Plasmin, Immunity, and Surgical Site Infection
Abstract
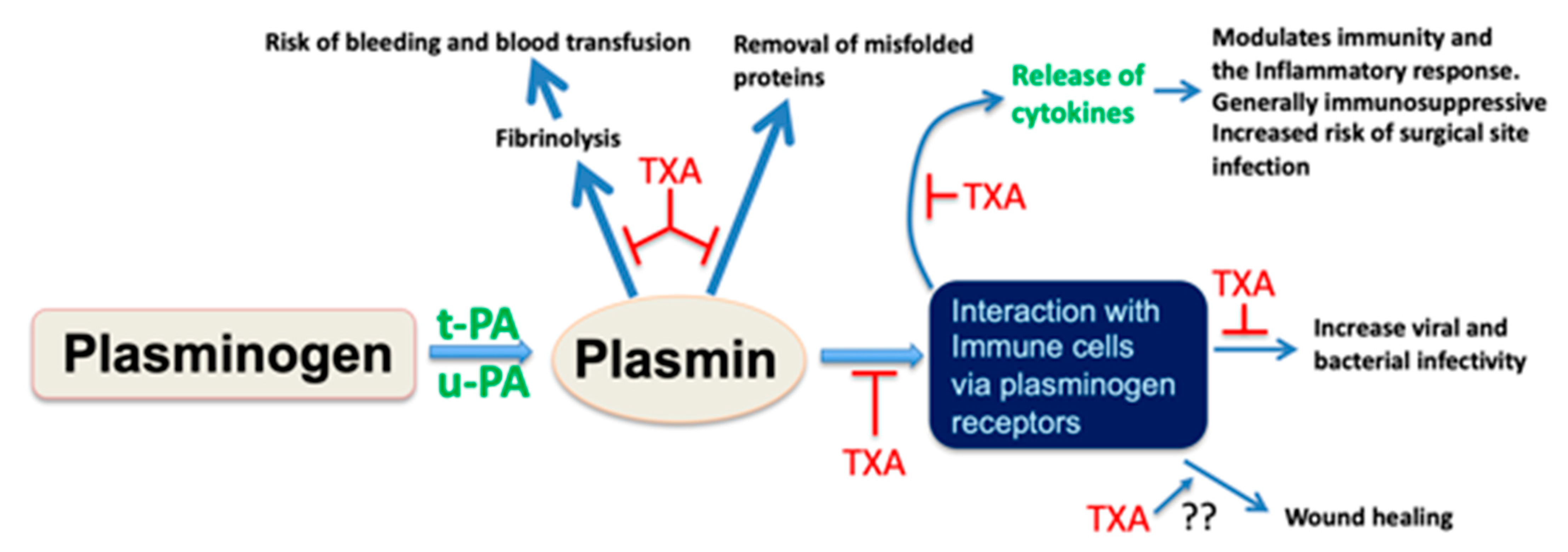
1. A Role for Plasmin as an Immune Modulator
2. Tranexamic Acid as an Agent to Mitigate Surgical Site Infection
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef]
- Healthcare-Associated Infections: Surgical Site Infections—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-surgical-site-infections-annual-1 (accessed on 24 April 2021).
- Bhangu, A.; O Ademuyiwa, A.; Aguilera, M.L.; Alexander, P.; Al-Saqqa, S.W.; Borda-Luque, G.; Costas-Chavarri, A.; Drake, T.M.; Ntirenganya, F.; Fitzgerald, J.E.; et al. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect. Dis. 2018, 18, 516–525. [Google Scholar]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence Guidelines—Surgical Site Infections; Prevention and Treatment 2020. Available online: www.nice.org.uk/guidance/ng125 (accessed on 25 April 2021).
- World Health Organisation Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed. Available online: https://apps.who.int/iris/handle/10665/277399?utm_medium=email&utm_source=transaction (accessed on 25 April 2021).
- Berríos-Torres, S.I.; Umscheid, C. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence (National Collaborating Centre for Women’s and Children’s Health)—Surgical Site Infection Prevention and Treatment of Surgical Site Infection. Available online: https://www.nice.org.uk/guidance/ng125/evidence/october-2008-full-guideline-pdf-6727105694 (accessed on 25 April 2021).
- Harper, N.J.N.; Cook, T.M. Anaesthesia, surgery, and life-threatening allergic reactions: Epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br. J. Anaesth. 2018, 121, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.M.; Springer, B. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients with Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthrit. Care Res. 2017, 69, 1111–1124. [Google Scholar] [CrossRef]
- World Health Organisation Global Guidelines on the Prevention of Surgical Site Infection Web Appendices, Appendix 12: Summary of the Systemic Review on the Perioperative Discontinuation of Immunosuppressive Agents. Available online: https://www.who.int/gpsc/ssi-web-appendices/en/ (accessed on 25 April 2021).
- Draxler, D.F.; Medcalf, R.L. The Fibrinolytic System—More Than Fibrinolysis? Transfus. Med. Rev. 2015, 29, 102–109. [Google Scholar] [CrossRef]
- Keragala, C.B.M.; Medcalf, R.L. Plasminogen: An enzymatic zymogen. Blood 2021, in press. [Google Scholar] [CrossRef]
- Foley, J.H. Examining coagulation-complement crosstalk: Complement activation and thrombosis. Thromb. Res. 2016, 141, S50–S54. [Google Scholar] [CrossRef]
- Pillemer, L.; Ratnoff, O.D. The inactivation of complement and its components by plasmin. J. Exp. Med. 1953, 97, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Barthel, D.; Schindler, S. Plasminogen Is a Complement Inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Hagström, E. Beneficial and Detrimental Effects of Plasmin(ogen) during Infection and Sepsis in Mice. PLoS ONE 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.W. Activation of plasminogen by human plasma kallikrein. Biochem. Biophys. Res. Commun. 1969, 35, 273–279. [Google Scholar] [CrossRef]
- Maas, C. Plasminflammation—An Emerging Pathway to Bradykinin Production. Front. Immunol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Amara, U.; Rittirsch, D. Interaction between the Coagulation and Complement System. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar]
- Bender, L.; Weidmann, H. Factor XII-Driven Inflammatory Reactions with Implications for Anaphylaxis. Front. Immunol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Khalil, N.; Corne, S. Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am. J. Respir. Cell Mol. 1996, 15, 252–259. [Google Scholar] [CrossRef]
- Gray, K.; Ellis, V. Activation of pro-BDNF by the pericellular serine protease plasmin. FEBS Lett. 2008, 582, 907–910. [Google Scholar] [CrossRef]
- Lijnen, H. Plasmin and matrix metalloproteinases in vascular remodelling. Thromb. Haemost. 2001, 86, 324–333. [Google Scholar]
- Keragala, C.B.; Draxler, D.F. Haemostasis and innate immunity—A complementary relationship. Br. J. Haematol. 2018, 180, 782–798. [Google Scholar] [CrossRef]
- Foley, J.H. Plasmin(ogen) at the Nexus of Fibrinolysis, Inflammation, and Complement. Semin. Thromb. Hemost. 2017, 43, 135–142. [Google Scholar] [CrossRef]
- Foley, J.H.; Peterson, E.A.; Lei, V.; Wan, L.W.; Krisinger, M.J.; Conway, E.M. Interplay between fibrinolysis and complement: Plasmin cleavage of iC3b modulates immune responses. J. Thromb. Haemost. 2015, 13, 610–618. [Google Scholar] [CrossRef]
- Mantuano, E.; Azmoon, P.; Brifault, C.; Banki, M.A.; Gilder, A.S.; Campana, W.M.; Gonias, S.L. Tissue-type plasminogen activator regulates macrophage activation and innate immunity. Blood 2017, 130, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Draxler, D.; Sashindranath, M. Plasmin: A Modulator of Immune Function. Semin. Thromb. Hemost. 2017, 43, 143–153. [Google Scholar] [CrossRef]
- Borg, R.J.; Samson, A. Dendritic Cell-Mediated Phagocytosis but Not Immune Activation Is Enhanced by Plasmin. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Ganapathy, S. Plasminogen promotes macrophage phagocytosis in mice. Blood 2014, 124, 679–688. [Google Scholar] [CrossRef]
- Li, Q.; Laumonnier, Y. Plasmin Triggers Cytokine Induction in Human Monocyte-Derived Macrophages. Arterioscler. Thromb. Vasc Biol. 2007, 27, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Syrovets, T. Plasmin Triggers Chemotaxis of Monocyte-Derived Dendritic Cells through an Akt2-Dependent Pathway and Promotes a T-Helper Type-1 Response. Arterioscler. Thromb. Vasc Biol. 2010, 30, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Lunov, O. Plasmin as a proinflammatory cell activator. J. Leukoc. Biol. 2012, 92, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Draxler, D.; Awas, M. Tranexamic Acid Influences the Immune Response, but not Bacterial Clearance in a Model of Post-Traumatic Brain Injury Pneumonia. J. Neurotraum 2019, 36, 3297–3308. [Google Scholar] [CrossRef]
- Draxler, D.F.; Daglas, M. Tranexamic acid modulates the cellular immune profile after traumatic brain injury in mice without hyperfibrinolysis. J. Thromb. Haemost. 2019, 17, 2174–2187. [Google Scholar] [CrossRef]
- Medcalf, R.; Keragala, C. Fibrinolysis and the Immune Response in Trauma. Semin. Thromb. Hemost. 2020, 46, 176–182. [Google Scholar] [CrossRef]
- Romer, J.; Bugge, T.H. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med. 1996, 2, 287–292. [Google Scholar] [CrossRef]
- Sulniute, R.; Shen, Y. Plasminogen is a critical regulator of cutaneous wound healing. Thromb. Haemost. 2016, 115, 1001–1009. [Google Scholar] [CrossRef]
- Shen, Y. Plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. J. Transl. Med. 2014, 12, 1–9. [Google Scholar] [CrossRef]
- Fallah, M.; Shen, Y. Plasminogen activation is required for the development of radiation-induced dermatitis. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Fallah, M.; Viklund, E. Plasminogen is a master regulator and a potential drug candidate for the healing of radiation wounds. Cell Death Dis. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Samson, A.L.; Borg, R. A nonfibrin macromolecular cofactor for tPA-mediated plasmin generation following cellular injury. Blood 2009, 114, 1937–1946. [Google Scholar] [CrossRef]
- Samson, A.L.; Knaupp, A. Nucleocytoplasmic Coagulation: An Injury-Induced Aggregation Event that Disulfide Crosslinks Proteins and Facilitates Their Removal by Plasmin. Cell Rep. 2012, 2, 889–901. [Google Scholar] [CrossRef]
- Ayón-Núñez, D.A.; Fragoso, G. Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci. Rep. 2018, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ringdahl, U. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 2004, 305, 1283–1286. [Google Scholar] [CrossRef]
- Gladysheva, I.P.; Turner, R.B. Coevolutionary patterns in plasminogen activation. Proc. Natl. Acad. Sci. USA 2003, 100, 9168–9172. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Okamoto, U. Amino-methyl-cyclohexane-carboxylic acid: AMCHA. Keio J. Med. 1962, 11, 105–115. [Google Scholar] [CrossRef]
- Ker, K.; Edwards, P. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ Br. Med. J. 2012, 344, 1–13. [Google Scholar] [CrossRef] [PubMed]
- CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. The never ending success story of tranexamic acid in acquired bleeding. Haematologica 2020, 105, 1201–1205. [Google Scholar] [CrossRef]
- Taeuber, I.; Weibel, S. Association of Intravenous Tranexamic Acid with Thromboembolic Events and Motality; A Systematic Review, Meta-analysis, and Meta-regression. JAMA Surg. 2021. [Google Scholar] [CrossRef]
- Remérand, F.; Cotton, M. Tranexamic acid decreases risk of haematomas but not pain after hip arthroplasty. Orthop. Traumatol. Surg. Res. 2013, 99, 667–673. [Google Scholar] [CrossRef]
- Johns, W.L.; Kempland, C. Tranexamic Acid Use in Foot and Ankle Surgery. Foot Ankle Orthop. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Kawakita, T.; Landy, H.J. Surgical site infections after cesarean delivery: Epidemiology, prevention and treatment. Matern. Health Neonatol. Perinatol. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brascia, D.; Garcia-Medina, N. Impact of transfusion on stroke after cardiovascular interventions: Meta-analysis of comparative studies. J. Crit. Care 2017, 38, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Reeves, B. Increased Mortality, Postoperative Morbidity, and Cost After Red Blood Cell Transfusion in Patients Having Cardiac Surgery. Circulation 2007, 116, 2544–2552. [Google Scholar] [CrossRef]
- Vlot, E.A.; Verwijmeren, L. Intra-operative red blood cell transfusion and mortality after cardiac surgery. BMC Anesthesiol. 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bower, W.F.; Jin, L. Peri-operative blood transfusion increases length of hospital stay and number of postoperative complications in non-cardiac surgical patients. Hong Kong Med. J. 2010, 16, 116–120. [Google Scholar]
- Zhang, L.; Liao, Q. Blood Transfusion is an Independent Risk Factor for Postoperative Serious Infectious Complications after Pancreaticoduodenectomy. World J. Surg. 2016, 40, 2507–2512. [Google Scholar] [CrossRef]
- Higgins, R.M.; Helm, M.C. Perioperative blood transfusion increases risk of surgical site infection after bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 582–587. [Google Scholar] [CrossRef]
- Remy, K.E.; Hall, M. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018, 58, 804–815. [Google Scholar] [CrossRef]
- Cata, J.P.; Wang, H. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. BJA Br. J. Anaesth. 2013, 110, 690–701. [Google Scholar] [CrossRef]
- Coobs, B.R.; Moskal, J.T. Tranexamic Acid: Indirect Benefits to the Standard of Care. J. Bone Jt. Surg. 2020, 102, e92. [Google Scholar] [CrossRef]
- Myles, P.S.; Smith, J. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N. Engl. J. Med. 2017, 376, 136–148. [Google Scholar] [CrossRef]
- Draxler, D.F.; Yep, K. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. 2019, 3, 1598–1609. [Google Scholar] [CrossRef]
- Alzahrani, S.; Ajjan, R. Coagulation and fibrinolysis in diabetes. Diabetes Vasc. Dis. Res. 2010, 7, 260–273. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Gamlen, T. Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme-specific activity. Blood 2013, 122, 134–142. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hastings, S.; Myles, P.S.; Medcalf, R.L. Plasmin, Immunity, and Surgical Site Infection. J. Clin. Med. 2021, 10, 2070. https://doi.org/10.3390/jcm10102070
Hastings S, Myles PS, Medcalf RL. Plasmin, Immunity, and Surgical Site Infection. Journal of Clinical Medicine. 2021; 10(10):2070. https://doi.org/10.3390/jcm10102070
Chicago/Turabian StyleHastings, Stuart, Paul S. Myles, and Robert L. Medcalf. 2021. "Plasmin, Immunity, and Surgical Site Infection" Journal of Clinical Medicine 10, no. 10: 2070. https://doi.org/10.3390/jcm10102070
APA StyleHastings, S., Myles, P. S., & Medcalf, R. L. (2021). Plasmin, Immunity, and Surgical Site Infection. Journal of Clinical Medicine, 10(10), 2070. https://doi.org/10.3390/jcm10102070





