Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Sample Collection and Processing
2.3. Treatment and Follow-Up Protocol
2.4. Clinical Evaluation and Statistical Analysis
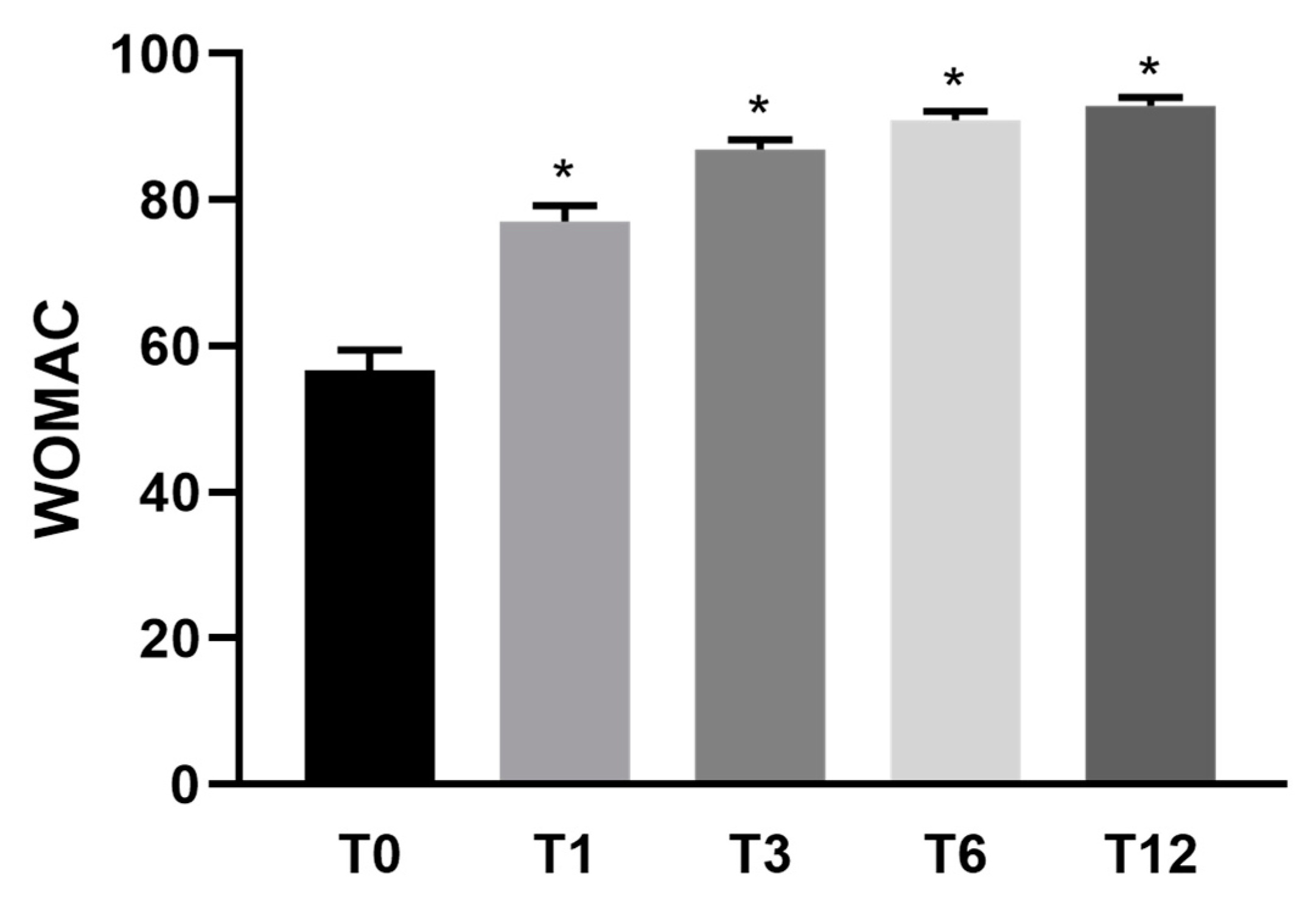
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Rev. Cient. Ordem Médicos Osteoarthr. Osteoartrite 2015, 28, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; van Dijk, N.; della Villa, S. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop. J. Sports Med. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyère, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E. PRP: Product Rich in Placebo? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3702–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys. Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.K.; Goyal, N.; Deirmengian, G.; Rangavajulla, A.; Parvizi, J.; Austin, M.S. Revision total knee arthroplasty in the young patient: Is there trouble on the horizon? J. Bone Jt. Surg. Ser. A 2014, 96, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Baer, P.C.; Geiger, H. Adipose-Derived Mesenchymal Stromal/Stem Cells: Tissue Localization, Characterization, and Heterogeneity. Stem Cells Int. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Cai, X.; Zhang, S.; Karperien, M.; Lin, Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: Perspectives from stem cell biology and molecular medicine. J. Cell. Physiol. 2013, 228, 938–944. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roato, I.; Mussano, F.; Reano, S.; Boriani, F.; Margara, A.; Ferracini, R.; Adriani, E.; Sabry, O.; Fiorini, M.; Fattori, P. A Novel Method to Optimize Autologous Adipose Tissue Recovery with Extracellular Matrix Preservation. Processes 2020, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Adriani, E.; Moio, M.; Di Paola, B.; Salustri, W.; Alfieri, A.; Parisi, P.; Ruggiero, M.; Borab, Z.; Carlesimo, B. Percutaneous fat transfer to treat knee osteoarthritis symptoms: Preliminary results. Joints 2017, 5, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Screpis, D.; Di Donato, S.L.; Bonetti, S.; Piovan, G.; Zorzi, C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: An update at 3 year follow-up. J. Exp. Orthop. 2018, 5, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef]
- Castellarin, G.; Mosca, S.; Micera, G.; Moroni, A. Intra-articular administration of purified autologous adipose tissue for knee osteoarthritis treatment. Minerva Ortop. Traumatol. 2020, 71, 93–97. [Google Scholar]
- Bistolfi, A.; Roato, I.; Fornelli, G.; Sabatini, L.; Massè, A.; Ferracini, R. Treatment of knee osteoarthritis by intra-articular injection of concentrated autologous adipose tissue: A twenty four month follow-up study. Int. Orthop. 2021, 45, 627–633. [Google Scholar] [CrossRef]
- Filardo, G.; Tschon, M.; Perdisa, F.; Brogini, S.; Cavallo, C.; Desando, G.; Giavaresi, G.; Grigolo, B.; Martini, L.; Nicoli Aldini, N.; et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: A comparative preclinical study. Knee Surg. Sports Traumatol. Arthrosc. 2021, 1–9. [Google Scholar] [CrossRef]
- London, N.J.; Miller, L.E.; Block, J.E. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med. Hypotheses 2011, 76, 887–892. [Google Scholar] [CrossRef]
- Law, G.W.; Lee, J.K.; Soong, J.; Lim, J.W.S.; Zhang, K.T.; Tan, A.H.C. Arthroscopic debridement of the degenerative knee—Is there still a role? Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2019, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [Green Version]
- Laupattarakasem, W.; Laopaiboon, M.; Laupattarakasem, P.; Sumananont, C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Orthop. 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Andriolo, L.; Di Laura Frattura, G.; Boffa, A.; Candrian, C.; Zaffagnini, S.; Filardo, G. Pain Trajectories in Knee Osteoarthritis—A Systematic Review and Best Evidence Synthesis on Pain Predictors. J. Clin. Med. 2020, 9, 2828. [Google Scholar] [CrossRef] [PubMed]


| Patient (n) | 30 |
|---|---|
| Age (year) | 60 (range 36–77) |
| Gender (n) | |
| Male | 17 |
| Female | 13 |
| Kellegren-Lawrence (n) | |
| II | 14 |
| III | 13 |
| IV | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caforio, M.; Nobile, C. Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms. J. Clin. Med. 2021, 10, 2053. https://doi.org/10.3390/jcm10102053
Caforio M, Nobile C. Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms. Journal of Clinical Medicine. 2021; 10(10):2053. https://doi.org/10.3390/jcm10102053
Chicago/Turabian StyleCaforio, Marco, and Carmelo Nobile. 2021. "Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms" Journal of Clinical Medicine 10, no. 10: 2053. https://doi.org/10.3390/jcm10102053





