Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection and Sources
2.3. Clinical Management of VA-ECMO
2.4. Anticoagulation and Bleeding Management
2.5. Sampling and Laboratory Assays
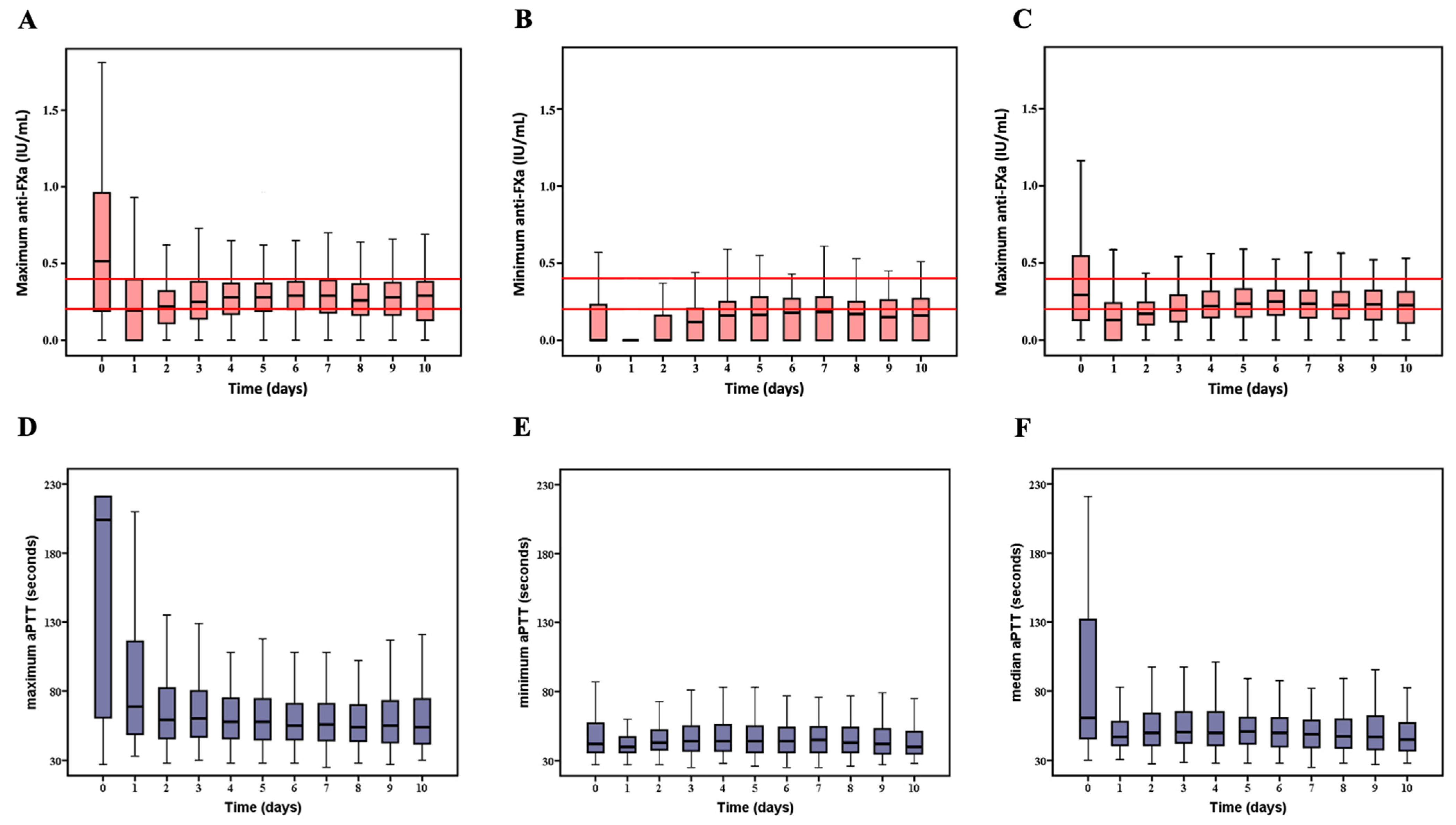
2.6. Description of aPTT and Anti-FXa Variables
2.7. Study Endpoints
2.8. Statistical Analysis
3. Results
3.1. Study Population
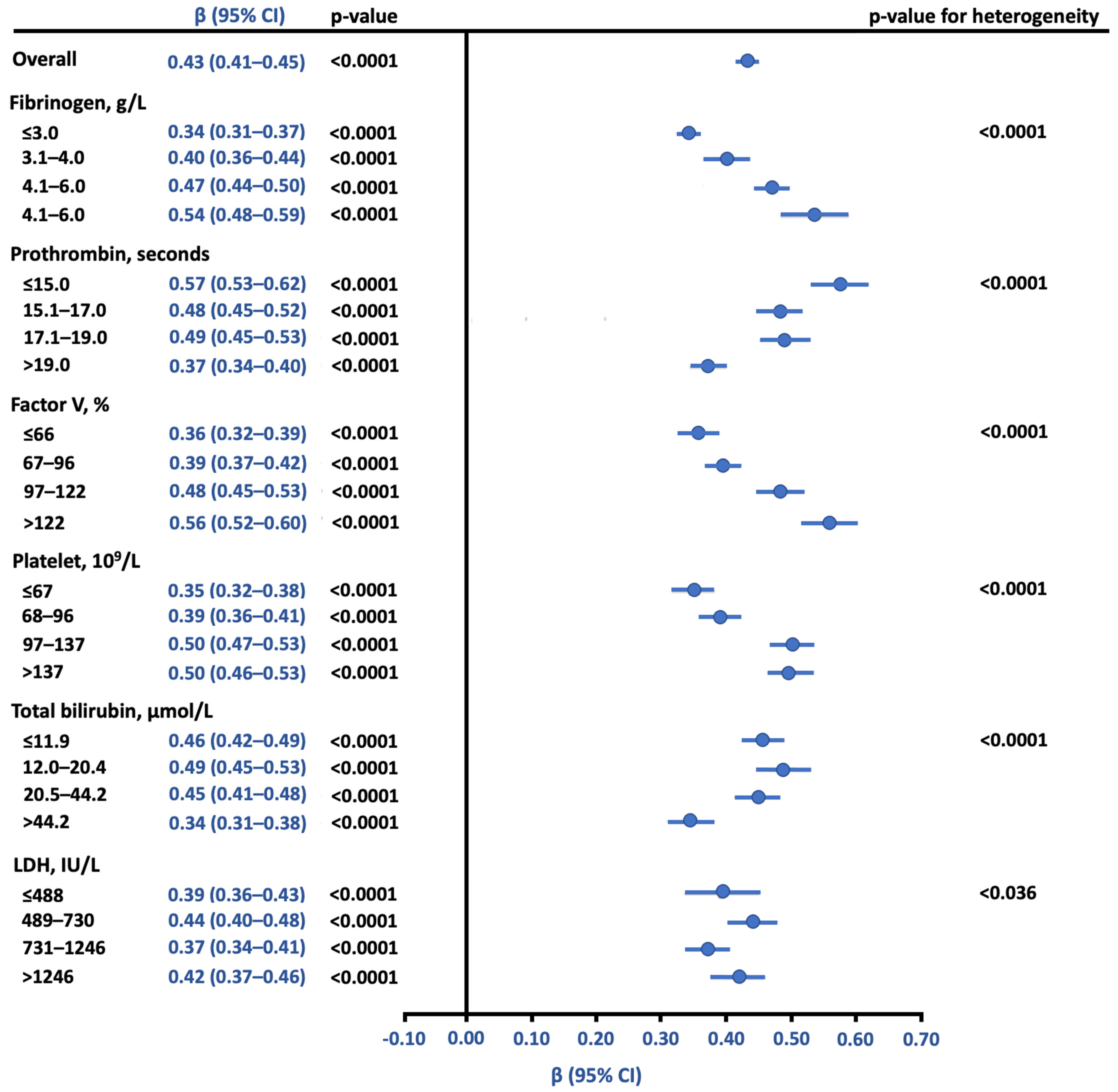
3.2. Relationship between Anti-FXa and aPTT Using Reference Samples
3.3. Association of Anti-FXa and aPTT with Serious Bleeding
3.4. Association of Anti-FXa and aPTT with Thrombotic Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavayas, Y.A.; Del Sorbo, L.; Fan, E. Intracranial Hemorrhage in Adults on ECMO. Perfusion 2018, 33, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Guimbretière, G.; Anselmi, A.; Roisne, A.; Lelong, B.; Corbineau, H.; Langanay, T.; Flécher, E.; Verhoye, J.-P. Prognostic Impact of Blood Product Transfusion in VA and VV ECMO. Perfusion 2019, 34, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.A.; Tanaka, K.; Roberts, A.; Rector, R.; Menaker, J.; Kon, Z.; Deatrick, K.B.; Kaczorowski, D.; Griffith, B.; Herr, D. Bleeding, Thrombosis, and Transfusion with Two Heparin Anticoagulation Protocols in Venoarterial ECMO Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1216–1220. [Google Scholar] [CrossRef]
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive Factors of Bleeding Events in Adults Undergoing Extracorporeal Membrane Oxygenation. Ann. Intensive Care 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Rayzman, V.; Nolte, M.W.; Nickel, K.F.; Björkqvist, J.; Jämsä, A.; Hardy, M.P.; Fries, M.; Schmidbauer, S.; Hedenqvist, P.; et al. A Factor XIIa Inhibitory Antibody Provides Thromboprotection in Extracorporeal Circulation without Increasing Bleeding Risk. Sci. Transl. Med. 2014, 6, 222ra17. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, G.; Berg, N.; Broman, L.M.; Prahl Wittberg, L. Flow-Induced Platelet Activation in Components of the Extracorporeal Membrane Oxygenation. Circuit. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- ELSO Guidelines General All ECLS Version1.4 Pdf. Available online: https://www.elso.org/Resources/Guidelines.aspx (accessed on 20 February 2021).
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2018, 44, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lequier, L.; Annich, G.; Al-Ibrahim, O. ELSO Anticoagulation Guideline. 2014. Extracorpor. Life Support Organ. ELSO 2016, 1–17. Available online: https://www.elso.org/Resources/Guidelines.aspx (accessed on 20 February 2021).
- Lorusso, R.; Whitman, G.; Milojevic, M.; Raffa, G.; McMullan, D.M.; Boeken, U.; Haft, J.; Bermudez, C.A.; Shah, A.S.; D’Alessandro, D.A. 2020 EACTS/ELSO/STS/AATS Expert Consensus on Post-Cardiotomy Extracorporeal Life Support in Adult Patients. Eur. J. Cardiothorac. Surg. 2021, 59, 12–53. [Google Scholar] [CrossRef]
- Irby, K.; Swearingen, C.; Byrnes, J.; Bryant, J.; Prodhan, P.; Fiser, R. Unfractionated Heparin Activity Measured by Anti-Factor Xa Levels Is Associated with the Need for Extracorporeal Membrane Oxygenation Circuit/Membrane Oxygenator Change: A Retrospective Pediatric Study. Pediatr. Crit. Care Med. 2014, 15, e175–e182. [Google Scholar] [CrossRef] [Green Version]
- Delmas, C.; Jacquemin, A.; Vardon-Bounes, F.; Georges, B.; Guerrero, F.; Hernandez, N.; Marcheix, B.; Seguin, T.; Minville, V.; Conil, J.-M.; et al. Anticoagulation Monitoring under ECMO Support: A Comparative Study between the Activated Coagulation Time and the Anti-Xa Activity Assay. J. Intensive Care Med. 2020, 35, 679–686. [Google Scholar] [CrossRef]
- Vandiver, J.W.; Vondracek, T.G. Antifactor Xa Levels versus Activated Partial Thromboplastin Time for Monitoring Unfractionated Heparin. Pharmacotherapy 2012, 32, 546–558. [Google Scholar] [CrossRef]
- Kostousov, V.; Nguyen, K.; Hundalani, S.G.; Teruya, J. The Influence of Free Hemoglobin and Bilirubin on Heparin Monitoring by Activated Partial Thromboplastin Time and Anti-Xa Assay. Arch. Pathol. Lab. Med. 2014, 138, 1503–1506. [Google Scholar] [CrossRef] [Green Version]
- Protti, A.; Iapichino, G.E.; Di Nardo, M.; Panigada, M.; Gattinoni, L. Anticoagulation Management and Antithrombin Supplementation Practice during Veno-Venous Extracorporeal Membrane Oxygenation: A Worldwide Survey. Anesthesiology 2020, 132, 562–570. [Google Scholar] [CrossRef]
- Krulder, J.W.; de Boer, A.; van den Besselaar, A.M.; Cohen, A.F.; Schoemaker, H.C.; Briët, E.; Meinders, A.E. Diurnal Rhythm in Anticoagulant Effect of Heparin during a Low Dose Constant Rate Infusion. A Study in Healthy Volunteers. Thromb. Haemost. 1992, 68, 30–32. [Google Scholar] [PubMed]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The Analysis of Failure Times in the Presence of Competing Risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral Anticoagulants. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef] [Green Version]
- Marlar, R.A. Clinical and Laboratory Standards Institute One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test: Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; ISBN 9781562386726. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Multiple Events per Subject. In Modeling Survival Data: Extending the Cox Model; Therneau, T.M., Grambsch, P.M., Eds.; Statistics for Biology and Health; Springer: New York, NY, USA, 2000; pp. 169–229. ISBN 9781475732948. [Google Scholar]
- McLaughlin, K.; Rimsans, J.; Sylvester, K.W.; Fanikos, J.; Dorfman, D.M.; Senna, P.; Connors, J.M.; Goldhaber, S.Z. Evaluation of Antifactor-Xa Heparin Assay and Activated Partial Thromboplastin Time Values in Patients on Therapeutic Continuous Infusion Unfractionated Heparin Therapy. Clin. Appl. Thromb. 2019, 25, 1076029619876030. [Google Scholar] [CrossRef]
- Adatya, S.; Uriel, N.; Yarmohammadi, H.; Holley, C.T.; Feng, A.; Roy, S.S.; Reding, M.T.; John, R.; Eckman, P.; Zantek, N.D. Anti-Factor Xa and Activated Partial Thromboplastin Time Measurements for Heparin Monitoring in Mechanical Circulatory Support. JACC Heart Fail. 2015, 3, 314–322. [Google Scholar] [CrossRef]
- Marlar, R.A.; Clement, B.; Gausman, J. Activated Partial Thromboplastin Time Monitoring of Unfractionated Heparin Therapy: Issues and Recommendations. Semin. Thromb. Hemost. 2017, 43, 253–260. [Google Scholar] [CrossRef]
- Meyer, A.D.; Gelfond, J.A.L.; Wiles, A.A.; Freishtat, R.J.; Rais-Bahrami, K. Platelet-Derived Microparticles Generated by Neonatal Extracorporeal Membrane Oxygenation Systems. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2015, 61, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Mazzeffi, M.; Hasan, S.; Abuelkasem, E.; Meyer, M.; Deatrick, K.; Taylor, B.; Kon, Z.; Herr, D.; Tanaka, K. Von Willebrand Factor-GP1bα Interactions in Venoarterial Extracorporeal Membrane Oxygenation Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2125–2132. [Google Scholar] [CrossRef]
- Wang, S.; Griffith, B.P.; Wu, Z.J. Device-Induced Hemostatic Disorders in Mechanically Assisted Circulation. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2021, 27, 1076029620982374. [Google Scholar] [CrossRef]
- Leytin, V.; Allen, D.J.; Mykhaylov, S.; Mis, L.; Lyubimov, E.V.; Garvey, B.; Freedman, J. Pathologic High Shear Stress Induces Apoptosis Events in Human Platelets. Biochem. Biophys. Res. Commun. 2004, 320, 303–310. [Google Scholar] [CrossRef]
- Ilkan, Z.; Wright, J.R.; Goodall, A.H.; Gibbins, J.M.; Jones, C.I.; Mahaut-Smith, M.P. Evidence for Shear-Mediated Ca2+ Entry through Mechanosensitive Cation Channels in Human Platelets and a Megakaryocytic Cell Line. J. Biol. Chem. 2017, 292, 9204–9217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, Z.; Liao, Y.; Zhang, W.; Shi, Q.; Yan, R.; Ruan, C.; Dai, K. The Glycoprotein Ibalpha-von Willebrand Factor Interaction Induces Platelet Apoptosis. J. Thromb. Haemost. JTH 2010, 8, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Balle, C.M.; Jeppesen, A.N.; Christensen, S.; Hvas, A.-M. Platelet Function During Extracorporeal Membrane Oxygenation in Adult Patients. Front. Cardiovasc. Med. 2019, 6, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzeffi, M.; Greenwood, J.; Tanaka, K.; Menaker, J.; Rector, R.; Herr, D.; Kon, Z.; Lee, J.; Griffith, B.; Rajagopal, K.; et al. Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. Ann. Thorac. Surg. 2016, 101, 682–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descamps, R.; Moussa, M.D.; Besnier, E.; Fischer, M.-O.; Preau, S.; Tamion, F.; Daubin, C.; Cousin, N.; Vincentelli, A.; Goutay, J.; et al. Anti-Xa Activity and Hemorrhagic Events under Extracorporeal Membrane Oxygenation (ECMO): A Multicenter Cohort Study. Crit. Care Lond. Engl. 2021, 25, 127. [Google Scholar] [CrossRef]
- Tauber, H.; Ott, H.; Streif, W.; Weigel, G.; Loacker, L.; Fritz, J.; Heinz, A.; Velik-Salchner, C. Extracorporeal Membrane Oxygenation Induces Short-Term Loss of High-Molecular-Weight von Willebrand Factor Multimers. Anesth. Analg. 2015, 120, 730–736. [Google Scholar] [CrossRef]
- Frimat, M.; Boudhabhay, I.; Roumenina, L.T. Hemolysis Derived Products Toxicity and Endothelium: Model of the Second Hit. Toxins 2019, 11, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, H.; Alexandridis, P.; Neelamegham, S. Aspects of Hydrodynamic Shear Regulating Shear-Induced Platelet Activation and Self-Association of von Willebrand Factor in Suspension. Blood 2003, 101, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- McGlasson, D.L.; Kaczor, D.A.; Krasuski, R.A.; Campbell, C.L.; Kostur, M.R.; Adinaro, J.T. Effects of Pre-Analytical Variables on the Anti-Activated Factor X Chromogenic Assay When Monitoring Unfractionated Heparin and Low Molecular Weight Heparin Anticoagulation. Blood Coagul. Fibrinolysis 2005, 16, 173–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaloro, E.J.; Kershaw, G.; Mohammed, S.; Lippi, G. How to Optimize Activated Partial Thromboplastin Time (APTT) Testing: Solutions to Establishing and Verifying Normal Reference Intervals and Assessing APTT Reagents for Sensitivity to Heparin, Lupus Anticoagulant, and Clotting Factors. Semin. Thromb. Hemost. 2019, 45, 22–35. [Google Scholar] [CrossRef]
- Cuker, A.; Ptashkin, B.; Konkle, B.A.; Pipe, S.W.; Whinna, H.C.; Zheng, X.L.; Cines, D.B.; Pollak, E.S. Interlaboratory Agreement in the Monitoring of Unfractionated Heparin Using the Anti-Factor Xa-Correlated Activated Partial Thromboplastin Time. J. Thromb. Haemost. 2009, 7, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Dovlatova, N.; Heptinstall, S. Platelet Aggregation Measured by Single-Platelet Counting and Using PFA-100 Devices. Platelets 2018, 29, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Panigada, M.; Iapichino, G.E.; Brioni, M.; Panarello, G.; Protti, A.; Grasselli, G.; Occhipinti, G.; Novembrino, C.; Consonni, D.; Arcadipane, A.; et al. Thromboelastography-Based Anticoagulation Management during Extracorporeal Membrane Oxygenation: A Safety and Feasibility Pilot Study. Ann. Intensive Care 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mazzeffi, M.; Strauss, E.; Meyer, M.; Hasan, S.; Judd, M.; Abuelkasem, E.; Chow, J.; Nandwani, V.; McCarthy, P.; Tanaka, K. Coagulation Factor Levels and Underlying Thrombin Generation Patterns in Adult Extracorporeal Membrane Oxygenation Patients. Anesth. Analg. 2019, 129, 659–666. [Google Scholar] [CrossRef]


| Characteristics | N | Values |
|---|---|---|
| Age, years | 265 | 55 ± 14 |
| Body mass index, kg/m2 | 258 | 27.5 ± 5.7 |
| Male gender | 265 | 183 (69.1) |
| Comorbidities | ||
| Strokes | 264 | 8 (3.0) |
| Atrial fibrillation | 263 | 95 (36.1) |
| Diabetes mellitus | 264 | 61 (23.1) |
| Hypertension | 264 | 144 (54.5) |
| Hypercholesterolaemia | 252 | 93 (36.9) |
| Chronic kidney disease | 256 | 97 (37.9) |
| P2Y12 inhibitors during ECMO | 257 | 21 (8.2) |
| Simplified acute physiology score II | 264 | 58.3 ± 22.0 |
| Lactate on admission, mmol/L | 176 | 4.5 (2.4 to 9.5) |
| Description of ECMO support | ||
| Aetiologies of refractory shocks | 265 | |
| Postoperative low cardiac output syndrome | 90 (34.0) | |
| Primary graft dysfunction | 15 (5.7) | |
| Myocardial infarction | 76 (28.7) | |
| Acute on chronic heart disease | 40 (15.1) | |
| Pulmonary embolism | 9 (3.4) | |
| Myocarditis | 16 (6.0) | |
| Poisoning | 7 (2.6) | |
| Others | 12 (4.5) | |
| Postcardiotomy shock | 265 | 103 (38.9) |
| Duration of ECMO support, days | 265 | 7 (3 to 11) |
| Peripheral ECMO | 265 | 263 (99.2) |
| Left ventricle unloading strategies | 265 | 51 (19.2) |
| Left ventricle venting | 10 (3.8) | |
| Cannulation upgrade | 10 (3.8) | |
| Intra-aortic balloon pumping | 4 (1.5) | |
| Impella CP/5.0 | 27 (10.2) | |
| Thrombotic complications | 265 | 87 (32.8) |
| Description of thrombosis sites ** | 102 | |
| Ischemic stroke | 46 (45.1) | |
| Limb ischaemia | 15 (14.7) | |
| Cannula/circuit thrombosis | 26 (25.5) | |
| Others | 15 (14.7) | |
| Serious bleeders | 265 | 150 (56.6) |
| Description of bleeding sites * | 206 | |
| Pericardial | 79 (38.4) | |
| Cannula | 46 (22.3) | |
| Pleural | 19 (9.2) | |
| Otorhinolaryngological area | 16 (7.8) | |
| Gastrointestinal tract | 10 (4.9) | |
| Haemoptysis | 9 (4.4) | |
| Intracerebral haemorrhage | 6 (2.9) | |
| Others * | 21 (10.2) | |
| Number of blood products | 265 | |
| Packed red blood cells, units | 250 | 10 (5 to 18) |
| Fresh frozen plasma, units | 169 | 7 (3 to 11) |
| Platelet concentrate, units | 176 | 3 (2 to 6) |
| Weaning categories | 265 | |
| Successful weaning | 120 (45.5) | |
| Heart transplantation | 22 (8.3) | |
| Left or bi-ventricular assist devices | 20 (7.6) | |
| Death under VA-ECMO support | 103 (38.9) | |
| Outcomes | 265 | |
| Intensive care unit length of stay (days) | 12 (6 to 25) | |
| Hospital length of stay (days) | 18 (8 to 41) | |
| 28-day mortality | 114 (43.0) | |
| Intensive care unit mortality | 126 (47.5) | |
| Hospital Mortality | 136 (51.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, M.D.; Soquet, J.; Lamer, A.; Labreuche, J.; Gantois, G.; Dupont, A.; Abou-Arab, O.; Rousse, N.; Liu, V.; Brandt, C.; et al. Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study. J. Clin. Med. 2021, 10, 2158. https://doi.org/10.3390/jcm10102158
Moussa MD, Soquet J, Lamer A, Labreuche J, Gantois G, Dupont A, Abou-Arab O, Rousse N, Liu V, Brandt C, et al. Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(10):2158. https://doi.org/10.3390/jcm10102158
Chicago/Turabian StyleMoussa, Mouhamed Djahoum, Jérôme Soquet, Antoine Lamer, Julien Labreuche, Guillaume Gantois, Annabelle Dupont, Osama Abou-Arab, Natacha Rousse, Vincent Liu, Caroline Brandt, and et al. 2021. "Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study" Journal of Clinical Medicine 10, no. 10: 2158. https://doi.org/10.3390/jcm10102158
APA StyleMoussa, M. D., Soquet, J., Lamer, A., Labreuche, J., Gantois, G., Dupont, A., Abou-Arab, O., Rousse, N., Liu, V., Brandt, C., Foulon, V., Leroy, G., Schurtz, G., Jeanpierre, E., Duhamel, A., Susen, S., Vincentelli, A., & Robin, E. (2021). Evaluation of Anti-Activated Factor X Activity and Activated Partial Thromboplastin Time Relations and Their Association with Bleeding and Thrombosis during Veno-Arterial ECMO Support: A Retrospective Study. Journal of Clinical Medicine, 10(10), 2158. https://doi.org/10.3390/jcm10102158







