Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Methods
2.2.1. Dermoscopy
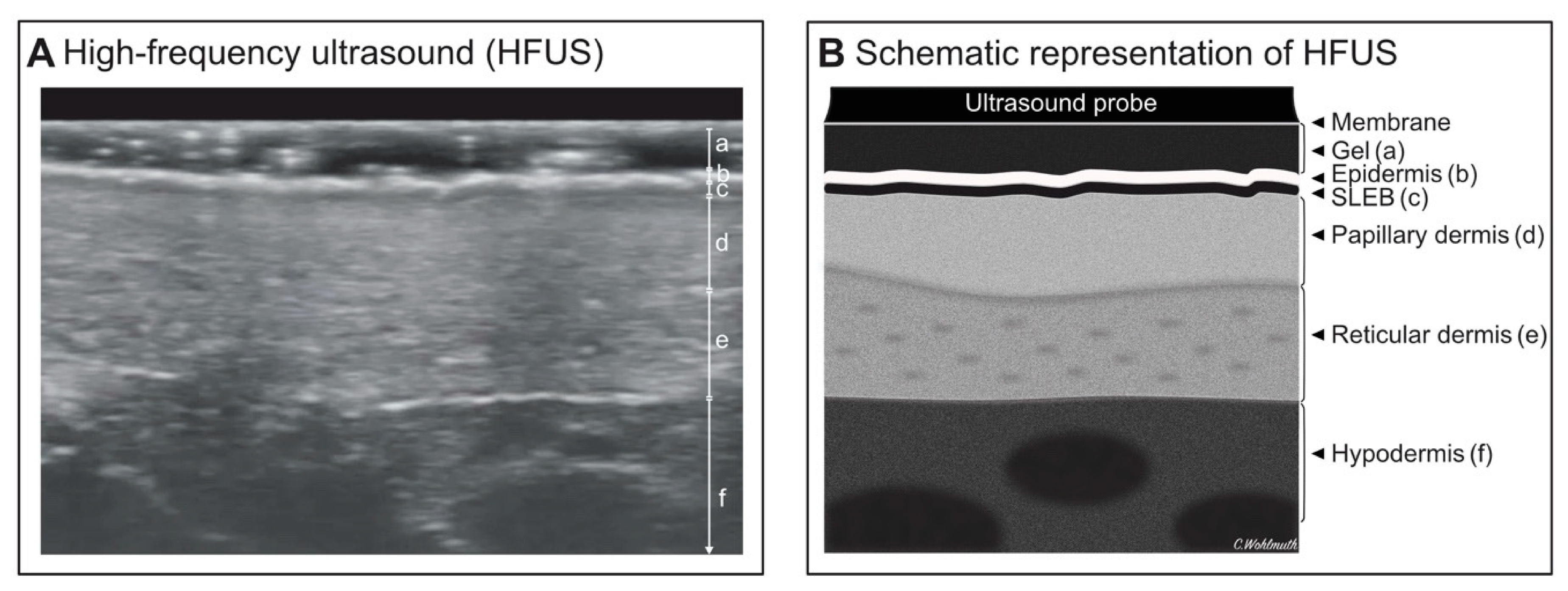
2.2.2. High-Frequency Ultrasound
2.3. Statistics
3. Results
3.1. Demographic Data
3.2. Dermoscopy
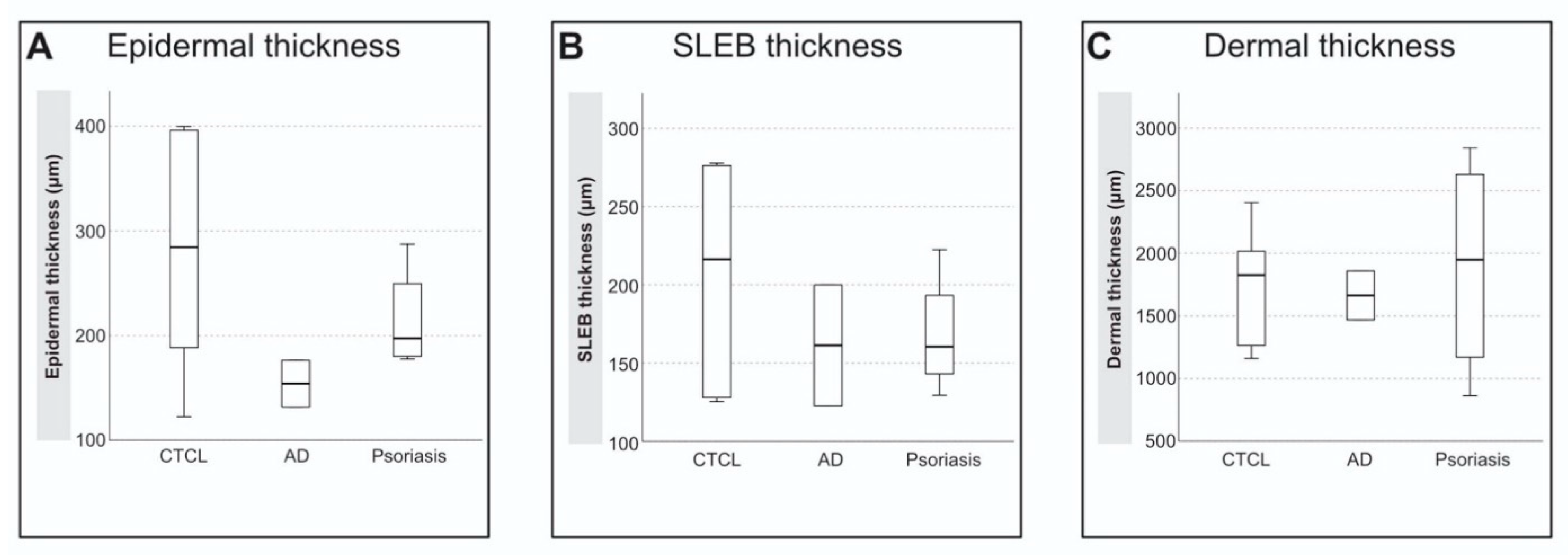
3.3. High-Frequency Ultrasound
4. Discussion
4.1. Dermoscopy
4.2. HFUS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. Faculty Opinions recommendation of WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed]
- Korgavkar, K.; Xiong, M.; Weinstock, M. Changing Incidence Trends of Cutaneous T-Cell Lymphoma. JAMA Dermatol. 2013, 149, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth-Wieser, I.; Wang, C.; Alberti-Violetti, S.; Lyons, G.; Tran, C.; Talpur, R.; Duvic, M. Clinical characteristics, risk factors and long-term outcome of 114 patients with folliculotropic mycosis fungoides. Arch. Dermatol. Res. 2017, 309, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Nashan, D.; Faulhaber, D.; Ständer, S.; Luger, T.; Stadler, R. Mycosis fungoides: A dermatological masquerader. Br. J. Dermatol. 2006, 156, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, J.J.; Quaglino, P.; Prince, H.; Papadavid, E.; Hodak, E.; Bagot, M.; Servitje, O.; Berti, E.; Ortiz-Romero, P.; Stadler, R.; et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br. J. Dermatol. 2019, 181, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Bombonato, C.; Pampena, R.; Lallas, A.; Pellacani, G.; Longo, C. Dermoscopy of Lymphomas and Pseudolymphomas. Dermatol. Clin. 2018, 36, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Polańska, A.; Dańczak-Pazdrowska, A.; Olek-Hrab, K.; Osmola-Mańkowska, A.; Bowszyc-Dmochowska, M.; Żaba, R.; Adamski, Z. High-frequency ultrasonography—New non-invasive method in assessment of skin lymphomas. Skin Res. Technol. 2018, 24, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Lallas, A.; Apalla, Z.; Lefaki, I.; Tzellos, T.; Karatolias, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D.; Zalaudek, I.; Argenziano, G. Dermoscopy of early stage mycosis fungoides. J. Eur. Acad. Dermatol. Venereol. 2012, 27, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Mandava, A.; Koppula, V.; Wortsman, X.; Catalano, O.; Alfageme, F. The clinical value of imaging in primary cutaneous lymphomas: Role of high resolution ultrasound and PET-CT. Br. J. Radiol. 2019, 92, 20180904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, Z.; Liu, J.; Zhu, Q.; Liu, Z.; Liu, Y.; Jin, H. Value of High-Frequency Ultrasound in Accurate Staging of Mycosis Fungoides/Sézary Syndrome. J. Ultrasound Med. 2020, 39, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, G.K.; Goetz, K.E.; Liu, V. Dermoscopic characterization of cutaneous lymphomas: A pilot survey. Int. J. Dermatol. 2018, 57, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Kyrgidis, A.; Tzellos, T.; Apalla, Z.; Karakyriou, E.; Karatolias, A.; Lefaki, I.; Sotiriou, E.; Ioannides, D.; Argenziano, G.; et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br. J. Dermatol. 2012, 166, 1198–1205. [Google Scholar] [CrossRef]
- Sandby-Moller, J.; Wulf, H.C. Ultrasonographic subepidermal low-echogenic band, dependence of age and body site. Skin Res. Technol. 2004, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Bosseila, M.; Sayed, K.S.; Sayed, S.S.E.-D.; El Monaem, N.A.A. Evaluation of Angiogenesis in Early Mycosis Fungoides Patients: Dermoscopic and Immunohistochemical Study. Dermatology 2015, 231, 82–86. [Google Scholar] [CrossRef]
- Giovagnorio, F. Sonography of cutaneous non-Hodgkin’s lymphomas. Clin. Radiol. 1997, 52, 301–303. [Google Scholar] [CrossRef]
- Tognetti, L.; Liso, F.G.; Nazzaro, G.; Provvidenziale, L.; De Piano, E.; Carraro, A.; Perrot, J.L. Ultrasound. In Technol Pract Dermatology; Fimiani, M., Rubegni, P., Cinotti, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 211–218. [Google Scholar]

| Dermoscopic Features | CTCL-Patch | CTCL-Plaque | Folliculotropic MF | |||||
|---|---|---|---|---|---|---|---|---|
| Own Cases | Ghahramani et al. [13] | Lallas et al. [10] | Own Cases | Ghahramani et al. [13] | Own Cases | Ghahramani et al. [13] | ||
| Vascular patterns | Fine short linear vessels | 2/4 (50%) | 5/6 (83.3%) | 30/32 (93.8%) | 0/1 | 0/1 | 1/1 (100%) | 2/5 (40%) |
| Dotted vessels | 2/4 (50%) | 1/6 (16.7%) | 18/32 (56.3%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 2/5 (40%) | |
| Spermatozoa-like structures | 2/4 (50%) | 4/6 (66.7%) | 16/32 (50.0%) | 0/1 | 0/1 | 0/1 | 1/5 (20%) | |
| Pseudopod-like vessels | 0/4 | 0/6 | - | 0/1 | 0/1 | 0/1 | 0/5 | |
| Arborizing vessels | 0/4 | 0/6 | - | 0/1 | 0/1 | 0/1 | 0/5 | |
| Polymorphous vascular pattern | 0/4 | 0/6 | - | 0/1 | 0/1 | 0/1 | 0/5 | |
| Background | Light red background | 3/4 (75%) | 1/6 (16.7%) | - | 0/1 | 0/1 | 0/1 | 1/5 (20%) |
| Dull red background | 1/4 (25%) | 3/6 (50%) | - | 1/1 (100%) | 1/1 (100%) | 0/1 | 4/5 (80%) | |
| Scale | White scale | 3/4 (75%) | 3/6 (50%) | 6/32 (18.8%) | 1/1 (100%) | 1/1 (100%) | 0/1 | 4/5 (80%) |
| Yellow scale | 0/4 | 0/6 | 0/32 | 0/1 | 0/1 | 0/1 | 0/5 | |
| Structureless patches | 2/4 (50%) | 6/6 (100%) | - | 0/1 | 1/1 (100%) | 0/1 | 3/5 (60%) | |
| Other features | Orange-yellowish patchy areas | 0/4 | 0/6 | 29/32 (90.6%) | 0/1 | 1/1 (100%) | 0/1 | 0/5 |
| Crystalline structures | 1/4 (25%) | 2/6 (33.3%) | - | 0/1 | 0/1 | 0/1 | 2/5 (40%) | |
| Yellow ulceration | 0/4 | 0/6 | - | 0/1 | 0/1 | 0/1 | 1/5 (20%) | |
| Perifollicular accentuation | 0/4 | 0/6 | - | 0/1 | 0/1 | 1/1 (100%) | 5/5 (100%) | |
| Comedo openings | 0/4 | 0/6 | - | 0/1 | 0/1 | 1/1 (100%) | 3/5 (60%) | |
| Dermoscopy Features | CTCL-Patch (n = 42) | AD (n = 37) | p-Value | CTCL-Plaque (n = 2) | Psoriasis (n = 88) | p-Value |
|---|---|---|---|---|---|---|
| Fine short linear vessels | 37/42 | 1/37 | <0.001 | 0/2 | 2/88 | 1.0 |
| Dotted vessels | 21/42 | 32/37 | 0.001 | 2/2 | 88/88 | n.a. |
| Spermatozoa-like structures | 22/42 | 0/37 | <0.001 | 0/2 | 0/5 * | n.a. |
| Pseudopod-like vessels | 0/42 | 0/37 | n.a. | 0/2 | 0/5 * | n.a. |
| Arborizing vessels | 0/42 | 0/37 | n.a. | 0/2 | 0/5 * | n.a. |
| Polymorphous vascular pattern | 0/42 | 0/37 | n.a. | 0/2 | 0/5 * | n.a. |
| Light red background | 4/10* | 2/2* | 0.46 | 0/2 | 37/88 | 0.51 |
| Dull red background | 4/10* | 0/2* | 0.52 | 2/2 | 51/88 | 0.51 |
| White scale | 12/42 | 25/37 | 0.001 | 2/2 | 65/88 | 1.0 |
| Yellow scale | 0/42 | 21/37 | <0.001 | 0/2 | 3/88 | 1.0 |
| Skin Thickness (µm) | CTCL-Patch (n = 5) | CTCL-Plaque (n = 1) | AD (n = 2) | Psoriasis (n = 4) | p-Value |
|---|---|---|---|---|---|
| Epidermis | 271 ± 124 | 322 | 154 ± 32 | 215 ± 51 | 0.381 |
| SLEB | 193 ± 78 | 274 | 161 ± 55 | 168 ± 39 | 0.571 |
| Dermis | 1847 ± 460 | 1265 | 1663 ± 276 | 1900 ± 897 | 0.571 |
| SLEB-Grade | 3 (2–3) | 3 | 2 (2) | 3 (2–3) | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlmuth-Wieser, I.; Ramjist, J.M.; Shear, N.; Alhusayen, R. Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound. J. Clin. Med. 2021, 10, 17. https://doi.org/10.3390/jcm10010017
Wohlmuth-Wieser I, Ramjist JM, Shear N, Alhusayen R. Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound. Journal of Clinical Medicine. 2021; 10(1):17. https://doi.org/10.3390/jcm10010017
Chicago/Turabian StyleWohlmuth-Wieser, Iris, Joel M. Ramjist, Neil Shear, and Raed Alhusayen. 2021. "Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound" Journal of Clinical Medicine 10, no. 1: 17. https://doi.org/10.3390/jcm10010017
APA StyleWohlmuth-Wieser, I., Ramjist, J. M., Shear, N., & Alhusayen, R. (2021). Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound. Journal of Clinical Medicine, 10(1), 17. https://doi.org/10.3390/jcm10010017





