ± 4.4 | 23 ± 5.8 | 0.041 | 16 ± 3.6 | 19 ± 4.4 | 22 ± 4.9 | <0.001 |
| FFMI (kg/m2) | 17 ± 2.3 | 21 ± 4.5 | 23 ± 5.8 | <0.001 | 14 ± 1.7 | 17 ± 2.0 | 19 ± 2.3 | <0.001 |
| Handgrip strength(kg) | 30 ± 10.2 | 31 ± 8.3 | 28 ± 4.8 | 0.637 | 23 ± 6.0 | 29 ± 9.9 | 32 ± 9.6 | 0.006 |
| Sarcopenia (%) | 58 (21.8) | 6 (20.7) | 6 (42.9) | 0.160 | 8 (80.0) | 49 (29.7) | 13 (10.2) | <0.001 |
| SGA (%) | <0.001 | <0.001 | ||||||
| A | 235 (90.0) | 25 (89.3) | 7 (50.0) | 5 (50.0) | 146 (88.5) | 112 (91.1) | ||
| B | 24 (9.2) | 3 (10.7) | 7 (50.0) | 4 (40.0) | 18 (10.9) | 11 (8.9) | ||
| C | 2 (0.8) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (0.6) | 0 (0.0) | ||
| Dietary intake | ||||||||
| Total energy (kcal) | 2086 ± 900 | 2099 ± 969 | 2137 ± 1067 | 0.982 | 1782 ± 504 | 2015 ± 888 | 2217 ± 938 | 0.151 |
| Inadequate (%) | 68 (28.0) | 12 (44.4) | 5 (38.5) | 0.167 | 2 (25.0) | 44 (16.3) | 37 (28.6) | 0.797 |
| Carbohydrate (g) | 309 ± 124 | 315 ± 120 | 335 ± 169 | 0.763 | 265 ± 73 | 300 ± 121 | 331 ± 131 | 0.095 |
| Protein (g) | 86 ± 45 | 88 ± 58 | 84 ± 51 | 0.969 | 71 ± 26 | 84 ± 46 | 90 ± 45 | 0.377 |
| Lipid (g) | 57 ± 34 | 57 ± 41 | 53 ± 33 | 0.939 | 48 ± 20 | 54 ± 34 | 60 ± 35 | 0.337 |
| Malnutrition (%) | ||||||||
| by ESPEN CPG | 27 (10.5) | 2 (6.9) | 4 (28.6) | 0.083 | 10 (100.0) | 21 (13.0) | 2 (1.6) | <0.001 |
| by EASL CPG | 117 (44.0) | 15 (51.7) | 11 (78.6) | 0.034 | 9 (90.0) | 81 (49.1) | 52 (40.6) | 0.007 |
| Classification | Sarcopenia | SGA | Inadequate Diet | Any | ||||
|---|---|---|---|---|---|---|---|---|
| Presence (n = 70) | p | Presence (n = 36) | p | Presence (n = 85) | p | Presence (n = 143) | p | |
| BMI (%, kg/m2) | <0.001 | 0.017 | 0.521 | 0.007 | ||||
| <18.5 | 8 (80.0) | 7 (70.0) | 2 (20.0) | 9 (90.0) | ||||
| 18.5–25 | 49 (29.7) | 18 (10.9) | 44 (26.7) | 81 (49.1) | ||||
| ≥25 | 13 (10.2) | 11 (8.6) | 37 (28.9) | 52 (40.6) | ||||
| Child–Pugh (%) | 0.160 | <0.001 | 0.111 | 0.034 | ||||
| A | 58 (21.8) | 25 (8.8) | 68 (25.6) | 117 (44.0) | ||||
| B | 6 (20.7) | 4 (26.7) | 12 (41.4) | 15 (51.7) | ||||
| C | 6 (42.9) | 7 (70.0) | 5 (35.7) | 11 (78.6) | ||||
| Etiology (%) | 0.339 | 0.055 | 0.510 | 0.962 | ||||
| Alcohol | 16 (18.2) | 15 (17.0) | 22 (25.0) | 39 (44.3) | ||||
| NASH | 9 (27.3) | 3 (9.1) | 9 (27.3) | 16 (48.5) | ||||
| Viral | 41 (23.8) | 18 (10.5) | 49 (28.5) | 80 (46.5) | ||||
| Others | 4 (25.0) | 0 (0.0) | 5 (31.3) | 8 (50.0) | ||||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Exp(ß) | p | Exp(ß) | p | |
| Age | 1.03 | 0.009 | 1.03 | 0.011 |
| BMI (kg/m2) | 0.90 | 0.002 | 0.84 | 0.002 |
| Etiology | 0.691 | |||
| Protein (g) | 0.22 | <0.001 | 0.18 | <0.001 |
| Child–Pugh classification | 0.017 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Kang, M.; Jun, D.-W.; Kim, M.; Kwak, J.-H.; Kang, B.-k. Determining Whether Low Protein Intake (<1.0 g/kg) Is a Risk Factor for Malnutrition in Patients with Cirrhosis. J. Clin. Med. 2021, 10, 2164. https://doi.org/10.3390/jcm10102164
Park J-H, Kang M, Jun D-W, Kim M, Kwak J-H, Kang B-k. Determining Whether Low Protein Intake (<1.0 g/kg) Is a Risk Factor for Malnutrition in Patients with Cirrhosis. Journal of Clinical Medicine. 2021; 10(10):2164. https://doi.org/10.3390/jcm10102164
Chicago/Turabian StylePark, Jin-Hwa, Minkoo Kang, Dae-Won Jun, Mimi Kim, Joo-Hee Kwak, and Bo-kyeong Kang. 2021. "Determining Whether Low Protein Intake (<1.0 g/kg) Is a Risk Factor for Malnutrition in Patients with Cirrhosis" Journal of Clinical Medicine 10, no. 10: 2164. https://doi.org/10.3390/jcm10102164
APA StylePark, J.-H., Kang, M., Jun, D.-W., Kim, M., Kwak, J.-H., & Kang, B.-k. (2021). Determining Whether Low Protein Intake (<1.0 g/kg) Is a Risk Factor for Malnutrition in Patients with Cirrhosis. Journal of Clinical Medicine, 10(10), 2164. https://doi.org/10.3390/jcm10102164






