Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Cohort
2.3. Statistical Analysis
2.4. Patient and Public Involvement
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roux, J.L.; Gallard, H.; Croué, J.-P.; Papot, S.; Deborde, M. Ndma formation by chloramination of ranitidine: Kinetics and mechanism. Environ. Sci. Technol. 2012, 46, 11095–11103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerecke, A.C.; Sedlak, D.L. Precursors of N-nitrosodimethylamine in natural waters. Environ. Sci. Technol. 2003, 37, 1331–1336. [Google Scholar] [CrossRef]
- Atsdr, U. Agency for Toxic Substances and Disease Registry; Case Studies in Environmental Medicine. 1997. Available online: http://www.atsdr.cdc.gov/HEC/CSEM/csem.html (accessed on 19 November 2020).
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; IARC: Lyon, France, 1987. [Google Scholar]
- Lipsy, R.J.; Fennerty, B.; Fagan, T.C. Clinical review of histamine2 receptor antagonists. Arch. Intern. Med. 1990, 150, 745–751. [Google Scholar] [CrossRef] [PubMed]
- El-Shaheny, R.; Radwan, M.; Yamada, K.; El-Maghrabey, M. Estimation of nizatidine gastric nitrosatability and product toxicity via an integrated approach combining hilic, in silico toxicology, and molecular docking. J. Food Drug Anal. 2019, 27, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, J.; Hinuma, K.; Tanida, N.; Tamura, K.; Ohno, T.; Kano, M.; Shimoyama, T. N-nitrosamines in gastric juice of patients with gastric ulcer before and during treatment with histamine H2-receptor antagonists. Gastroenterol. Jpn. 1990, 25, 162–168. [Google Scholar] [CrossRef]
- Shen, R.; Andrews, S.A. Formation of NDMA from ranitidine and sumatriptan: The role of pH. Water Res. 2013, 47, 802–810. [Google Scholar] [CrossRef]
- Zeng, T.; Mitch, W.A. Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine. Carcinogenesis 2016, 37, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Michaud, D.S.; Mysliwiec, P.A.; Aldoori, W.; Willett, W.C.; Giovannucci, E. Peptic ulcer disease and the risk of bladder cancer in a prospective study of male health professionals. Cancer Epidemiol. Prev. Biomark. 2004, 13, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Vermeer, I.T.; Engels, L.G.; Pachen, D.M.; Dallinga, J.W.; Kleinjans, J.C.; Van Maanen, J.M. Intragastric volatile N-nitrosamines, nitrite, pH, and Helicobacter pylori during long-term treatment with omeprazole. Gastroenterology 2001, 121, 517–525. [Google Scholar] [CrossRef]
- Pottegård, A.; Kristensen, K.B.; Ernst, M.T.; Johansen, N.B.; Quartarolo, P.; Hallas, J. Use of n-nitrosodimethylamine (ndma) contaminated valsartan products and risk of cancer: Danish nationwide cohort study. BMJ 2018, 362, k3851. [Google Scholar] [CrossRef] [Green Version]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.-Y.; Kwon, H.-S.; Cha, B.S.; Park, J.-Y.; Lee, K.-U.; Ko, K.S.; Lee, B.-W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef]
- Lijinsky, W.; Reuber, M.D. Carcinogenesis in rats by nitrosodimethylamine and other nitrosomethylalkylamines at low doses. Cancer Lett. 1984, 22, 83–88. [Google Scholar] [CrossRef]
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Nitrosamine carcinogenesis in 5120 rodents: Chronic administration of sixteen different concentrations of ndea, ndma, npyr and npip in the water of 4440 inbred rats, with parallel studies on ndea alone of the effect of age of starting (3, 6 or 20 weeks) and of species (rats, mice or hamsters). IARC Sci. Publ. 1984, 57, 627–665. [Google Scholar]
- Seong, S.C. National Health Insurance System of Korea. 2015. Available online: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiyneiVi4DuAhVHPnAKHRqGAnAQFjAAegQIAxAC&url=http%3A%2F%2Fwww.kobia.kr%2Fskin%2Fbbs%2Fdownloads_e2%2Fdownload.php%3Ftbl%3Dpolicy_report%26no%3D401&usg=AOvVaw2UvT38upoP4Ka4J_a34H3J (accessed on 19 November 2020).
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. 2013. Available online: www.whocc.no/filearchive/publications/1_2013guidelines.pdf (accessed on 20 August 2015).
- Black, J.; Duncan, W.; Durant, C.J.; Ganellin, C.R.; Parsons, E. Definition and antagonism of histamine h2-receptors. Nature 1972, 236, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.G.; Koch, K.M.; Webster, C.; Sirgo, M.A.; Fitzgerald, K.; Wood, J.R. The safety of ranitidine in over a decade of use. Aliment. Pharmacol. Ther. 1997, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wormsley, K.G. Safety profile of ranitidine: A review. Drugs 1993, 46, 976–985. [Google Scholar] [CrossRef]
- Liu, Y.D.; Selbes, M.; Zeng, C.; Zhong, R.; Karanfil, T. Formation mechanism of ndma from ranitidine, trimethylamine, and other tertiary amines during chloramination: A computational study. Environ. Sci. Technol. 2014, 48, 8653–8663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegelhalder, B.; Eisenbrand, G.; Preussmann, R. Urinary excretion of n-nitrosamines in rats and humans. IARC Sci. Publ. 1982, 41, 443–449. [Google Scholar]
- Fan, C.-C.; Lin, T.-F. N-nitrosamines in drinking water and beer: Detection and risk assessment. Chemosphere 2018, 200, 48–56. [Google Scholar] [CrossRef]
- Mulhern, R.E. Removal of N-Nitrosodimethylamine and Otherdisinfection by-Product Precursors from Tertiary Wastewater Effluent by Activatedcarbon. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2016. [Google Scholar]
- Schwarzenegger, A.; Adams, L.S.; Denton, J.E. N-nitrosodimethylamine. 2006. Available online: https://www.josorge.com/publications/Citations/Toxicology/021.pdf (accessed on 23 November 2020).
- Bartsch, H.; O’Neill, I.K. Ninth International Meeting on N-Nitroso Compounds: Exposures, Mechanisms, and Relevance to Human Cancer; AACR: Philadelphia, PA, USA, 1988. [Google Scholar]
- Iwagami, M.; Kumazawa, R.; Miyamoto, Y.; Ito, Y.; Ishimaru, M.; Morita, K.; Hamada, S.; Tamiya, N.; Yasunaga, H. Risk of cancer in association with ranitidine and nizatidine vs other h2 blockers: Analysis of the japan medical data center claims database 2005–2018. Drug Saf. 2020, 1–11. [Google Scholar]
- Ducrotté, P.; Guillemot, F.; Elouaer-Blanc, L.; Hirschauer, C.; Thorel, J.M.; Petit, A.; Hochain, P.; Michel, P.; Cortot, A.; Colin, R. Comparison of omeprazole and famotidine on esophageal ph in patients with moderate to severe esophagitis: A cross-over study. Am. J. Gastroenterol. 1994, 89, 717–721. [Google Scholar] [PubMed]

| Characteristics | Ranitidine (n = 88,416) | Famotidine (n = 10,129) |
|---|---|---|
| Male (n, %) | 43,217 (48.9) | 5307 (52.4) |
| Age (years, mean ± SD) | 61.0 ± 11.3 | 59.8 ± 11.8 |
| 30–49 (years, n, %) | 14,305 (16.2) | 1966 (19.4) |
| 50–59 (years, n, %) | 23,356 (26.4) | 2810 (27.7) |
| 60–69 (years, n, %) | 26,914 (30.4) | 2850 (28.2) |
| 70–79 (years, n, %) | 23,841 (27.0) | 2503 (24.7) |
| Diabetes mellitus | 32,051 (36.3) | 3501 (34.6) |
| Diabetes mellitus period | ||
| ≤3 years | 9529 (10.8) | 1006 (9.9) |
| ≥4 years | 22,522 (25.5) | 2495 (24.6) |
| Dosage | ||
| mg, (mean ± SD) | 204,919 ± 104,366 | 29,579 ± 15,544 |
| DDD (mean ± SD) | 683.1 ± 347.9 | 739.5 ± 388.6 |
| Cumulative duration | ||
| 12–17 months | 40,557 (45.9) | 4023 (39.7) |
| 18–23 months | 19,361 (21.9) | 2133 (21.0) |
| 24–29 months | 11,126 (12.6) | 1364 (13.5) |
| ≥30 months | 17,372 (19.6) | 2609 (25.8) |
| Characteristics | Ranitidine (n = 40,488) | Famotidine (n = 10,122) | p-Value |
|---|---|---|---|
| Male (n, %) | 21,208 (52.4) | 5302 (52.4) | >0.999 |
| Age (years, mean ± SD) | 59.9 ± 11.8 | 59.8 ± 11.8 | 0.765 |
| 30–49 (years, n, %) | 7836 (19.3) | 1959 (19.3) | |
| 50–59 (years, n, %) | 11,240 (27.8) | 2810 (27.8) | |
| 60–69 (years, n, %) | 11,400 (28.2) | 2850 (28.2) | |
| 70–79 (years, n, %) | 10,012 (24.7) | 2503 (24.7) | |
| Diabetes mellitus | 13,976 (34.5) | 3494 (34.5) | >0.999 |
| Diabetes mellitus period | >0.999 | ||
| ≤3 years | 4020 (9.9) | 1005 (9.9) | |
| ≥4 years | 9956 (24.6) | 2489 (24.6) | |
| Dosage | |||
| mg, mean ± SD) | 220,395 ± 112,850 | 29578 ± 15549 | |
| DDD (mean ± SD) | 734.6 ± 376.2 | 739.4 ± 388.7 | 0.254 |
| Cumulative duration | >0.999 | ||
| 12–17 months | 16,092 (39.8) | 4023 (39.8) | |
| 18–23 months | 8524 (21.0) | 2131 (21.0) | |
| 24–29 months | 5440 (13.4) | 1360 (13.4) | |
| ≥30 months | 10,432 (25.8) | 2608 (25.8) |
| Cancer | Ranitidine (n, %) | Famotidine (n, %) | p-Value | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
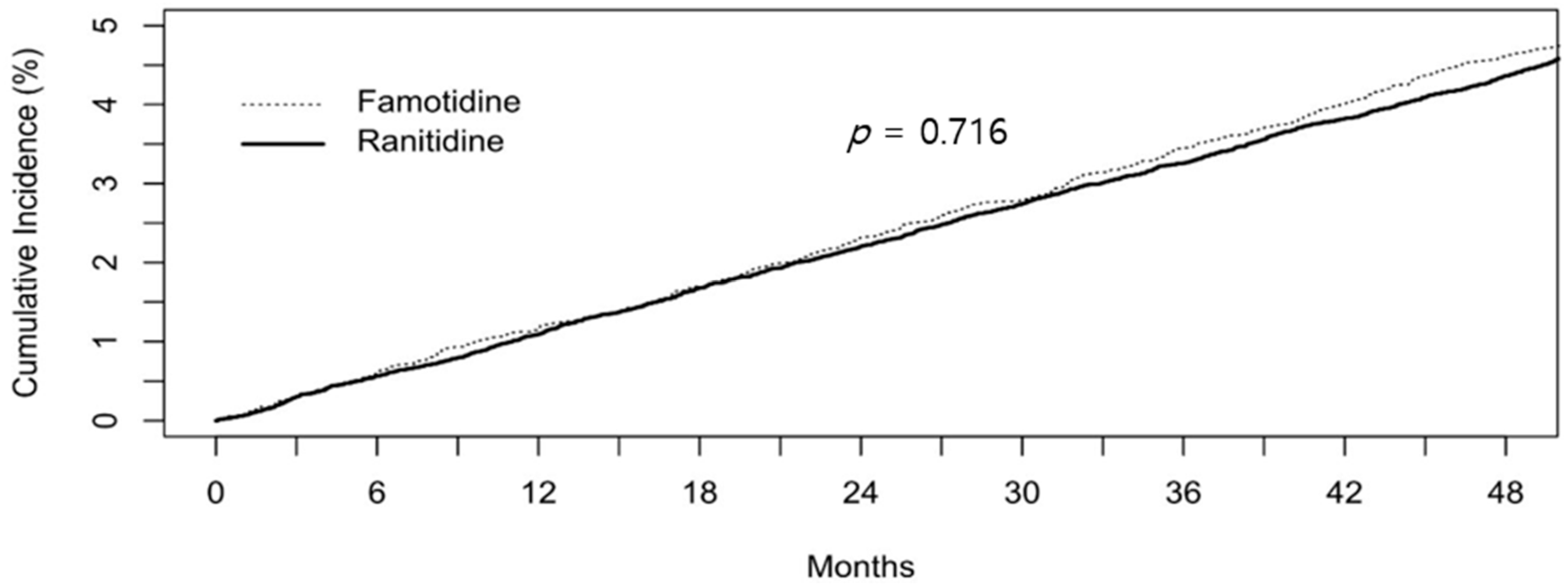
| Overall | 3018 (7.45) | 765 (7.56) | 0.739 | 0.99 (0.91–1.07) | 0.716 |
| Liver | 400 (0.99) | 117 (1.16) | 0.148 | 0.85 (0.69–1.05) | 0.133 |
| Colorectal | 359 (0.89) | 92 (0.91) | 0.878 | 0.98 (0.78–1.23) | 0.832 |
| Biliary | 93 (0.23) | 19 (0.19) | 0.493 | 1.22 (0.75–2.00) | 0.422 |
| Stomach | 450 (1.11) | 106 (1.05) | 0.616 | 1.06 (0.86–1.31) | 0.580 |
| Lung | 473 (1.17) | 119 (1.18) | 0.992 | 0.99 (0.81–1.21) | 0.951 |
| Prostate | 173 (0.43) | 43 (0.42) | >0.999 | 1.01 (0.72–1.40) | 0.974 |
| Kidney | 72 (0.18) | 23 (0.23) | 0.369 | 0.78 (0.49–1.25) | 0.306 |
| Bladder | 118 (0.29) | 21 (0.21) | 0.181 | 1.41 (0.88–2.24) | 0.151 |
| Uterine | 46 (0.11) | 10 (0.10) | 0.815 | 1.15 (0.58–2.28) | 0.689 |
| Breast | 108 (0.27) | 33 (0.33) | 0.365 | 0.82 (0.55–1.21) | 0.313 |
| Thyroid | 159 (0.39) | 37 (0.37) | 0.761 | 1.07 (0.75–1.54) | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.J.; Kim, J.-H.; Seo, G.H.; Park, H. Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021, 10, 153. https://doi.org/10.3390/jcm10010153
Yoon HJ, Kim J-H, Seo GH, Park H. Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea. Journal of Clinical Medicine. 2021; 10(1):153. https://doi.org/10.3390/jcm10010153
Chicago/Turabian StyleYoon, Hong Jin, Jie-Hyun Kim, Gi Hyeon Seo, and Hyojin Park. 2021. "Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea" Journal of Clinical Medicine 10, no. 1: 153. https://doi.org/10.3390/jcm10010153





