The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD)
Abstract
1. Introduction
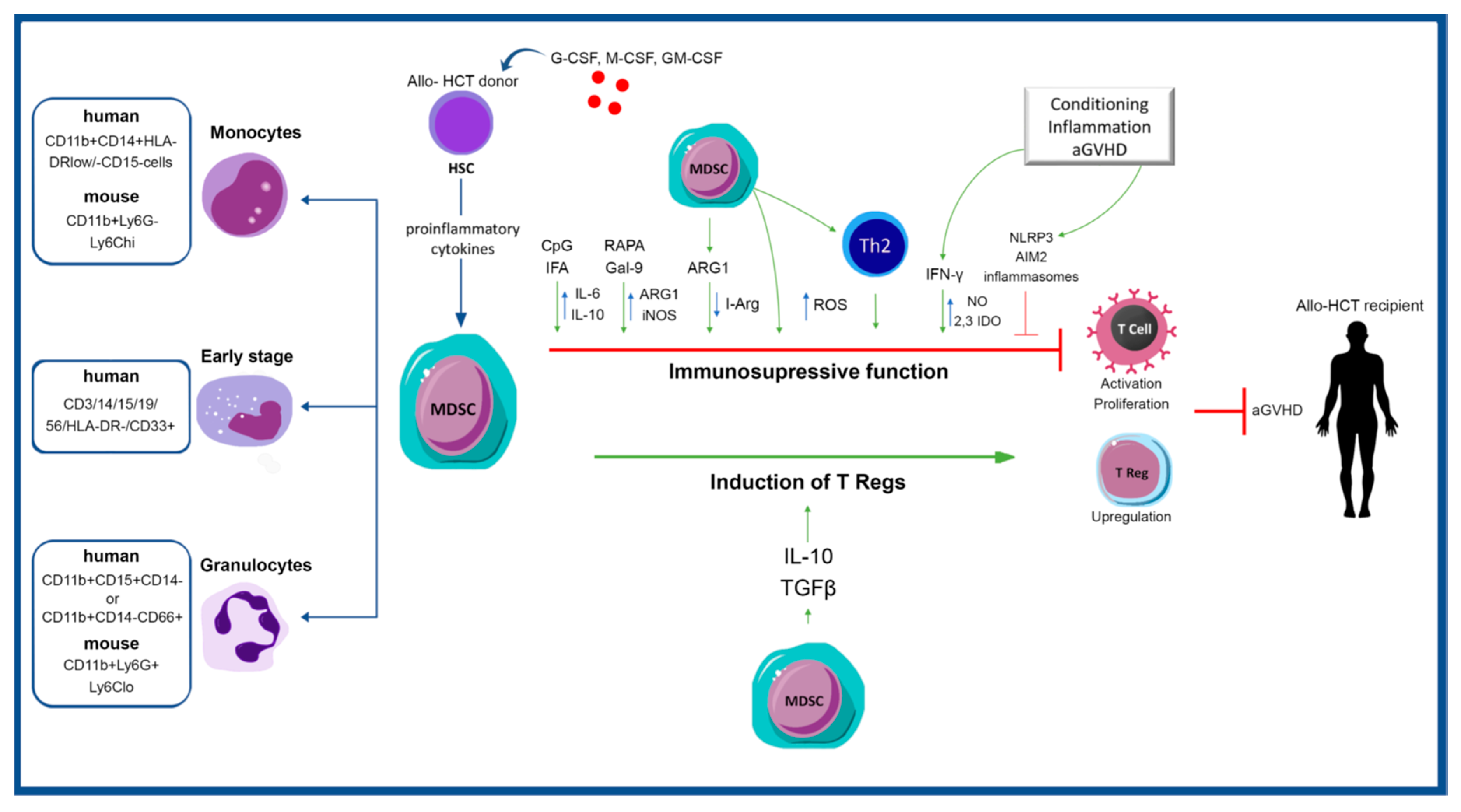
2. Key Functions of MDSCs
3. GVHD Pathophysiology and Implications for MDSCs
3.1. Early Studies in Murine Models
3.2. Studies in Humans
4. MDSCs as Diagnostic or Therapeutic Targets in GVHD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Socie, G.; Ritz, J. Current issues in chronic graft-versus-host disease. Blood 2014, 124, 374–384. [Google Scholar] [CrossRef]
- Wingard, J.R.; Majhail, N.S.; Brazauskas, R.; Wang, Z.; Sobocinski, K.A.; Jacobsohn, D.; Sorror, M.L.; Horowitz, M.M.; Bolwell, B.; Rizzo, J.D.; et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Dignan, F.L.; Clark, A.; Amrolia, P.; Cornish, J.; Jackson, G.; Mahendra, P.; Scarisbrick, J.J.; Taylor, P.C.; Hadzic, N.; Shaw, B.E.; et al. Diagnosis and management of acute graft-versus-host disease. Br. J. Haematol. 2012, 158, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Anasetti, C.; Pidala, J. Have we improved in preventing and treating acute graft-versus-host disease? Curr. Opin. Hematol. 2011, 18, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Perales, M.A.; Schroeder, M.A.; Ali, H.; Shah, N.N.; Chen, Y.B.; Fazal, S.; Dawkins, F.W.; Arbushites, M.C.; Tian, C.; et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): A multicenter, open-label phase 2 trial. Blood 2020, 135, 1739–1749. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Ruutu, T.; Gratwohl, A.; de Witte, T.; Afanasyev, B.; Apperley, J.; Bacigalupo, A.; Dazzi, F.; Dreger, P.; Duarte, R.; Finke, J.; et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014, 49, 168–173. [Google Scholar] [CrossRef]
- Martin, P.J.; Rizzo, J.D.; Wingard, J.R.; Ballen, K.; Curtin, P.T.; Cutler, C.; Litzow, M.R.; Nieto, Y.; Savani, B.N.; Schriber, J.R.; et al. First- and second-line systemic treatment of acute graft-versus-host disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1150–1163. [Google Scholar] [CrossRef]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef]
- Mielcarek, M.; Furlong, T.; O’Donnell, P.V.; Storer, B.E.; McCune, J.S.; Storb, R.; Carpenter, P.A.; Flowers, M.E.; Appelbaum, F.R.; Martin, P.J. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016, 127, 1502–1508. [Google Scholar] [CrossRef]
- Luyckx, A.; Schouppe, E.; Rutgeerts, O.; Lenaerts, C.; Fevery, S.; Devos, T.; Dierickx, D.; Waer, M.; Van Ginderachter, J.A.; Billiau, A.D. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin. Immunol. 2012, 143, 83–87. [Google Scholar] [CrossRef]
- Bizymi, N.; Bjelica, S.; Kittang, A.O.; Mojsilovic, S.; Velegraki, M.; Pontikoglou, C.; Roussel, M.; Ersvaer, E.; Santibanez, J.F.; Lipoldova, M.; et al. Myeloid-Derived Suppressor Cells in Hematologic Diseases: Promising Biomarkers and Treatment Targets. Hemasphere 2019, 3, e168. [Google Scholar] [CrossRef]
- Wang, P.F.; Song, S.Y.; Wang, T.J.; Ji, W.J.; Li, S.W.; Liu, N.; Yan, C.X. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology 2018, 7, e1494113. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, K.; Huang, X.J. Myeloid-derived suppressor cells in hematological malignancies: Friends or foes. J. Hematol. Oncol. 2019, 12, 105. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Rao, V.S.; Mitchell, M.S. Systemic bacillus Calmette-Guerin (BCG) activates natural suppressor cells. Proc. Natl. Acad. Sci. USA 1978, 75, 5142–5144. [Google Scholar] [CrossRef] [PubMed]
- Oseroff, A.; Okada, S.; Strober, S. Natural suppressor (NS) cells found in the spleen of neonatal mice and adult mice given total lymphoid irradiation (TLI) express the null surface phenotype. J. Immunol. 1984, 132, 101–110. [Google Scholar] [PubMed]
- Weigensberg, M.; Morecki, S.; Weiss, L.; Fuks, Z.; Slavin, S. Suppression of cell-mediated immune responses after total lymphoid irradiation (TLI). I. Characterization of suppressor cells of the mixed lymphocyte reaction. J. Immunol. 1984, 132, 971–978. [Google Scholar] [PubMed]
- Maier, T.; Holda, J.H.; Claman, H.N. Synergism between T and non-T cells in the in vivo induction and in vitro expression of graft-vs.-host disease-induced natural suppressor cells. J. Exp. Med. 1985, 162, 979–992. [Google Scholar] [CrossRef]
- Strober, S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: Exploring obscure relationships. Annu. Rev. Immunol. 1984, 2, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Schwadron, R.B.; Gandour, D.M.; Strober, S. Cloned natural suppressor cell lines derived from the spleens of neonatal mice. J. Exp. Med. 1985, 162, 297–310. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Lu, C.; Redd, P.S.; Lee, J.R.; Savage, N.; Liu, K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1247135. [Google Scholar] [CrossRef]
- Mastio, J.; Condamine, T.; Dominguez, G.; Kossenkov, A.V.; Donthireddy, L.; Veglia, F.; Lin, C.; Wang, F.; Fu, S.; Zhou, J.; et al. Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs. J. Exp. Med. 2019, 216, 2150–2169. [Google Scholar] [CrossRef]
- Sangaletti, S.; Talarico, G.; Chiodoni, C.; Cappetti, B.; Botti, L.; Portararo, P.; Gulino, A.; Consonni, F.M.; Sica, A.; Randon, G.; et al. SPARC Is a New Myeloid-Derived Suppressor Cell Marker Licensing Suppressive Activities. Front. Immunol. 2019, 10, 1369. [Google Scholar] [CrossRef]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Cotter, M.J.; Cheng, P.; Cheng, F.; Kusmartsev, S.; Sotomayor, E.; Padhya, T.; McCaffrey, T.V.; McCaffrey, J.C.; Gabrilovich, D.I. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009, 182, 5693–5701. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Poschke, I.; Mougiakakos, D.; Hansson, J.; Masucci, G.V.; Kiessling, R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010, 70, 4335–4345. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Jitschin, R.; von Bahr, L.; Poschke, I.; Gary, R.; Sundberg, B.; Gerbitz, A.; Ljungman, P.; Le Blanc, K. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia 2013, 27, 377–388. [Google Scholar] [CrossRef]
- Oberholtzer, N.; Atkinson, C.; Nadig, S.N. Adoptive Transfer of Regulatory Immune Cells in Organ Transplantation. Front. Immunol. 2021, 12, 631365. [Google Scholar] [CrossRef]
- Hoechst, B.; Ormandy, L.A.; Ballmaier, M.; Lehner, F.; Kruger, C.; Manns, M.P.; Greten, T.F.; Korangy, F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008, 135, 234–243. [Google Scholar] [CrossRef]
- Huang, B.; Pan, P.Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef]
- Yang, R.; Cai, Z.; Zhang, Y.; Yutzy, W.H.t.; Roby, K.F.; Roden, R.B. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006, 66, 6807–6815. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.P.; Rowe, V.; Clouston, A.D.; Welply, J.K.; Kuns, R.D.; Ferrara, J.L.; Thomas, R.; Hill, G.R. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J. Immunol. 2005, 174, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef]
- Ball, L.M.; Egeler, R.M.; Party, E.P.W. Acute GvHD: Pathogenesis and classification. Bone Marrow Transpl. 2008, 41 (Suppl. 2), S58–S64. [Google Scholar] [CrossRef] [PubMed]
- Koehn, B.H.; Apostolova, P.; Haverkamp, J.M.; Miller, J.S.; McCullar, V.; Tolar, J.; Munn, D.H.; Murphy, W.J.; Brickey, W.J.; Serody, J.S.; et al. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood 2015, 126, 1621–1628. [Google Scholar] [CrossRef]
- Koehn, B.H.; Saha, A.; McDonald-Hyman, C.; Loschi, M.; Thangavelu, G.; Ma, L.; Zaiken, M.; Dysthe, J.; Krepps, W.; Panthera, J.; et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood 2019, 134, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Messmann, J.J.; Reisser, T.; Leithauser, F.; Lutz, M.B.; Debatin, K.M.; Strauss, G. In vitro-generated MDSCs prevent murine GVHD by inducing type 2 T cells without disabling antitumor cytotoxicity. Blood 2015, 126, 1138–1148. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Haarberg, K.; Fu, J.; Kaosaard, K.; Nagaraj, S.; Anasetti, C.; Gabrilovich, D.; Yu, X.Z. Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol. Blood Marrow Transpl. 2013, 19, 692–702. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.M.; Ma, G.; Zhou, Z.; Raulet, D.; Rivera, A.L.; Chen, S.H.; Pan, P.Y. The mechanistic study behind suppression of GVHD while retaining GVL activities by myeloid-derived suppressor cells. Leukemia 2019, 33, 2078–2089. [Google Scholar] [CrossRef]
- Wang, K.; Lv, M.; Chang, Y.J.; Zhao, X.Y.; Zhao, X.S.; Zhang, Y.Y.; Sun, Y.Q.; Wang, Z.D.; Suo, P.; Zhou, Y.; et al. Early myeloid-derived suppressor cells (HLA-DR(-)/(low)CD33(+)CD16(-)) expanded by granulocyte colony-stimulating factor prevent acute graft-versus-host disease (GVHD) in humanized mouse and might contribute to lower GVHD in patients post allo-HSCT. J. Hematol. Oncol. 2019, 12, 31. [Google Scholar] [CrossRef]
- Morecki, S.; Gelfand, Y.; Yacovlev, E.; Eizik, O.; Shabat, Y.; Slavin, S. CpG-induced myeloid CD11b+Gr-1+ cells efficiently suppress T cell-mediated immunoreactivity and graft-versus-host disease in a murine model of allogeneic cell therapy. Biol. Blood Marrow Transpl. 2008, 14, 973–984. [Google Scholar] [CrossRef]
- Joo, Y.D.; Lee, S.M.; Lee, S.W.; Lee, W.S.; Lee, S.M.; Park, J.K.; Choi, I.W.; Park, S.G.; Choi, I.; Seo, S.K. Granulocyte colony-stimulating factor-induced immature myeloid cells inhibit acute graft-versus-host disease lethality through an indoleamine dioxygenase-independent mechanism. Immunology 2009, 128, e632–e640. [Google Scholar] [CrossRef] [PubMed]
- Highfill, S.L.; Rodriguez, P.C.; Zhou, Q.; Goetz, C.A.; Koehn, B.H.; Veenstra, R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Serody, J.S.; Munn, D.H.; et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010, 116, 5738–5747. [Google Scholar] [CrossRef] [PubMed]
- D’Aveni, M.; Rossignol, J.; Coman, T.; Sivakumaran, S.; Henderson, S.; Manzo, T.; Santos e Sousa, P.; Bruneau, J.; Fouquet, G.; Zavala, F.; et al. G-CSF mobilizes CD34+ regulatory monocytes that inhibit graft-versus-host disease. Sci. Transl. Med. 2015, 7, 281ra242. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, Y.K.; Lee, S.E.; Ju, J.M.; Eom, K.S.; Kim, Y.J.; Chung, N.G.; Jeong, D.C.; Park, G.; Choi, E.Y.; et al. MyD88 in donor bone marrow cells is critical for protection from acute intestinal graft-vs.-host disease. Mucosal Immunol. 2016, 9, 730–743. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, B.; Shan, W.; Tan, Y.; Feng, J.; Xu, L.; Wang, L.; Han, B.; Zhang, M.; Yu, J.; et al. mTOR inhibitor rapamycin induce polymorphonuclear myeloid-derived suppressor cells mobilization and function in protecting against acute graft-versus-host disease after bone marrow transplantation. Clin. Immunol. 2018, 187, 122–131. [Google Scholar] [CrossRef]
- Scheurer, J.; Reisser, T.; Leithauser, F.; Messmann, J.J.; Holzmann, K.; Debatin, K.M.; Strauss, G. Rapamycin-based graft-versus-host disease prophylaxis increases the immunosuppressivity of myeloid-derived suppressor cells without affecting T cells and anti-tumor cytotoxicity. Clin. Exp. Immunol. 2020, 202, 407–422. [Google Scholar] [CrossRef]
- Yin, J.; Li, L.; Wang, C.; Zhang, Y. Increased Galectin-9 expression, a prognostic biomarker of aGVHD, regulates the immune response through the Galectin-9 induced MDSC pathway after allogeneic hematopoietic stem cell transplantation. Int. Immunopharmacol. 2020, 88, 106929. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Baek, J.A.; Kim, S.Y.; Jung, K.A.; Choi, J.W.; Park, S.H.; Kwok, S.K.; Cho, M.L. Myeloid-derived suppressor cells therapy enhance immunoregulatory properties in acute graft versus host disease with combination of regulatory T cells. J. Transl. Med. 2020, 18, 483. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Martin, P.J.; Torok-Storb, B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood 1997, 89, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Varney, M.L.; Buyukberber, S.; Ino, K.; Ageitos, A.G.; Reed, E.; Tarantolo, S.; Talmadge, J.E. Fas-FasL-mediated CD4+ T-cell apoptosis following stem cell transplantation. Cancer Res. 1999, 59, 3107–3111. [Google Scholar] [PubMed]
- Schmidt-Wolf, I.G.; Dejbakhsh-Jones, S.; Ginzton, N.; Greenberg, P.; Strober, S. T-cell subsets and suppressor cells in human bone marrow. Blood 1992, 80, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.; Sharabi, Y.; Sachs, D.H. Achieving alloengraftment without graft-versus-host disease: Approaches using mixed allogeneic bone marrow transplantation. Bone Marrow Transpl. 1988, 3, 379–386. [Google Scholar]
- Sykes, M.; Sharabi, Y.; Sachs, D.H. Natural suppressor cells in spleens of irradiated, bone marrow-reconstituted mice and normal bone marrow: Lack of Sca-1 expression and enrichment by depletion of Mac1-positive cells. Cell Immunol. 1990, 127, 260–274. [Google Scholar] [CrossRef]
- Craddock, C.F.; Apperley, J.F.; Wright, E.G.; Healy, L.E.; Bennett, C.A.; Evans, M.; Grimsley, P.G.; Gordon, M.Y. Circulating stem cells in mice treated with cyclophosphamide. Blood 1992, 80, 264–269. [Google Scholar] [CrossRef]
- Hill, G.R.; Crawford, J.M.; Cooke, K.R.; Brinson, Y.S.; Pan, L.; Ferrara, J.L. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood 1997, 90, 3204–3213. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, L.; Zhang, K.; Sun, W.; Yang, S.; Zhang, B.; Salzman, P.; Wang, W.; Liu, C.; Vidyasagar, S.; et al. Response patterns of cytokines/chemokines in two murine strains after irradiation. Cytokine 2012, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef]
- Van der Veen, R.C.; Dietlin, T.A.; Pen, L.; Gray, J.D.; Hofman, F.M. Antigen presentation to Th1 but not Th2 cells by macrophages results in nitric oxide production and inhibition of T cell proliferation: Interferon-gamma is essential but insufficient. Cell Immunol. 2000, 206, 125–135. [Google Scholar] [CrossRef]
- Jankovic, D.; Ganesan, J.; Bscheider, M.; Stickel, N.; Weber, F.C.; Guarda, G.; Follo, M.; Pfeifer, D.; Tardivel, A.; Ludigs, K.; et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J. Exp. Med. 2013, 210, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Ferrara, J.L. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.R.; Gerbitz, A.; Crawford, J.M.; Teshima, T.; Hill, G.R.; Tesolin, A.; Rossignol, D.P.; Ferrara, J.L. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J. Clin. Investig. 2001, 107, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Ehrhardt, M.J.; Lees, C.J.; Panoskaltsis-Mortari, A.; Krieg, A.M.; Sharpe, A.H.; Murphy, W.J.; Serody, J.S.; Hemmi, H.; Akira, S.; et al. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood 2008, 112, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Abouelnasr, A.; Roy, J.; Cohen, S.; Kiss, T.; Lachance, S. Defining the role of sirolimus in the management of graft-versus-host disease: From prophylaxis to treatment. Biol. Blood Marrow Transpl. 2013, 19, 12–21. [Google Scholar] [CrossRef]
- Lim, J.Y.; Ryu, D.B.; Park, M.Y.; Lee, S.E.; Park, G.; Kim, T.G.; Min, C.K. Ex Vivo Generated Human Cord Blood Myeloid-Derived Suppressor Cells Attenuate Murine Chronic Graft-versus-Host Diseases. Biol. Blood Marrow Transpl. 2018, 24, 2381–2396. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.C.; Gross, T.G.; Varney, M.L.; Heimann, D.G.; Reed, E.C.; Kessinger, A.; Talmadge, J.E. Immunologic phenotype and function in human bone marrow, blood stem cells and umbilical cord blood. Bone Marrow Transpl. 1996, 18, 53–61. [Google Scholar]
- Talmadge, J.E.; Reed, E.C.; Kessinger, A.; Kuszynski, C.A.; Perry, G.A.; Gordy, C.L.; Mills, K.C.; Thomas, M.L.; Pirruccello, S.J.; Letheby, B.A.; et al. Immunologic attributes of cytokine mobilized peripheral blood stem cells and recovery following transplantation. Bone Marrow Transpl. 1996, 17, 101–109. [Google Scholar]
- Sica, S.; Rutella, S.; Di Mario, A.; Salutari, P.; Rumi, C.; Ortu la Barbera, E.; Etuk, B.; Menichella, G.; D’Onofrio, G.; Leone, G. rhG-CSF in healthy donors: Mobilization of peripheral hemopoietic progenitors and effect on peripheral blood leukocytes. J. Hematother. 1996, 5, 391–397. [Google Scholar] [CrossRef]
- Vendramin, A.; Gimondi, S.; Bermema, A.; Longoni, P.; Rizzitano, S.; Corradini, P.; Carniti, C. Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol. Blood Marrow Transpl. 2014, 20, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhao, X.S.; Hu, Y.; Chang, Y.J.; Zhao, X.Y.; Kong, Y.; Zhang, X.H.; Xu, L.P.; Liu, K.Y.; Huang, X.J. Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2015, 90, E9–E16. [Google Scholar] [CrossRef]
- Schneidawind, C.; Jahnke, S.; Schober-Melms, I.; Schumm, M.; Handgretinger, R.; Faul, C.; Kanz, L.; Bethge, W.; Schneidawind, D. G-CSF administration prior to donor lymphocyte apheresis promotes anti-leukaemic effects in allogeneic HCT patients. Br. J. Haematol. 2019, 186, 60–71. [Google Scholar] [CrossRef]
- Ward, D.M. Extracorporeal photopheresis: How, when, and why. J. Clin. Apher. 2011, 26, 276–285. [Google Scholar] [CrossRef]
- Lamioni, A.; Parisi, F.; Isacchi, G.; Giorda, E.; Di Cesare, S.; Landolfo, A.; Cenci, F.; Bottazzo, G.F.; Carsetti, R. The immunological effects of extracorporeal photopheresis unraveled: Induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation 2005, 79, 846–850. [Google Scholar] [CrossRef]
- Sakellari, I.; Gavriilaki, E.; Batsis, I.; Mallouri, D.; Panteliadou, A.K.; Lazaridou, A.; Vardi, A.; Constantinou, V.; Yannaki, E.; Papalexandri, A.; et al. Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: Prospective single-center study. J. Clin. Apher. 2018, 33, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Rieber, N.; Wecker, I.; Neri, D.; Fuchs, K.; Schafer, I.; Brand, A.; Pfeiffer, M.; Lang, P.; Bethge, W.; Amon, O.; et al. Extracorporeal photopheresis increases neutrophilic myeloid-derived suppressor cells in patients with GvHD. Bone Marrow Transpl. 2014, 49, 545–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Ni, M.; Huckelhoven-Krauss, A.; Sellner, L.; Hoffmann, J.M.; Neuber, B.; Luft, T.; Hegenbart, U.; Schonland, S.; Kleist, C.; et al. Modulation of B Cells and Homing Marker on NK Cells Through Extracorporeal Photopheresis in Patients With Steroid-Refractory/Resistant Graft-Vs.-Host Disease Without Hampering Anti-viral/Anti-leukemic Effects. Front. Immunol. 2018, 9, 2207. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Karafoulidou, I.; Pasteli, N.; Batsis, I.; Mallouri, D.; Lazaridou, A.; Iskas, M.; Vardi, A.; Papalexandri, A.; et al. Intestinal thrombotic microangiopathy: A distinct entity in the spectrum of graft-versus-host disease. Int. J. Hematol. 2019, 110, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Park, S.S.; Lim, J.Y.; Min, G.J.; Park, S.; Jeon, Y.W.; Yahng, S.A.; Shin, S.H.; Lee, S.E.; Yoon, J.H.; et al. Predictive Role of Circulating Immune Cell Subtypes Early after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Acute Leukemia. Int. J. Stem Cells 2018, 12, 73–83. [Google Scholar] [CrossRef]
- Lee, S.E.; Lim, J.Y.; Kim, T.W.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Kim, H.J.; Lee, S.; et al. Matrix Metalloproteinase-9 in Monocytic Myeloid-Derived Suppressor Cells Correlate with Early Infections and Clinical Outcomes in Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2018, 24, 32–42. [Google Scholar] [CrossRef] [PubMed]
- D’Aveni, M.; Notarantonio, A.B.; Bertrand, A.; Boulange, L.; Pochon, C.; Rubio, M.T. Myeloid-Derived Suppressor Cells in the Context of Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lim, J.Y.; Ryu, D.B.; Kim, T.W.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; et al. Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2019, 25, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Sakellari, I.; Anagnostopoulos, A.; Gavriilaki, E. The Impact of Antibiotic-Mediated Modification of the Intestinal Microbiome on Outcomes of Allogeneic Hematopoietic Cell Transplantation: Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2020, 26, 1738–1746. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010, 238, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 receptor: A key player in IL-1 processing and release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Muller, T.; Durr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Juttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Lee, C.R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.H.; Ye, M.B.; Lee, W.; Sim, K.Y.; Kang, J.A.; Kim, Y.C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef]
- Maury, S.; Lemoine, F.M.; Hicheri, Y.; Rosenzwajg, M.; Badoual, C.; Cherai, M.; Beaumont, J.L.; Azar, N.; Dhedin, N.; Sirvent, A.; et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci. Transl. Med. 2010, 2, 41ra52. [Google Scholar] [CrossRef]
- Franssen, L.E.; van de Donk, N.W.; Emmelot, M.E.; Roeven, M.W.; Schaap, N.; Dolstra, H.; Hobo, W.; Lokhorst, H.M.; Mutis, T. The impact of circulating suppressor cells in multiple myeloma patients on clinical outcome of DLIs. Bone Marrow Transpl. 2015, 50, 822–828. [Google Scholar] [CrossRef]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Heaphy, C.E.; Costinean, S.; Stauffer, N.; Na, C.; Hamadani, M.; Santhanam, R.; Mao, C.; Taylor, P.A.; Sandhu, S.; et al. Regulation of acute graft-versus-host disease by microRNA-155. Blood 2012, 119, 4786–4797. [Google Scholar] [CrossRef]
- Chen, S.; Smith, B.A.; Iype, J.; Prestipino, A.; Pfeifer, D.; Grundmann, S.; Schmitt-Graeff, A.; Idzko, M.; Beck, Y.; Prinz, G.; et al. MicroRNA-155-deficient dendritic cells cause less severe GVHD through reduced migration and defective inflammasome activation. Blood 2015, 126, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Liu, H.; Liang, X.; Yang, T.; Fan, Z.; Huang, F.; Ling, Y.; Liao, X.; Xuan, L.; Xu, N.; et al. Superior GVHD-free, relapse-free survival for G-BM to G-PBSC grafts is associated with higher MDSCs content in allografting for patients with acute leukemia. J. Hematol. Oncol. 2017, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Sakellari, I.; Gavriilaki, M.; Anagnostopoulos, A. A New Era in Endothelial Injury Syndromes: Toxicity of CAR-T Cells and the Role of Immunity. Int. J. Mol. Sci. 2020, 21, 3886. [Google Scholar] [CrossRef]
| MDSC Immunophenotype | Clinical Finding | Mechanism | Reference |
|---|---|---|---|
| CD11clow, MHCIIlow, F4/80int | Exposure to inflammasome-stimulating mediators negates the suppressive function of cultured murine and human-derived MDSCs | MDSC-IL13 were activated for NLRP3 or AIM2 inflammasomes using either LPS plus ATP or LPS plus poly(dT) transfection | Koehn et al. [46] |
| Murine MDSC-IL13s | Inhibition of the inflammasome pathway resulted in maintained MDSC function and improved survival after HSCT in the aGVHD model | Inflammasome activation was reduced via P2x7 knockout (KO) or suppression of ATP binding to the receptor (exhibited with extracellular ATP depletion via apyrase and pharmacologically via administration of A-438079, a highly specific P2x7R inhibitor) | Koehn et al. [47] |
| G-MDSCs expressing CD11b+Gr-1+, Ly-6ClowLy-6G+ and M-MDSCs expressing CD11b+Gr-1+, Ly-6ChighLy-6G− | MDSCs prevented GVHD-induced death and diminished histologic GVHD | MDSCs induce Th2, while anti-tumor cytotoxicity of alloantigen-specific T cells was preserved | Messmann et al. [48] |
| H-2Kb+CD11b+Gr-1+ | Addition of functional MDSCs in donor graft-attenuated GVHD, while the removal of MDSCs in vivo exacerbated GVHD. MDSCs derived from recipients with GVHD demonstrated induced suppressive potency compared with those from recipients without GVHD. Tumor relapse allowed progressive accumulation of MDSCs in the peripheral blood and spleens of recipients after allo-HCT. Thus, monitoring blood MDSCs may predict relapse | MDSCs suppress alloreactive T cell responses | Wang et al. [49] |
| CD115+Gr-1+F4/80+ | MDSCs effectively attenuated GVHD but did not significantly compromise GVL effects | MDSC demonstrated cytolytic activities against allogeneic leukemia cells via induction of NKG2D+ CD8 T cells, whereas suppressed GVHD through upregulation of T Regs | Zhang et al. [50] |
| HLA-DR−/lowCD33+CD16- cells | eMDSCs prevented GVHD in humanized mouse model and suppressed the occurrence of grade II-IV aGVHD in allo-HCT patients | eMDSCs are implicated in T Reg upregulation and polarization of T cells from Th1/Th17 to Th2 | Wang et al. [51] |
| CD11bhighGr-1low | MDSCs induce IL-10-producing T Reg that inhibit GVHD through MHC class II restriction | Indirect presentation of host (H-2d) peptides throughMHC class II donor molecules | McDonald et al. [43] |
| CD11b+Gr-1+ | MDSCs inhibit T cell mediated immunoreactivity and GVHD | Decreased number and dysfunction of T cells, the presence of enriched MSCs and/or the increased IL-10, IL-6 cytokine secretion | Morecki et al. [52] |
| CD11b+Gr-1+ | Suppression of acute GVHD by inhibiting alloreactive donor T cell expansion | MDSC suppress GVHD via an IDO-independent manner | Joo et al. [53] |
| CD11b+Gr-1+ | MDSCs suppress allogeneic T cell responses, both in vitro and in vivo | MDSCs triggered arginase-1 activity, which depleted T cell L-arginine | Highfill et al. [54] |
| CD11bintCD34+ | CD34+ M-MDSCs producing NO mediate apoptosis in alloreactive T cell | CD34+ monocytes mobilized with G-CSF require T cell–mediated IFN-γ to yield NO that attenuates T cell activation and proliferation | D’Aveni et al. [55] |
| CD11b+Gr-1+ | MyD88 signaling in donor BM cells demonstrated a protective role via allowing the amplification of MDSCs derived from the donor TCD-BM | GVHD was induced with T cell-depleted BM (TCD-BM) collected from MyD88KO C57BL/6 (B6) mice and T cells collected from WT B6 mice | Lim et al. [56] |
| M-MDSCs (CD11b+ Ly-6GnegLy-6Chigh) and G-MDSCs (CD11b+Ly-6Gpos Ly-6Clow) | RAPA can significantly alleviate acute graft-versus-host disease | RAPA enhances the immunosuppressive function of PMN-MDSCs via induction of ARG1 and iNOS and stimulation of regulatory T cells in vivo | Lin et al. [57] |
| M-MDSCs (CD11b+ Ly-6GnegLy-6Chigh) and G-MDSCs (CD11b+Ly-6Gpos Ly-6Clow) | RAPA treatment induced the immunosuppressive role of MDSCs and inhibited GVHD, while GVT effect was maintained | MDSCs from RAPA-treated mice showed increased immunosuppressive potential, which was primarily iNOS-dependent | Scheurer et al. [58] |
| HLA-DR+CD33+CD14+ | Treatment with gal-9 inhibited GVHD | Treatment with gal-9 increased G-MDSCs through stimulations of iNOS and ARG1 | Yin et al. [59] |
| CD11c–CD11b + and Gr-1 + | Infusion of MDSCs and T Regs inhibited aGVHD | Combined treatment modulated differentiation of allogeneic T cells toward T Regs and IL-10-secreting regulatory B cells | Park et al. [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demosthenous, C.; Sakellari, I.; Douka, V.; Papayanni, P.G.; Anagnostopoulos, A.; Gavriilaki, E. The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD). J. Clin. Med. 2021, 10, 2050. https://doi.org/10.3390/jcm10102050
Demosthenous C, Sakellari I, Douka V, Papayanni PG, Anagnostopoulos A, Gavriilaki E. The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD). Journal of Clinical Medicine. 2021; 10(10):2050. https://doi.org/10.3390/jcm10102050
Chicago/Turabian StyleDemosthenous, Christos, Ioanna Sakellari, Vassiliki Douka, Penelope Georgia Papayanni, Achilles Anagnostopoulos, and Eleni Gavriilaki. 2021. "The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD)" Journal of Clinical Medicine 10, no. 10: 2050. https://doi.org/10.3390/jcm10102050
APA StyleDemosthenous, C., Sakellari, I., Douka, V., Papayanni, P. G., Anagnostopoulos, A., & Gavriilaki, E. (2021). The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD). Journal of Clinical Medicine, 10(10), 2050. https://doi.org/10.3390/jcm10102050







