Combined Administration of Fibrinogen and Factor XIII Concentrate Does Not Improve Dilutional Coagulopathy Superiorly Than Sole Fibrinogen Therapy: Results of an In-Vitro Thrombelastographic Study
Abstract
1. Introduction
2. Materials and Methods
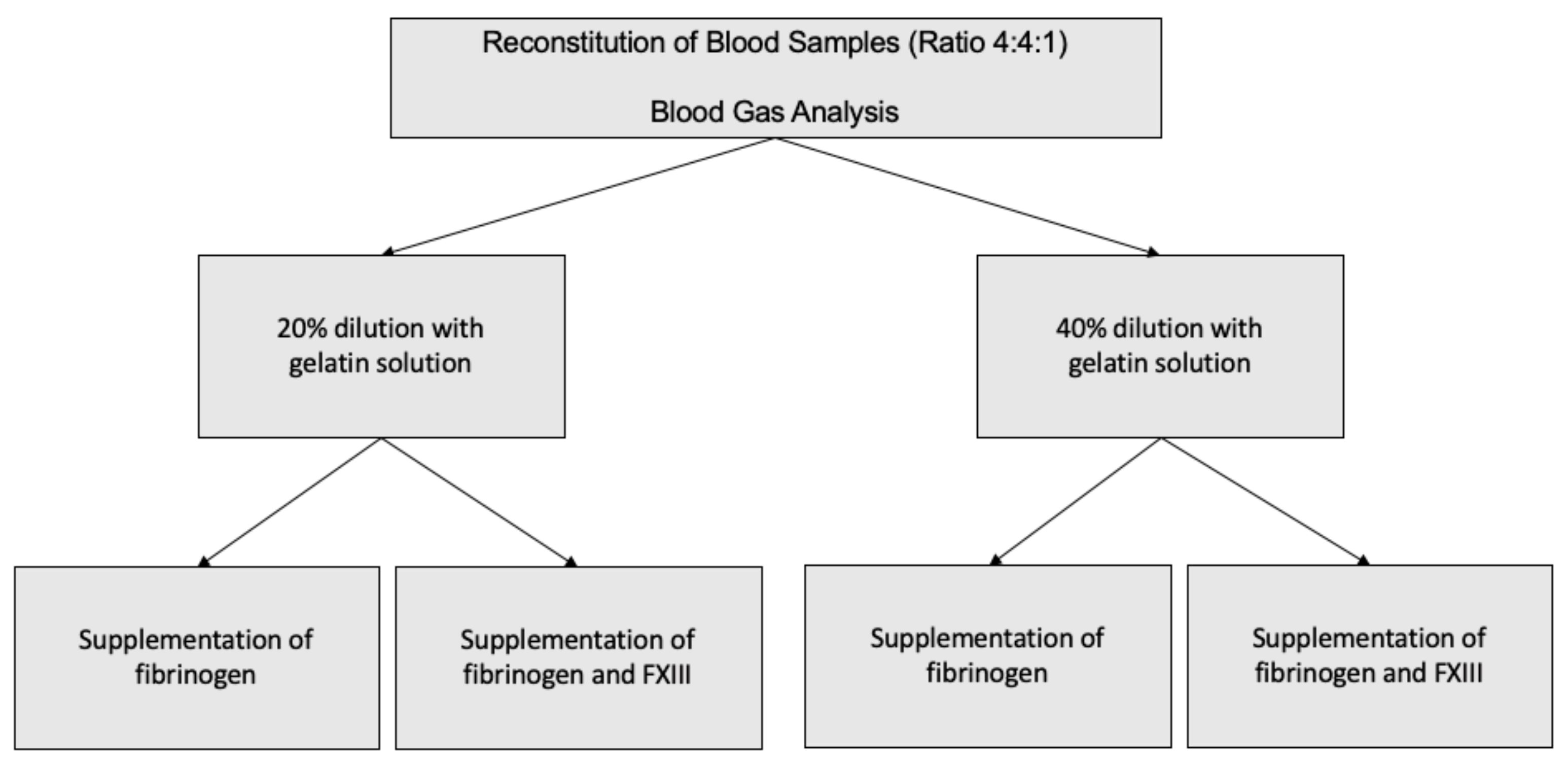
2.1. Study Design and Recruitment of Study Probands
2.2. Processing of Blood Products
2.3. Experimental Setup
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Probands
3.2. Effects of Hemodilution
3.3. Treatment of Dilutional Coagulopathy with Fibrinogen or Combination of Fibrinogen and FXIII
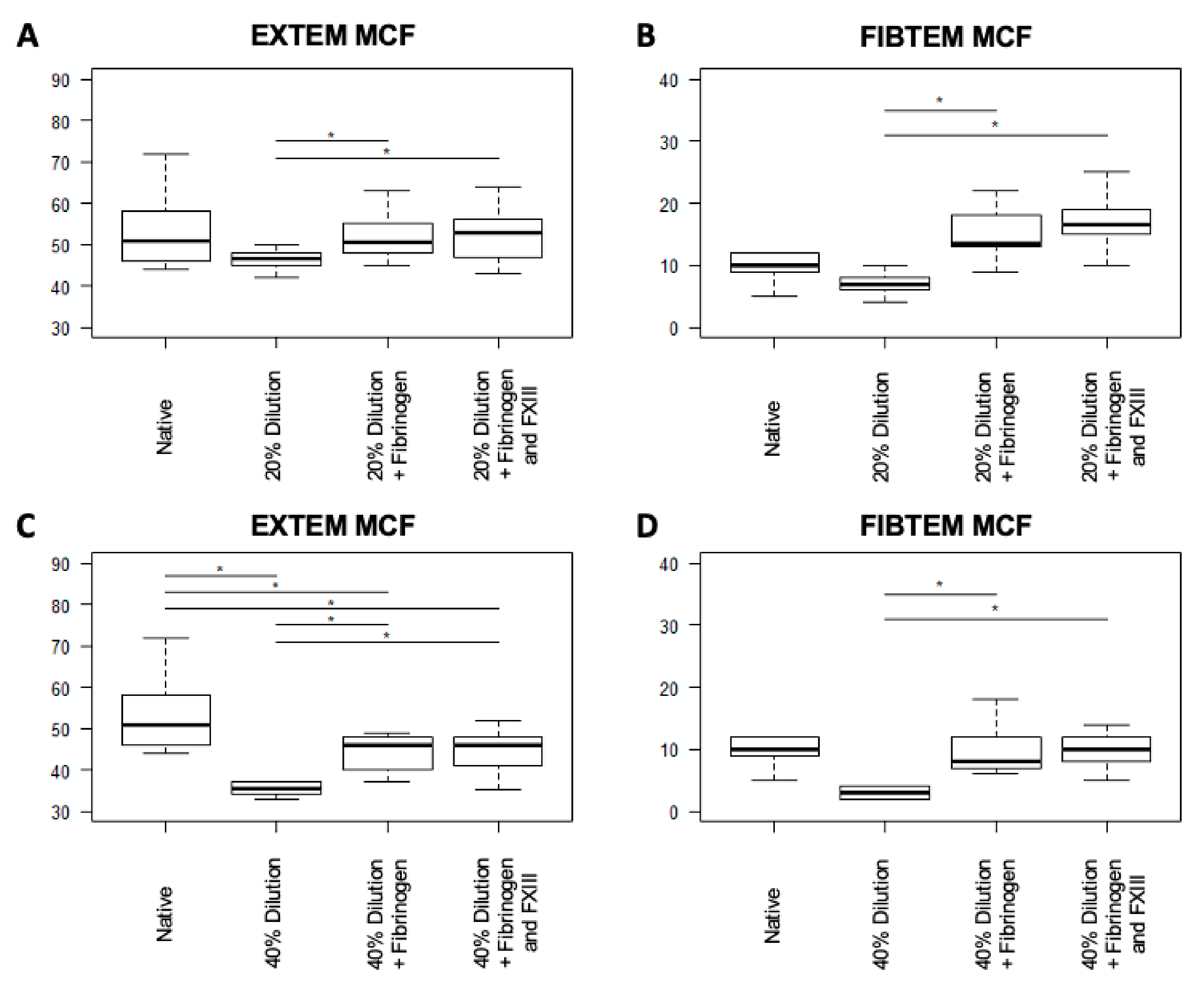
3.3.1. Clot Properties
3.3.2. Clot Formation
3.3.3. Lysis Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Chatrath, V.; Khetarpal, R.; Ahuja, J. Fluid management in patients with trauma: Restrictive versus liberal approach. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 308. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Nicholl, J.; Webber, L.; Cox, H.; Dixon, S.; Yates, D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol. Assess. 2000, 4, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.A.; Carrick, M.M.; Norman, M.A.; Scott, B.G.; Welsh, F.J.; Tsai, P.; Liscum, K.R.; Wall, M.J.; Mattox, K.L. Hypotensive Resuscitation Strategy Reduces Transfusion Requirements and Severe Postoperative Coagulopathy in Trauma Patients with Hemorrhagic Shock: Preliminary Results of a Randomized Controlled Trial. J. Trauma Inj. Infect. Crit. Care 2011, 70, 652–663. [Google Scholar] [CrossRef]
- Frith, D.; Goslings, J.C.; Gaarder, C.; Maegele, M.; Cohen, M.J.; Allard, S.; Johansson, P.I.; Stanworth, S.; Thiemermann, C.; Brohi, K. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J. Thromb. Haemost. 2010, 8, 1919–1925. [Google Scholar] [CrossRef]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early coagulopathy predicts mortality in trauma. J. Trauma 2003, 55, 39–44. [Google Scholar] [CrossRef]
- Maegele, M.; Lefering, R.; Yucel, N.; Tjardes, T.; Rixen, D.; Paffrath, T.; Simanski, C.; Neugebauer, E.; Bouillon, B. Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 2007, 38, 298–304. [Google Scholar] [CrossRef]
- Cap, A.; Hunt, B.J. The pathogenesis of traumatic coagulopathy. Anaesthesia 2015, 70, 96–101. [Google Scholar] [CrossRef]
- Simmons, J.W.; Powell, M.F. Acute traumatic coagulopathy: Pathophysiology and resuscitation. Br. J. Anaesth. 2016, 117, iii31–iii43. [Google Scholar] [CrossRef]
- Simurda, T.; Snahnicanova, Z.; Loderer, D.; Sokol, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Fibrinogen Martin: A Novel Mutation in FGB (Gln180Stop) Causing Congenital Afibrinogenemia. Semin. Thromb. Hemost. 2016, 42, 455–458. [Google Scholar]
- Stein, P.; Kaserer, A.; Sprengel, K.; Wanner, G.A.; Seifert, B.; Theusinger, O.M.; Spahn, D.R. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia 2017, 72, 1317–1326. [Google Scholar] [CrossRef]
- Innerhofer, P.; Fries, D.; Mittermayr, M.; Innerhofer, N.; von Langen, D.; Hell, T.; Gruber, G.; Schmid, S.; Friesenecker, B.; Lorenz, I.H.; et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): A single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017, 4, e258–e271. [Google Scholar] [CrossRef]
- Ågren, A.; Edgren, G.; Kardell, M.; Östlund, A.; Wikman, A.T. In vitro combinations of red blood cell, plasma and platelet components evaluated by thromboelastography. Blood Transfus. 2014, 12, 491–496. [Google Scholar]
- Ågren, A.; Edgren, G.; Ambrosio, D.; Gryfelt, G.; Östlund, A.; Wikman, A. Haemostasis monitored in stored red blood cells, plasma and platelet concentrates in the proportion of 4:4:1 diluted with crystalloids and colloids. Blood Coagul. Fibrinolysis 2016, 27, 334–339. [Google Scholar] [CrossRef]
- Kind, S.L.; Spahn-Nett, G.H.; Emmert, M.Y.; Eismon, J.; Seifert, B.; Spahn, D.R.; Theusinger, O.M. Is dilutional coagulopathy induced by different colloids reversible by replacement of fibrinogen and factor xiii concentrates? Anesth. Analg. 2013, 117, 1063–1071. [Google Scholar] [CrossRef]
- Schlimp, C.J.; Cadamuro, J.; Solomon, C.; Redl, H.; Schöchl, H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, gelatine, hydroxyethyl starch or saline in vitro. Blood Transfus. 2013, 11, 510–517. [Google Scholar]
- Winstedt, D.; Thomas, O.D.; Nilsson, F.; Olanders, K.; Schött, U. Correction of hypothermic and dilutional coagulopathy with concentrates of fibrinogen and factor XIII: An in vitro study with ROTEM. Scand. J. Trauma. Resusc. Emerg. Med. 2014, 22, 73. [Google Scholar] [CrossRef]
- Bundesärztekammer. Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives, 4th ed.; Deutscher Aerzteverlag: Cologne, Germany, 2014; pp. 10–278. [Google Scholar]
- Simurda, T.; Zolkova, J.; Snahnicanova, Z.; Loderer, D.; Skornova, I.; Sokol, J.; Hudecek, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Identification of Two Novel Fibrinogen Bβ Chain Mutations in Two Slovak Families with Quantitative Fibrinogen Disorders. Int. J. Mol. Sci. 2017, 19, 100. [Google Scholar] [CrossRef]
- Hartmann, J.; Walsh, M.; Grisoli, A.; Thomas, A.V.; Shariff, F.; McCauley, R.; Vande Lune, S.; Zackariya, N.; Patel, S.; Farrell, M.S.; et al. Diagnosis and Treatment of Trauma-Induced Coagulopathy by Viscoelastography. Semin. Thromb. Hemost. 2020, 46, 134–146. [Google Scholar] [CrossRef]
- Schäfer, N.; Driessen, A.; Bauerfeind, U.; Fröhlich, M.; Ofir, J.; Stürmer, E.K.; Maegele, M. In vitro effects of different sources of fibrinogen supplementation on clot initiation and stability in a model of dilutional coagulopathy. Transfus. Med. 2016, 26, 373–380. [Google Scholar] [CrossRef]
- Nagashima, F.; Inoue, S.; Koami, H.; Miike, T.; Sakamoto, Y.; Kai, K. High-dose Factor XIII administration induces effective hemostasis for trauma-associated coagulopathy (TAC) both in vitro and in rat hemorrhagic shock in vivo models. J. Trauma Acute Care Surg. 2018, 85, 588–597. [Google Scholar] [CrossRef]
- Budnik, I.; Shenkman, B.; Morozova, O.; Einav, Y. Thromboelastometry assessment of the effects of fibrinogen, activated prothrombin complex concentrate, and tranexamic acid on clot formation and fibrinolysis in a model of trauma-induced coagulopathy. Eur. J. Trauma Emerg. Surg. 2020. [Google Scholar] [CrossRef]
- Budnik, I.; Shenkman, B.; Morozova, O.; Einav, Y. In-vitro assessment of the effects of fibrinogen, recombinant factor VIIa and factor XIII on trauma-induced coagulopathy. Blood Coagul. Fibrinolysis 2020, 31, 253–257. [Google Scholar] [CrossRef]
- Lier, H.; Vorweg, M.; Hanke, A.; Görlinger, K. Thromboelastometry guided therapy of severe bleeding. Hamostaseologie 2013, 33, 51–61. [Google Scholar]
- Schöchl, H.; Maegele, M.; Solomon, C.; Görlinger, K.; Voelckel, W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma. Resusc. Emerg. Med. 2012, 20, 15. [Google Scholar] [CrossRef]
- Görlinger, K.; Dirkmann, D.; Solomon, C.; Hanke, A.A. Fast interpretation of thromboelastometry in non-cardiac surgery: Reliability in patients with hypo-, normo-, and hypercoagulability. Br. J. Anaesth. 2013, 110, 222–230. [Google Scholar] [CrossRef]
- Shin, H.; Lee, H.; Na, H. The effect of a mixture of 2.7% sorbitol-0.54% mannitol solution on blood coagulation: An invitro, observational healthyvolunteer study using rotational thromboelastometry (ROTEM). Korean J. Anesthesiol. 2019, 72, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Park, H.-Y.; Na, H.-S.; Hong, J.-P.; Lee, G.-W.; Do, S.-H. The effects of Plasma-Lyte 148 solution on blood coagulation. Blood Coagul. Fibrinolysis 2018, 29, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.C.A.; Varanasi, S.; Yang, K.X. The Effects of Haemodilution with Succinylated Gelatin Solution on Coagulation in Vitro as Assessed by Thromboelastometry and Impedance (Multiple Electrode) Aggregometry. Anaesth. Intensive Care 2018, 46, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Behring Information to Factor XIII Concentrate (Fibrogammin®). Available online: https://www.corifact.com/important-safety-information.aspx (accessed on 7 December 2020).
- Theusinger, O.; Baulig, W.; Asmis, L.; Seifert, B.; Spahn, D. In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM®). Thromb. Haemost. 2010, 104, 385–391. [Google Scholar]
- Hanna, J.; Winstedt, D.; Schött, U. Fibrinogen and FXIII dose response effects on albumin-induced coagulopathy. Scand. J. Clin. Lab. Investig. 2013, 73, 553–562. [Google Scholar] [CrossRef][Green Version]
- Winstedt, D.; Hanna, J.; Schött, U. Albumin-induced coagulopathy is less severe and more effectively reversed with fibrinogen concentrate than is synthetic colloid-induced coagulopathy. Scand. J. Clin. Lab. Investig. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Thaler, U.; Deusch, E.; Kozek-Langenecker, S.A. In vitro effects of gelatin solutions on platelet function: A comparison with hydroxyethyl starch solutions. Anaesthesia 2005, 60, 554–559. [Google Scholar] [CrossRef]
- Dirkmann, D.; Hanke, A.A.; Görlinger, K.; Peters, J. Hypothermia and Acidosis Synergistically Impair Coagulation in Human Whole Blood. Anesth. Analg. 2008, 106, 1627–1632. [Google Scholar] [CrossRef]
- Martini, W.Z. Coagulopathy by Hypothermia and Acidosis: Mechanisms of Thrombin Generation and Fibrinogen Availability. J. Trauma Inj. Infect. Crit. Care 2009, 67, 202–209. [Google Scholar] [CrossRef]
- Rijken, D.C.; Uitte de Willige, S. Inhibition of Fibrinolysis by Coagulation Factor XIII. Biomed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Sucker, C.; Tharra, K.; Litmathe, J.; Scharfv, R.E.; Zotz, R.B. Rotation thromboelastography (ROTEM) parameters are influenced by age, gender, and oral contraception. Perfusion 2011, 26, 334–340. [Google Scholar] [CrossRef]
- Lang, T.; Bauters, A.; Braun, S.L.; Pöizsch, B.; Von Pape, K.W.; Kolde, H.J.; Lakner, M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul. Fibrinolysis 2005, 16, 301–310. [Google Scholar] [CrossRef]
- Ramaker, A.J.D.W.R.; Meyer, P.; Van Der Meer, J.; Struys, M.M.R.F.; Lisman, T.; Van Oeveren, W.; Hendriks, H.G.D. Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: Results of point of care testing with the thromboelastography analyser. Blood Coagul. Fibrinolysis 2009, 20, 436–439. [Google Scholar] [CrossRef]
- Adams, F.; Bellairs, G.; Bird, A.R.; Oguntibeju, O.O. Biochemical Storage Lesions Occurring in Nonirradiated and Irradiated Red Blood Cells: A Brief Review. Biomed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
| Parameter | Native | Dilution 20% | Dilution 40% | p-Value Native vs. 20% | p-Value Native vs. 40% |
|---|---|---|---|---|---|
| INTEM CT (s) | 220 (214–255) | 196.5 (180–207.3) | 250 (236–268.8) | 0.11 | 0.57 |
| INTEM CFT (s) | 173 (147–186) | 196.5 (153.3–235.8) | 462.5 (414.3–593.3) | 0.16 | <0.01 |
| INTEM MCF (mm) | 52 (50–53) | 45.5 (42.3–47.8) | 30 (29–32.5) | 0.12 | 0.01 |
| INTEM LI60 (%) | 99 (98–100) | 97 (96–99) | 95 (95–99) | 0.71 | 0.28 |
| EXTEM CT (s) | 92 (67–102) | 72.5 (66.3–94) | 119 (88.3–125.8) | 0.55 | 0.55 |
| EXTEM CFT (s) | 160 (119–242) | 213.5 (203.3–255) | 388 (367–434) | 0.29 | 0.29 |
| EXTEM MCF (mm) | 51 (46–58) | 46.5 (45.3–47.8) | 35.5 (34.3–36.8) | 0.03 | 0.01 |
| EXTEM LI60 (%) | 100 (97.5–100) | 99 (96.8–100) | 99 (96–100) | 1.0 | 1.0 |
| FIBTEM CT (s) | 71 (59–86) | 63.5 (58.5–87.5) | 265 (130–570) | 0.81 | 0.05 |
| FIBTEM MCF (mm) | 10 (9–12) | 7 (6.3–7.8) | 3 (2–3.8) | 0.06 | <0.01 |
| FIBTEM LI60 (%) | 100 (100) | 100 (100) | 100 (100) | n.c. | 1.0 |
| APTEM CT (s) | 67 (63–83) | 70.5 (64.5–79.3) | 100 (89–145) | 0.86 | 0.02 |
| APTEM CFT (s) | 175 (146–236) | 226 (201.3–264.5) | 413 (325–455) | 0.57 | 0.29 |
| APTEM MCF (mm) | 49 (46–52) | 46 (42.3–47.8) | 40 (35–40) | 0.48 | 0.22 |
| APTEM LI60 (%) | 100 (100) | 99 (97.3–100) | 100 (97–100) | 0.56 | 1.0 |
| Parameter | Dilution 20% | Dilution 20% Fibrinogen | Dilution 20% Fibrinogen + FXIII | p-Value Dilution 20% vs. Fibrinogen | p-Value Dilution 20% vs. Fibrinogen + FXIII | p-Value Dilution 20% Fibrinogen vs. Fibrinogen + FXIII |
|---|---|---|---|---|---|---|
| INTEM CT (s) | 196.5 (180–207.3) | 224 (211.5–267.5) | 213.5 (203–246.5) | 0.19 | 0.19 | 0.49 |
| INTEM CFT (s) | 196.5 (153.3–235.8) | 155 (128.5–182.5) | 140 (114.5–194.8) | 0.08 | 0.16 | 0.77 |
| INTEM MCF (mm) | 45.5 (42.3–47.8) | 49.5 (46.3–53.8) | 51 (44.8–54.8) | 0.04 | 0.12 | 0.34 |
| INTEM LI60 (%) | 97 (96–99) | 99 (98–99) | 100 (99.8–100) | 0.30 | 0.30 | 0.37 |
| EXTEM CT (s) | 72.5 (66.3–94) | 54 (50.3–57.3) | 51 (45.5–53.5) | 0.03 | 0.06 | 0.36 |
| EXTEM CFT (s) | 213.5 (203.3–255) | 175 (136.5–217.3) | 141.5 (117.3–192.5) | 0.04 | 0.04 | 0.11 |
| EXTEM MCF (mm) | 46.5 (45.3–47.8) | 50.5 (48.3–54.8) | 53 (48.3–55.8) | 0.02 | 0.02 | 0.36 |
| EXTEM LI60 (%) | 99 (96.8–100) | 100 (100) | 100 (100) | 0.09 | 0.09 | n.c. |
| FIBTEM CT (s) | 63.5 (58.5–87.5) | 48.5 (46.5–50) | 50.5 (43–57) | 0.65 | 0.04 | 0.84 |
| FIBTEM MCF (mm) | 7 (6.3–7.8) | 13.5 (13–17.3) | 16.5 (15.3–18.8) | <0.01 | <0.01 | 0.1 |
| FIBTEM LI60 (%) | 100 (100) | 100 (100) | 100 (100) | n.c. | n.c. | n.c. |
| APTEM CT (s) | 70.5 (64.5–79.3) | 53 (51–59) | 49 (48.3–52.8) | 0.02 | 0.02 | 0.81 |
| APTEM CFT (s) | 226 (201.3–264.5) | 191 (119–219) | 132.5 (93–176) | 0.01 | <0.01 | 0.01 |
| APTEM MCF (mm) | 46 (42.3–47.8) | 50 (49–55) | 56.5 (51.8–60.8) | 0.03 | 0.01 | 0.01 |
| APTEM LI60 (%) | 99 (97.3–100) | 100 (99.5–100) | 100 (100) | 0.09 | 0.09 | 1.0 |
| Parameter | Dilution 40% | Dilution 40% Fibrinogen | Dilution 40% Fibrinogen + FXIII | p-Value Dilution 40% vs. Fibrinogen | p-Value Dilution 40% vs. Fibrinogen + FXIII | p-Value Dilution 40% Fibrinogen vs. Fibrinogen + FXIII |
|---|---|---|---|---|---|---|
| INTEM CT (s) | 250 (236–268.8) | 218 (206.3–234) | 231.5 (208.3–256)) | 0.02 | 0.06 | 0.84 |
| INTEM CFT (s) | 462.5 (414.3–593) | 265 (212.8–354) | 259 (213–400) | <0.01 | <0.01 | 0.82 |
| INTEM MCF (mm) | 30 (29–32.5) | 42 (38–45.8) | 41.5 (36–44.5) | 0.02 | 0.18 | 1.0 |
| INTEM LI60 (%) | 95 (95–99) | 100 (98.8–100) | 100 (99.8–100) | 0.16 | 0.16 | 1.0 |
| EXTEM CT (s) | 119 (88.3–125.8) | 52 (51–59.8) | 51.5 (51–53.8) | <0.01 | <0.01 | 0.29 |
| EXTEM CFT (s) | 388 (367–434) | 286.5 (246.5–403.3) | 286.5 (228.3–355.5) | 0.11 | 0.08 | 0.31 |
| EXTEM MCF (mm) | 35.5 (34.3–36.8) | 46 (40.3–47.8) | 46 (41.5–48) | <0.01 | <0.01 | 0.21 |
| EXTEM LI60 (%) | 99 (96–100) | 100 (100) | 100 (100) | 0.41 | 0.29 | 1.0 |
| FIBTEM CT (s) | 265 (130–570) | 53 (52–57.3) | 54 (46.3–60) | 0.02 | 0.02 | 0.76 |
| FIBTEM MCF (mm) | 3 (2–3.8) | 8 (7–11.3) | 10 (8.3–11.8) | <0.01 | <0.01 | 0.78 |
| FIBTEM LI60 (%) | 100 (100) | 100 (100) | 99 (83.5–100) | n.c. | 0.36 | 0.2 |
| APTEM CT (s) | 100 (89–145) | 55 (51–59.8) | 53 (50.3–56.8) | <0.01 | <0.01 | 0.88 |
| APTEM CFT (s) | 413 (325–455) | 300.5 (262.8–455.8) | 313 (228.8–381) | 0.07 | 0.04 | 0.07 |
| APTEM MCF (mm) | 40 (35–40) | 45 (40.3–48.3) | 45 (42–48.8) | 0.02 | 0.02 | 0.38 |
| APTEM LI60 (%) | 100 (97–100) | 100 (100) | 100 (100) | 0.37 | 0.37 | n.c. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneck, E.; Muelich, M.; Markmann, M.; Edinger, F.; Cooper, N.; Moeller, A.; Bein, G.; Hecker, A.; Koch, C.; Sander, M.; et al. Combined Administration of Fibrinogen and Factor XIII Concentrate Does Not Improve Dilutional Coagulopathy Superiorly Than Sole Fibrinogen Therapy: Results of an In-Vitro Thrombelastographic Study. J. Clin. Med. 2021, 10, 2068. https://doi.org/10.3390/jcm10102068
Schneck E, Muelich M, Markmann M, Edinger F, Cooper N, Moeller A, Bein G, Hecker A, Koch C, Sander M, et al. Combined Administration of Fibrinogen and Factor XIII Concentrate Does Not Improve Dilutional Coagulopathy Superiorly Than Sole Fibrinogen Therapy: Results of an In-Vitro Thrombelastographic Study. Journal of Clinical Medicine. 2021; 10(10):2068. https://doi.org/10.3390/jcm10102068
Chicago/Turabian StyleSchneck, Emmanuel, Marcus Muelich, Melanie Markmann, Fabian Edinger, Nina Cooper, Annette Moeller, Gregor Bein, Andreas Hecker, Christian Koch, Michael Sander, and et al. 2021. "Combined Administration of Fibrinogen and Factor XIII Concentrate Does Not Improve Dilutional Coagulopathy Superiorly Than Sole Fibrinogen Therapy: Results of an In-Vitro Thrombelastographic Study" Journal of Clinical Medicine 10, no. 10: 2068. https://doi.org/10.3390/jcm10102068
APA StyleSchneck, E., Muelich, M., Markmann, M., Edinger, F., Cooper, N., Moeller, A., Bein, G., Hecker, A., Koch, C., Sander, M., & Wolff, M. (2021). Combined Administration of Fibrinogen and Factor XIII Concentrate Does Not Improve Dilutional Coagulopathy Superiorly Than Sole Fibrinogen Therapy: Results of an In-Vitro Thrombelastographic Study. Journal of Clinical Medicine, 10(10), 2068. https://doi.org/10.3390/jcm10102068









