Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review
Abstract
1. Introduction
2. Overview of Thrombogenic Potential of Exogenous Androgens
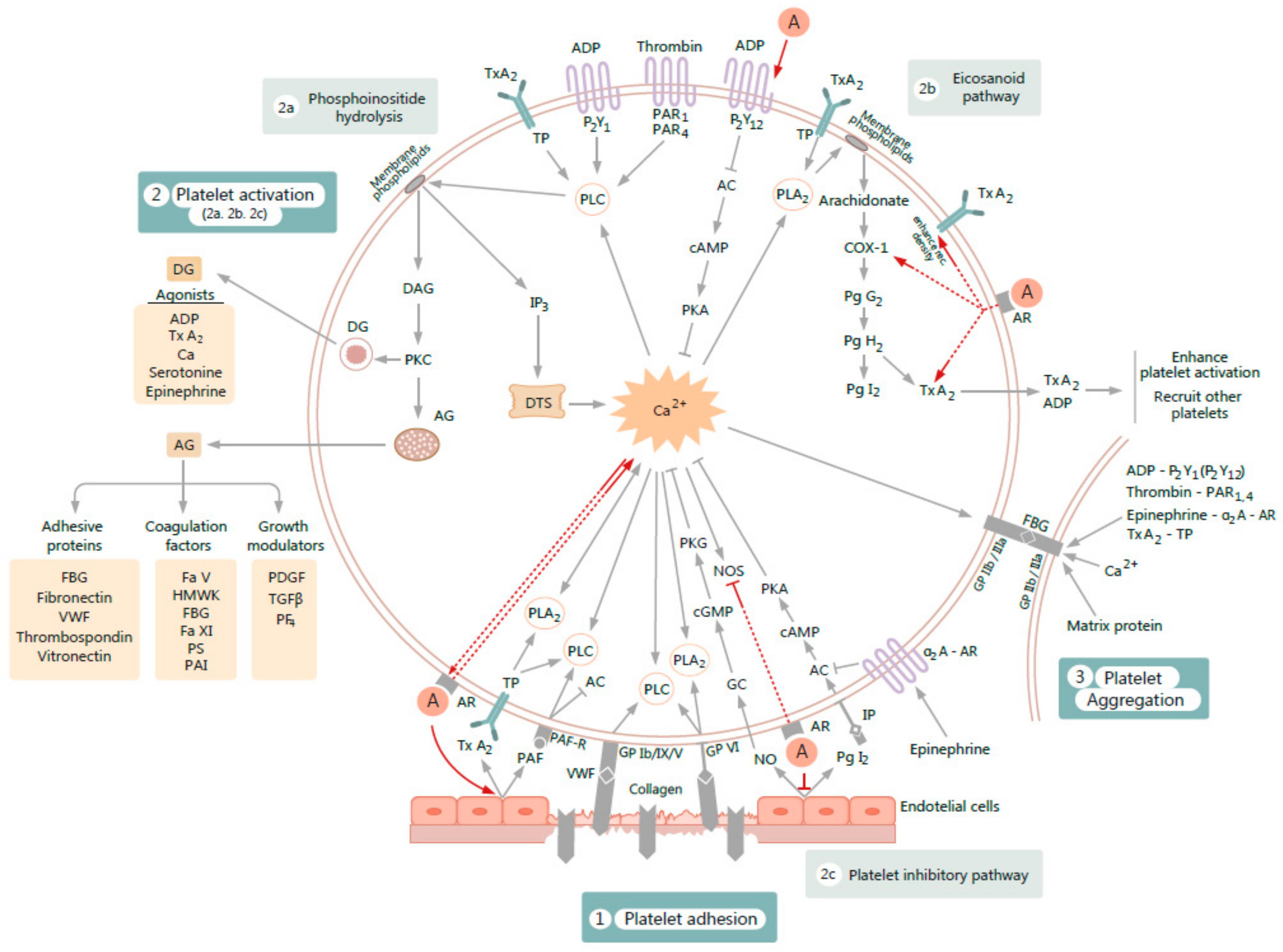
3. Overview of Platelet Function
4. Influence of Exogenous Androgens on Platelet Hemostatic Activity and Thrombopoiesis
4.1. Evidence from Animal Studies
4.2. Evidence from Human Studies
4.2.1. Studies Using In Vitro Platelet Function Assay
4.2.2. Studies Using Ex Vivo Platelet Function Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef]
- Kuhn, C.M. Anabolic steroids. Recent Prog. Horm. Res. 2002, 57, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Wahlstrom, J.T.; Dobs, A.S. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001, 86, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, N.T. A Review of the Chemistry, Biological Action, and Clinical Applications of Anabolic-Androgenic Steroids. Clin. Ther. 2001, 23, 1355–1390. [Google Scholar] [CrossRef]
- Achar, S.; Rostamian, A.; Narayan, S.M. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am. J. Cardiol. 2010, 106, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.I. Anabolic steroids: A fatal attraction? J. Neuroendocrinol. 2006, 18, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.I. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front. Neuroendocrinol. 2008, 29, 490–506. [Google Scholar] [CrossRef]
- Kanayama, G.; Hudson, J.I.; Pope, H.G.J. Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: A looming public health concern? Drug Alcohol Depend. 2008, 98, 1–12. [Google Scholar] [CrossRef]
- Pope, H.G.J.; Wood, R.I.; Rogol, A.; Nyberg, F.; Bowers, L.; Bhasin, S. Adverse health consequences of performance-enhancing drugs: An Endocrine Society scientific statement. Endocr. Rev. 2014, 35, 341–375. [Google Scholar] [CrossRef]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef]
- Hauger, L.E.; Westlye, L.T.; Bjørnebekk, A. Anabolic androgenic steroid dependence is associated with executive dysfunction. Drug Alcohol. Depend. 2020, 208, 107874. [Google Scholar] [CrossRef] [PubMed]
- Van Amsterdam, J.; Opperhuizen, A.; Hartgens, F. Adverse health effects of anabolic-androgenic steroids. Regul. Toxicol. Pharmacol. 2010, 57, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Kaufman, M.J.; Pope, H.G.J. Public health impact of androgens. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.; Whalley, B.J.; Schifano, F.; Baker, J.S. Anabolic androgenic steroid abuse in the United Kingdom: An update. Br. J. Pharmacol. 2020, 177, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev. Endocr. Metab. Disord. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hartgens, F.; Kuipers, H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004, 34, 513–554. [Google Scholar] [CrossRef]
- Melchert, R.B.; Welder, A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1995, 27, 1252–1262. [Google Scholar] [CrossRef]
- Fineschi, V.; Baroldi, G.; Monciotti, F.; Paglicci Reattelli, L.; Turillazzi, E. Anabolic steroid abuse and cardiac sudden death: A pathologic study. Arch. Pathol. Lab. Med. 2001, 125, 253–255. [Google Scholar]
- McNutt, R.A.; Ferenchick, G.S.; Kirlin, P.C.; Hamlin, N.J. Acute myocardial infarction in a 22-year-old world class weight lifter using anabolic steroids. Am. J. Cardiol. 1988, 62, 164. [Google Scholar] [CrossRef]
- Ferenchick, G.S.; Adelman, S. Myocardial infarction associated with anabolic steroid use in a previously healthy 37-year-old weight lifter. Am. Heart J. 1992, 124, 507–508. [Google Scholar] [CrossRef]
- Flo, F.J.; Kanu, O.; Teleb, M.; Chen, Y.; Siddiqui, T. Anabolic androgenic steroid-induced acute myocardial infarction with multiorgan failure. Proc. (Bayl. Univ. Med. Cent.) 2018, 31, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Rämö, M.P.; Viitasalo, M.; Heikkilä, P.; Karjalainen, J.; Mäntysaari, M.; Heikkilä, J. Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. Eur. Heart J. 1996, 17, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, R.D.; Schaller, F.; Prather, I.; McConathy, W.J. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology 1995, 86, 172–173. [Google Scholar] [CrossRef]
- Winkler, U.H. Effects of androgens on haemostasis. Maturitas 1996, 24, 147–155. [Google Scholar] [CrossRef]
- Ferenchick, G.S. Anabolic/androgenic steroid abuse and thrombosis: Is there a connection? Med. Hypotheses 1991, 35, 27–31. [Google Scholar] [CrossRef]
- Dhar, R.; Stout, C.W.; Link, M.S.; Homoud, M.K.; Weinstock, J.; Estes, N.A.M., 3rd. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin. Proc. 2005, 80, 1307–1315. [Google Scholar] [CrossRef]
- Christou, G.A.; Christou, K.A.; Nikas, D.N.; Goudevenos, J.A. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur. J. Prev. Cardiol. 2016. [Google Scholar] [CrossRef]
- Ferenchick, G.; Schwartz, D.; Ball, M.; Schwartz, K. Androgenic-anabolic steroid abuse and platelet aggregation: A pilot study in weight lifters. Am. J. Med. Sci. 1992, 303, 78–82. [Google Scholar] [CrossRef]
- Hourigan, L.A.; Rainbird, A.J.; Dooris, M. Intracoronary stenting for acute myocardial infarction (AMI) in a 24-year-old man using anabolic androgenic steroids. Aust. N. Z. J. Med. 1998, 28, 838–839. [Google Scholar] [CrossRef]
- Ment, J.; Ludman, P.F. Coronary thrombus in a 23 year old anabolic steroid user. Heart 2002, 88, 342. [Google Scholar] [CrossRef] [PubMed]
- Tischer, K.-H.; Heyny-von Haussen, R.; Mall, G.; Doenecke, P. Coronary thrombosis and ectasia of coronary arteries after long-term use of anabolic steroids. Z. Kardiol. 2003, 92, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Güneş, Y.; Erbaş, C.; Okuyan, E.; Babalik, E.; Gürmen, T. Myocardial infarction with intracoronary thrombus induced by anabolic steroids. Anadolu Kardiyol. Derg. 2004, 4, 357–358. [Google Scholar] [PubMed]
- Halvorsen, S.; Thorsby, P.M.; Haug, E. [Acute myocardial infarction in a young man who had been using androgenic anabolic steroids]. Tidsskr. Nor. Laegeforen. 2004, 124, 170–172. [Google Scholar]
- Wysoczanski, M.; Rachko, M.; Bergmann, S.R. Acute myocardial infarction in a young man using anabolic steroids. Angiology 2008, 59, 376–378. [Google Scholar] [CrossRef]
- Stergiopoulos, K.; Brennan, J.J.; Mathews, R.; Setaro, J.F.; Kort, S. Anabolic steroids, acute myocardial infarction and polycythemia: A case report and review of the literature. Vasc. Health Risk Manag. 2008, 4, 1475–1480. [Google Scholar] [CrossRef]
- Ilhan, E.; Demirci, D.; Güvenç, T.S.; Calık, A.N. Acute myocardial infarction and renal infarction in a bodybuilder using anabolic steroids. Turk Kardiyol. Dern. Ars. 2010, 38, 275–278. [Google Scholar]
- García-Esperón, C.; Hervás-García, J.V.; Jiménez-González, M.; Pérez de la Ossa-Herrero, N.; Gomis-Cortina, M.; Dorado-Bouix, L.; López-Cancio Martinez, E.; Castaño-Duque, C.H.; Millán-Torné, M.; Dávalos, A. Ingestion of anabolic steroids and ischaemic stroke. A clinical case report and review of the literature. Rev. Neurol. 2013, 56, 327–331. [Google Scholar]
- Poorzand, H.; Jafarzadeh Esfehani, R.; Hosseinzadeh, P.; Vojdanparast, M. Acute myocardial infarction in a young male wrestler: A case report. ARYA Atheroscler. 2015, 11, 366–369. [Google Scholar]
- Lehmann, S.; Thomas, A.; Schiwy-Bochat, K.-H.; Geyer, H.; Thevis, M.; Glenewinkel, F.; Rothschild, M.A.; Andresen-Streichert, H.; Juebner, M. Death after misuse of anabolic substances (clenbuterol, stanozolol and metandienone). Forensic Sci. Int. 2019, 303, 109925. [Google Scholar] [CrossRef]
- Sonmez, E.; Turkdogan, K.A.; Yilmaz, C.; Kucukbuzcu, S.; Ozkan, A.; Sogutt, O. Chronic anabolic androgenic steroid usage associated with acute coronary syndrome in bodybuilder. Turk. J. Emerg. Med. 2016, 16, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Alrabadi, N.; Jarrah, M.I.; Alzoubi, K.H. Acute myocardial infarction with cardiogenic shock in a young physically active physician concurrently using the anabolic steroid sustanon: A case report. Biomed. Rep. 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Menkis, A.H.; Daniel, J.K.; McKenzie, F.N.; Novick, R.J.; Kostuk, W.J.; Pflugfelder, P.W. Cardiac Transplantation After Myocardial Infarction in a 24-Year-Old Bodybuilder Using Anabolic Steroids. Clin. J. Sport Med. 1991, 1, 138–140. [Google Scholar] [CrossRef]
- Capezzuto, A.; Achilli, A.; Serra, N. Myocardial infarction in a 21-year-old body builder. Am. J. Cardiol. 1989, 63, 1539. [Google Scholar] [CrossRef]
- Lyngberg, K.K. Myocardial infarction and death of a body builder after using anabolic steroids. Ugeskr. Laeger 1991, 153, 587–588. [Google Scholar]
- Kennedy, M.C.; Lawrence, C. Anabolic steroid abuse and cardiac death. Med. J. Aust. 1993, 158, 346–348. [Google Scholar] [CrossRef]
- Appleby, M.; Fisher, M.; Martin, M. Myocardial infarction, hyperkalaemia and ventricular tachycardia in a young male body-builder. Int. J. Cardiol. 1994, 44, 171–174. [Google Scholar] [CrossRef]
- Huie, M.J. An acute myocardial infarction occurring in an anabolic steroid user. Med. Sci. Sports Exerc. 1994, 26, 408–413. [Google Scholar] [CrossRef]
- Fisher, M.; Appleby, M.; Rittoo, D.; Cotter, L. Myocardial infarction with extensive intracoronary thrombus induced by anabolic steroids. Br. J. Clin. Pract. 1996, 50, 222–223. [Google Scholar]
- Mewis, C.; Spyridopoulos, I.; Kühlkamp, V.; Seipel, L. Manifestation of severe coronary heart disease after anabolic drug abuse. Clin. Cardiol. 1996, 19, 153–155. [Google Scholar] [CrossRef]
- McCarthy, K.; Tang, A.T.; Dalrymple-Hay, M.J.; Haw, M.P. Ventricular thrombosis and systemic embolism in bodybuilders: Etiology and management. Ann. Thorac. Surg. 2000, 70, 658–660. [Google Scholar] [CrossRef]
- Frankle, M.A.; Eichberg, R.; Zachariah, S.B. Anabolic androgenic steroids and a stroke in an athlete: Case report. Arch. Phys. Med. Rehabil. 1988, 69, 632–633. [Google Scholar] [PubMed]
- Hashmi, A.; Kim, P.; Ahmad, S.W.; Faucheux, J.; Gandikal, N. Superior Sagittal Venous Sinus Thrombosis in a Patient with Illicit Testosterone Use. Cureus 2019, 11, e5491. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, R.M.; Richter, K.J. Cardiomyopathy and Cerebrovascular Accident Associated With Anabolic-Androgenic Steroid Use. Phys. Sportsmed. 1988, 16, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Laroche, G.P. Steroid anabolic drugs and arterial complications in an athlete--a case history. Angiology 1990, 41, 964–969. [Google Scholar] [CrossRef]
- Jaillard, A.S.; Hommel, M.; Mallaret, M. Venous sinus thrombosis associated with androgens in a healthy young man. Stroke 1994, 25, 212–213. [Google Scholar] [CrossRef]
- Sahraian, M.A.; Mottamedi, M.; Azimi, A.R.; Moghimi, B. Androgen-induced cerebral venous sinus thrombosis in a young body builder: Case report. BMC Neurol. 2004, 4, 22. [Google Scholar] [CrossRef]
- Santamarina, R.D.; Besocke, A.G.; Romano, L.M.; Ioli, P.L.; Gonorazky, S.E. Ischemic stroke related to anabolic abuse. Clin. Neuropharmacol. 2008, 31, 80–85. [Google Scholar] [CrossRef]
- Shimada, Y.; Yoritaka, A.; Tanaka, Y.; Miyamoto, N.; Ueno, Y.; Hattori, N.; Takao, U. Cerebral infarction in a young man using high-dose anabolic steroids. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2012, 21, 906.e9–11. [Google Scholar] [CrossRef]
- Sveinsson, O.; Herrman, L. Cortical venous thrombosis following exogenous androgen use for bodybuilding. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]
- Richard, S.; Lacour, J.-C.; Frotscher, B.; Enea, A.; Mione, G.; Ducrocq, X. Report of a recurrent cerebral venous thrombosis in a young athlete. BMC Neurol. 2014, 14, 182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falkenberg, M.; Karlsson, J.; Ortenwall, P. Peripheral arterial thrombosis in two young men using anabolic steroids. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 1997, 13, 223–226. [Google Scholar] [CrossRef]
- Alvarado, R.G.; Liu, J.Y.; Zwolak, R.M. Danazol and limb-threatening arterial thrombosis: Two case reports. J. Vasc. Surg. 2001, 34, 1123–1126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCulloch, N.A.; Abbas, J.R.; Simms, M.H. Multiple arterial thromboses associated with anabolic androgenic steroids. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2014, 24, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Gaede, J.T.; Montine, T.J. Massive pulmonary embolus and anabolic steroid abuse. JAMA 1992, 267, 2328–2329. [Google Scholar]
- Liljeqvist, S.; Helldén, A.; Bergman, U.; Söderberg, M. Pulmonary embolism associated with the use of anabolic steroids. Eur. J. Intern. Med. 2008, 19, 214–215. [Google Scholar] [CrossRef]
- Choe, H.; Elfil, M.; DeSancho, M.T. Inherited antithrombin deficiency and anabolic steroids: A risky combination. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2016, 27, 717–719. [Google Scholar] [CrossRef]
- Alhadad, A.; Acosta, S.; Sarabi, L.; Kölbel, T. Pulmonary embolism associated with protein C deficiency and abuse of anabolic-androgen steroids. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Damasceno, E.F.; Neto, A.M.; Damasceno, N.A.P.; Horowitz, S.A.P.; de Moraes Junior, H.V. Branch retinal vein occlusion and anabolic steroids abuse in young bodybuilders. Acta Ophthalmol. 2009, 87, 580–581. [Google Scholar] [CrossRef]
- Ammatuna, E.; Nijziel, M.R. Polycythemia and renal infarction in a bodybuilder. QJM 2014, 107, 661–662. [Google Scholar] [CrossRef][Green Version]
- Colburn, S.; Childers, W.K.; Chacon, A.; Swailes, A.; Ahmed, F.M.; Sahi, R. The cost of seeking an edge: Recurrent renal infarction in setting of recreational use of anabolic steroids. Ann. Med. Surg. 2017, 14, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.L.; Farb, A.; Virmani, R.; Sample, R.H. Sudden cardiac death during exercise in a weight lifter using anabolic androgenic steroids: Pathological and toxicological findings. J. Forensic. Sci. 1990, 35, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Hammer, S.; Betz, P. Performance enhancing drugs (doping agents) and sudden death--a case report and review of the literature. Int. J. Legal Med. 1998, 111, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, M.; Agozzino, M.; Toni, C.; Luciani, A.B.; Molendini, L.; Scaglione, M.; Inzani, F.; Pasotti, M.; Buzzi, F.; Arbustini, E. Sudden anabolic steroid abuse-related death in athletes. Int. J. Cardiol. 2007, 114, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Riezzo, I.; Centini, F.; Silingardi, E.; Licata, M.; Beduschi, G.; Karch, S.B. Sudden cardiac death during anabolic steroid abuse: Morphologic and toxicologic findings in two fatal cases of bodybuilders. Int. J. Legal Med. 2007, 121, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Montisci, M.; El Mazloum, R.; Cecchetto, G.; Terranova, C.; Ferrara, S.D.; Thiene, G.; Basso, C. Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012, 217, e13–e18. [Google Scholar] [CrossRef]
- Frati, P.; Busardò, F.P.; Cipolloni, L.; De Dominicis, E.; Fineschi, V. Anabolic Androgenic Steroid (AAS) related deaths: Autoptic, histopathological and toxicological findings. Curr. Neuropharmacol. 2015, 13, 146–159. [Google Scholar] [CrossRef]
- Hernández-Guerra, A.I.; Tapia, J.; Menéndez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic steroids abuse: Case report and literature review. Forensic Sci. Res. 2019, 4, 267–273. [Google Scholar] [CrossRef]
- Pärssinen, M.; Kujala, U.; Vartiainen, E.; Sarna, S.; Seppälä, T. Increased premature mortality of competitive powerlifters suspected to have used anabolic agents. Int. J. Sports Med. 2000, 21, 225–227. [Google Scholar] [CrossRef]
- Thiblin, I.; Garmo, H.; Garle, M.; Holmberg, L.; Byberg, L.; Michaëlsson, K.; Gedeborg, R. Anabolic steroids and cardiovascular risk: A national population-based cohort study. Drug Alcohol Depend. 2015, 152, 87–92. [Google Scholar] [CrossRef]
- Chu, K.; Kang, D.W.; Kim, D.E.; Roh, J.K. Cerebral venous thrombosis associated with tentorial subdural hematoma during oxymetholone therapy. J. Neurol. Sci. 2001, 185, 27–30. [Google Scholar] [CrossRef]
- Shiozawa, Z.; Yamada, H.; Mabuchi, C.; Hotta, T.; Saito, M.; Sobue, I.; Huang, Y.P. Superior sagittal sinus thrombosis associated with androgen therapy for hypoplastic anemia. Ann. Neurol. 1982, 12, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.A.; Mathur, R.; Halushka, P.V. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation 1995, 91, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Nagelberg, S.B.; Laue, L.; Loriaux, D.L.; Liu, L.; Sherins, R.J. Cerebrovascular accident associated with testosterone therapy in a 21-year-old hypogonadal man. N. Engl. J. Med. 1986, 314, 649–650. [Google Scholar] [PubMed]
- Xu, L.; Freeman, G.; Cowling, B.J.; Schooling, C.M. Testosterone therapy and cardiovascular events among men: A systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013, 11, 108. [Google Scholar] [CrossRef]
- Martinez, C.; Suissa, S.; Rietbrock, S.; Katholing, A.; Freedman, B.; Cohen, A.T.; Handelsman, D.J. Testosterone treatment and risk of venous thromboembolism: Population based case-control study. BMJ 2016, 355, i5968. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Miner, M.M.; Caliber, M.; Guay, A.T.; Khera, M.; Traish, A.M. Testosterone Therapy and Cardiovascular Risk: Advances and Controversies. Mayo Clin. Proc. 2015, 90, 224–251. [Google Scholar] [CrossRef]
- Kloner, R.A.; Carson, C., 3rd; Dobs, A.; Kopecky, S.; Mohler, E.R., 3rd. Testosterone and Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 67, 545–557. [Google Scholar] [CrossRef]
- Corona, G.; Maseroli, E.; Rastrelli, G.; Isidori, A.M.; Sforza, A.; Mannucci, E.; Maggi, M. Cardiovascular risk associated with testosterone-boosting medications: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2014, 13, 1327–1351. [Google Scholar] [CrossRef]
- Houghton, D.E.; Alsawas, M.; Barrioneuvo, P.; Tello, M.; Farah, W.; Beuschel, B.; Prokop, L.J.; Layton, J.B.; Murad, M.H.; Moll, S. Testosterone therapy and venous thromboembolism: A systematic review and meta-analysis. Thromb. Res. 2018, 172, 94–103. [Google Scholar] [CrossRef]
- Pawlowitzki, I.H.; Diekstall, P.; Miny, P.; Balleisen, L. Abnormal platelet function in Kallmann syndrome. Lancet 1986, 2, 166. [Google Scholar] [CrossRef]
- Iijima, M.; Shigehara, K.; Sugimoto, K.; Kouji, I.; Fukushima, M.; Maeda, Y.; Konaka, H.; Mizokami, A.; Koh, E.; Namiki, M. Myelodysplastic syndrome treated effectively with testosterone enanthate. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2011, 18, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, A.; Ramey, E.; Ramwell, P.W. Effect of testosterone, sex and age on experimentally induced arterial thrombosis. Nature 1976, 261, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, A.D.; Ramey, E.R.; Ramwell, P.W. Arachidonate-induced thrombosis in mice: Effects of gender or testosterone and estradiol administration. Prostaglandins 1977, 13, 995–1002. [Google Scholar] [CrossRef]
- Uzunova, A.D.; Ramey, E.R.; Ramwell, P.W. Gonadal hormones and pathogenesis of occlusive arterial thrombosis. Am. J. Physiol. 1978, 234, H454–H459. [Google Scholar] [CrossRef]
- Rosenblum, W.I.; el-Sabban, F.; Nelson, G.H.; Allison, T.B. Effects in mice of testosterone and dihydrotestosterone on platelet aggregation in injured arterioles and ex vivo. Thromb. Res. 1987, 45, 719–728. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Li, J.; Deng, X.; Li, Y.; Cong, Y. Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thromb. Res. 2007, 121, 127–134. [Google Scholar] [CrossRef]
- Chan, M.Y.; Andreotti, F.; Becker, R.C. Hypercoagulable states in cardiovascular disease. Circulation 2008, 118, 2286–2297. [Google Scholar] [CrossRef]
- Campo, G.; Valgimigli, M.; Ferraresi, P.; Malagutti, P.; Baroni, M.; Arcozzi, C.; Gemmati, D.; Percoco, G.; Parrinello, G.; Ferrari, R.; et al. Tissue factor and coagulation factor VII levels during acute myocardial infarction: Association with genotype and adverse events. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2800–2806. [Google Scholar] [CrossRef][Green Version]
- Doggen, C.J.M.; Rosendaal, F.R.; Meijers, J.C.M. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood 2006, 108, 4045–4051. [Google Scholar] [CrossRef]
- Koenig, W. Haemostatic risk factors for cardiovascular diseases. Eur. Heart J. 1998, 19 (Suppl. C), C39–C43. [Google Scholar] [PubMed]
- Juhan-Vague, I.; Pyke, S.D.; Alessi, M.C.; Jespersen, J.; Haverkate, F.; Thompson, S.G. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation 1996, 94, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Friedman, J.; Hafeez, A.; Hassan, A.; Wang, P. Testosterone, thrombophilia, thrombosis. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2014, 25, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Münster, A.-M.B.; Gram, J.; Sidelmann, J.J. Anabolic Androgenic Steroid Abuse: The Effects on Thrombosis Risk, Coagulation, and Fibrinolysis. Semin. Thromb. Hemost. 2018, 44, 734–746. [Google Scholar] [CrossRef]
- Lippi, G.; Banfi, G. Doping and thrombosis in sports. Semin. Thromb. Hemost. 2011, 37, 918–928. [Google Scholar] [CrossRef]
- Király, C.L. Androgenic-anabolic steroid effects on serum and skin surface lipids, on red cells, and on liver enzymes. Int. J. Sports Med. 1988, 9, 249–252. [Google Scholar] [CrossRef]
- Ghorbanihaghjo, A.; Argani, H.; Rohbaninoubar, M.; Rashtchizadeh, N. Effect of Nandrolone Decanoate on serum lipoprotein (a) and its isoforms in hemodialysis patients. Lipids Health Dis. 2004, 3, 16. [Google Scholar] [CrossRef][Green Version]
- Teruel, J.L.; Lasuncion, M.A.; Rivera, M.; Aguilera, A.; Ortega, H.; Tato, A.; Marcen, R.; Ortuño, J. Nandrolone decanoate reduces serum lipoprotein(a) concentrations in hemodialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1997, 29, 569–575. [Google Scholar] [CrossRef]
- Rosca, A.E.; Stancu, C.S.; Badiu, C.; Popescu, B.O.; Mirica, R.; Căruntu, C.; Gologan, S.; Voiculescu, S.E.; Zagrean, A.-M. Lipid Profile Changes Induced by Chronic Administration of Anabolic Androgenic Steroids and Taurine in Rats. Medicina (Kaunas) 2019, 55, 540. [Google Scholar] [CrossRef]
- Graham, M.R.; Grace, F.M.; Boobier, W.; Hullin, D.; Kicman, A.; Cowan, D.; Davies, B.; Baker, J.S. Homocysteine induced cardiovascular events: A consequence of long term anabolic-androgenic steroid (AAS) abuse. Br. J. Sports Med. 2006, 40, 644–648. [Google Scholar] [CrossRef]
- Vanberg, P.; Atar, D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb. Exp. Pharmacol. 2010, 411–457. [Google Scholar] [CrossRef]
- dos Santos, M.A.P.; de Oliveira, C.V.C.; Silva, A.S. Adverse cardiovascular effects from the use of anabolic-androgenic steroids as ergogenic resources. Subst. Use Misuse 2014, 49, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Skogastierna, C.; Hotzen, M.; Rane, A.; Ekström, L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur. J. Prev. Cardiol. 2014, 21, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Shen, W.; Zhong, M.; Wu, P.; Chen, H.; Lu, A. Nandrolone attenuates aortic adaptation to exercise in rats. Cardiovasc. Res. 2013, 97, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Roşca, A.E.; Stoian, I.; Badiu, C.; Gaman, L.; Popescu, B.O.; Iosif, L.; Mirica, R.; Tivig, I.C.; Stancu, C.S.; Căruntu, C.; et al. Impact of chronic administration of anabolic androgenic steroids and taurine on blood pressure in rats. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2016, 49, e5116. [Google Scholar] [CrossRef]
- O’Connell, B.J.; Genest, J.J. High-density lipoproteins and endothelial function. Circulation 2001, 104, 1978–1983. [Google Scholar] [CrossRef]
- Van der Stoep, M.; Korporaal, S.J.A.; Van Eck, M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res. 2014, 103, 362–371. [Google Scholar] [CrossRef]
- Baron, A.D. Vascular reactivity. Am. J. Cardiol. 1999, 84, 25J–27J. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Iatrogenic hyperviscosity and thrombosis. Semin. Thromb. Hemost. 2012, 38, 854–864. [Google Scholar] [CrossRef]
- Chan, L.W.; Luo, X.P.; Ni, H.C.; Shi, H.M.; Liu, L.; Wen, Z.C.; Gu, X.Y.; Qiao, J.; Li, J. High levels of LDL-C combined with low levels of HDL-C further increase platelet activation in hypercholesterolemic patients. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2015, 48, 167–173. [Google Scholar] [CrossRef][Green Version]
- Fowler, B. Homocystein--an independent risk factor for cardiovascular and thrombotic diseases. Ther. Umsch. 2005, 62, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelium and haemostasis. Hamostaseologie 2015, 35, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide Biol. Chem. 2013, 35, 175–185. [Google Scholar] [CrossRef]
- Baszczuk, A.; Kopczyński, Z.; Thielemann, A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postepy Hig. Med. Dosw. (Online) 2014, 68, 91–100. [Google Scholar] [CrossRef]
- Sudano, I.; Roas, S.; Noll, G. Vascular abnormalities in essential hypertension. Curr. Pharm. Des. 2011, 17, 3039–3044. [Google Scholar] [CrossRef]
- Fraer, M.; Kilic, F. Serotonin: A different player in hypertension-associated thrombosis. Hypertens. (Dallas Tex. 1979) 2015, 65, 942–948. [Google Scholar] [CrossRef]
- Higgins, J.P.; Heshmat, A.; Higgins, C.L. Androgen abuse and increased cardiac risk. South. Med. J. 2012, 105, 670–674. [Google Scholar] [CrossRef]
- Nascimento, J.H.M.; Medei, E. Cardiac effects of anabolic steroids: Hypertrophy, ischemia and electrical remodelling as potential triggers of sudden death. Mini Rev. Med. Chem. 2011, 11, 425–429. [Google Scholar] [CrossRef]
- Riezzo, I.; De Carlo, D.; Neri, M.; Nieddu, A.; Turillazzi, E.; Fineschi, V. Heart disease induced by AAS abuse, using experimental mice/rats models and the role of exercise-induced cardiotoxicity. Mini Rev. Med. Chem. 2011, 11, 409–424. [Google Scholar] [CrossRef]
- Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Li Rosi, G.; Di Nunno, N.; et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina (Kaunas) 2020, 56, 587. [Google Scholar] [CrossRef] [PubMed]
- Middlebrook, I.; Schoener, B. Anabolic Steroid Toxicity; Treasure Island (FL): StatPearls Publishing (LLC): Florida, FL, USA, 2020; pp. 1–30. [Google Scholar]
- Nieswandt, B.; Pleines, I.; Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 92–104. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009, 102, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Marder, V.J.; Aird, W.C.; Bennett, J.S.; Schulman, S.; White, G.C., II. Hemostasis and Thrombosis: Basic Principles and Clinical Practice; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2012; pp. 402–403. [Google Scholar]
- Marder, V.J.; Aird, W.C.; Bennett, J.S.; Schulman, S.; White, G.C., II. Hemostasis and Thrombosis: Basic Principles and Clinical Practice; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2012; p. 428. ISBN 978-1-60-831906-0. [Google Scholar]
- Marder, V.J.; Aird, W.C.; Bennett, J.S.; Schulman, S.; White, G.C., II. Hemostasis and Thrombosis: Basic Principles and Clinical Practice; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2012; pp. 449–461. ISBN 978-1-60-831906-0. [Google Scholar]
- Nurden, A.T.; Nurden, P. Inherited disorders of platelet function: Selected updates. J. Thromb. Haemost. 2015, 13, S2–S9. [Google Scholar] [CrossRef]
- Kendall, D. Overview of phosphoinositide hydrolysis. Curr. Protoc. Pharmacol. 2001. Chapter 2, Unit2.3. [Google Scholar] [CrossRef]
- Estevez, B.; Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef]
- Gachet, C.; Hechler, B. Platelet Purinergic Receptors in Thrombosis and Inflammation. Hamostaseologie 2020, 40, 145–152. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Ritter, J.; Ferro, A. Platelet-derived nitric oxide signaling and regulation. Circ. Res. 2007, 101, 654–662. [Google Scholar] [CrossRef]
- Schwarz, U.R.; Walter, U.; Eigenthaler, M. Taming platelets with cyclic nucleotides. Biochem. Pharmacol. 2001, 62, 1153–1161. [Google Scholar] [CrossRef]
- Johnson, M.; Ramey, E.; Ramwell, P.W. Androgen-mediated sensitivity in platelet aggregation. Am. J. Physiol. 1977, 232, H381–H385. [Google Scholar] [CrossRef]
- Skjaerlund, J.M.; Deitemeyer, D.; Yunker, R.L.; Subbiah, M.T. Effect of testosterone and oxandrolone on thrombocyte aggregation and synthesis of prostaglandins in thrombocytes and aorta of atherosclerosis-susceptible pigeons. Andrologia 1983, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, K.; Komatz, Y.; Ikeda, M.; Sugimoto, T.; Saito, K. Platelet-activating factor (PAF) in male reproductive organs of guinea pigs and rats: Effect of androgen on PAF in seminal vesicles. Biol. Reprod. 1993, 48, 386–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, K.; Ruff, A.; Morinelli, T.A.; Mathur, R.S.; Halushka, P.V. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am. J. Physiol. 1994, 267, H887–H893. [Google Scholar] [CrossRef] [PubMed]
- Bednarek-Tupikowska, G.; Gosk, I.; Szuba, A.; Bohdanowicz-Pawlak, A.; Kosowska, B.; Bidzińska, B.; Milewicz, A. Influence of dehydroepiandrosterone on platelet aggregation, superoxide dismutase activity and serum lipid peroxide concentrations in rabbits with induced hypercholesterolemia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2000, 6, 40–45. [Google Scholar]
- Aydilek, N.; Aksakal, M. Effects of testosterone on lipid peroxidation, lipid profiles and some coagulation parameters in rabbits. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2005, 52, 436–439. [Google Scholar] [CrossRef]
- Sullivan, P.S.; Jackson, C.W.; McDonald, T.P. Castration decreases thrombocytopoiesis and testosterone restores platelet production in castrated BALB/c mice: Evidence that testosterone acts on a bipotential hematopoietic precursor cell. J. Lab. Clin. Med. 1995, 125, 326–333. [Google Scholar]
- Alhawiti, N.M.; Alqahtani, S.A. Chronic testosterone administration improves cardiac contractility and has a beneficial effect on the haemostatic system by enhancing fibrinolytic activity and inducing hypocoagulation in healthy rats. Arch. Physiol. Biochem. 2018, 125, 311–320. [Google Scholar] [CrossRef]
- Roşca, A.E.; Badiu, C.; Uscătescu, V.; Stoian, I.; Mirică, R.; Braga, R.I.; Pavel, B.; Zăgrean, L. Influence of chronic administration of anabolic androgenic steroids and taurine on haemostasis profile in rats: A thrombelastographic study. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2013, 24, 256–260. [Google Scholar] [CrossRef]
- Rosca, A.; Badiu, C.; Uscatescu, V.; Mirica, R.; Braga, R.; Pavel, B.; Zagrean, L. Effect of chronic administration of anabolic androgenic steroids and taurine on platelet agregation in rats. Acta Endocrinol. 2013, 9, 33–39. [Google Scholar] [CrossRef]
- Schafer, A.I. The hypercoagulable states. Ann. Intern. Med. 1985, 102, 814–828. [Google Scholar] [CrossRef]
- Appleby, N.; Angelov, D. Clinical and laboratory assessment of a patient with thrombocytosis. Br. J. Hosp. Med. 2017, 78, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Li, J.; Deng, X.; Li, Y. Inhibition of oxidative-stress-induced platelet aggregation by androgen at physiological levels via its receptor is associated with the reduction of thromboxane A2 release from platelets. Steroids 2007, 72, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Cutini, P.H.; Campelo, A.E.; Agriello, E.; Sandoval, M.J.; Rauschemberger, M.B.; Massheimer, V.L. The role of sex steroids on cellular events involved in vascular disease. J. Steroid Biochem. Mol. Biol. 2012, 132, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Campelo, A.E.; Cutini, P.H.; Massheimer, V.L. Cellular actions of testosterone in vascular cells: Mechanism independent of aromatization to estradiol. Steroids 2012, 77, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Campelo, A.E.; Cutini, P.H.; Massheimer, V.L. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J. Endocrinol. 2012, 213, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Ramey, E.; Ramwell, P.W. Sex and age differences in human platelet aggregation. Nature 1975, 253, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Pilo, R.; Aharony, D.; Raz, A. Testosterone potentiation of ionophore and ADP induced platelet aggregation: Relationship to arachidonic acid metabolism. Thromb. Haemost. 1981, 46, 538–542. [Google Scholar] [CrossRef]
- Togna, G.I.; Togna, A.R.; Graziani, M.; Franconi, M. Testosterone and cocaine: Vascular toxicity of their concomitant abuse. Thromb. Res. 2003, 109, 195–201. [Google Scholar] [CrossRef]
- Banerjee, D.; Mazumder, S.; Bhattacharya, S.; Sinha, A.K. The sex specific effects of extraneous testosterone on ADP induced platelet aggregation in platelet-rich plasma from male and female subjects. Int. J. Lab. Hematol. 2014, 36, e74–e77. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, J.; Cho, S.; Jarrar, Y.B.; Shin, J. Effects of testosterone and 17 b -oestradiol on expression of the G protein-coupled receptor P2Y12 in megakaryocytic DAMI cells. Platelets. 2012, 23, 579–585. [Google Scholar] [CrossRef]
- Matsuda, K.; Mathur, R.S.; Duzic, E.; Halushka, P.V. Androgen regulation of thromboxane A2/prostaglandin H2 receptor expression in human erythroleukemia cells. Am. J. Physiol. 1993, 265, E928–E934. [Google Scholar] [CrossRef] [PubMed]
- Halushka, P.V.; Masuda, A.; Matsuda, K. The Gordon Wilson Lecture. Regulation of thromboxane A2 receptors by testosterone: Implications for steroid abuse and cardiovascular disease. Trans. Am. Clin. Climatol. Assoc. 1994, 105, 95–103. [Google Scholar] [PubMed]
- Zucker, T.P.; Higashiura, K.; Mathur, R.S.; Halushka, P.V. Androstenedione increases thromboxane A2 receptors in human erythroleukemia cells. Life Sci. 1996, 58, 683–690. [Google Scholar] [CrossRef]
- Jesse, R.L.; Loesser, K.; Eich, D.M.; Qian, Y.Z.; Hess, M.L.; Nestler, J.E. Dehydroepiandrosterone inhibits human platelet aggregation in vitro and in vivo. Ann. N. Y. Acad. Sci. 1995, 774, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Rastoldo, A.; Sarasso, C.; Di, C.; Sampietro, S.; Nalin, M.; Bagarotti, A.; Sinigaglia, F. Dehydroepiandrosterone-sulfate inhibits thrombin-induced platelet aggregation. Steroids 2012, 77, 260–268. [Google Scholar] [CrossRef]
- Muñoz, Y.C.; Gomez, G.I.; Moreno, M.; Solis, C.L.; Valladares, L.E.; Velarde, V. Dehydroepiandrosterone prevents the aggregation of platelets obtained from postmenopausal women with type 2 diabetes mellitus through the activation of the PKC/eNOS/NO pathway. Horm. Metab. Res. Horm. Stoffwechselforsch. Horm. Metab. 2012, 44, 625–631. [Google Scholar] [CrossRef]
- Kahn, N.N.; Sinha, A.K.; Spungen, A.M.; Bauman, W.A. Effects of oxandrolone, an anabolic steroid, on hemostasis. Am. J. Hematol. 2006, 81, 95–100. [Google Scholar] [CrossRef]
- Liu, W.; Gu, X.; Fu, R.; Li, Y.; Lv, M.; Sun, T.; Lv, C.; Liu, X.; Xue, F.; Zhang, L.; et al. The Effect of Danazol in Primary Immune Thrombocytopenia: An Analysis of a Large Cohort from a Single Center in China. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2016, 22, 727–733. [Google Scholar] [CrossRef]
- Colunga-Pedraza, P.R.; Colunga-Pedraza, J.E.; Garza-Ledezma, M.A.; Jaime-Pérez, J.C.; Cantú-Rodríguez, O.G.; Gutiérrez-Aguirre, C.H.; Rendón-Ramírez, E.J.; López-García, Y.K.; Lozano-Morales, R.E.; Gómez-De León, A.; et al. Danazol as First-Line Therapy for Myelodysplastic Syndrome. Clin. Lymphoma. Myeloma Leuk. 2018, 18, e109–e113. [Google Scholar] [CrossRef]
- Ansell, J.E.; Tiarks, C.; Fairchild, V.K. Coagulation abnormalities associated with the use of anabolic steroids. Am. Heart J. 1993, 125, 367–371. [Google Scholar] [CrossRef]
- Severo, C.B.; Ribeiro, J.P.; Umpierre, D.; Da Silveira, A.D.; Padilha, M.C.; De Aquino Neto, F.R.; Stein, R. Increased atherothrombotic markers and endothelial dysfunction in steroid users. Eur. J. Prev. Cardiol. 2013, 20, 195–201. [Google Scholar] [CrossRef]
- Zitzmann, M.; Junker, R.; Kamischke, A.; Nieschlag, E. Contraceptive Steroids Influence the Hemostatic Activation State in Healthy Men. J. Androl. 2002, 23, 503–511. [Google Scholar] [PubMed]
- Khetawat, G.; Faraday, N.; Nealen, M.L.; Vijayan, K.V.; Bolton, E.; Noga, S.J.; Bray, P.F. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): Testosterone regulates AR expression. Blood 2000, 95, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, S.F.; Raya, J.L.; Hoogeveen, R.C.; Bray, P.F. Human platelets differentially concentrate estradiol, estrone and testosterone. J. Thromb. Haemost. 2008, 6, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Nealen, M.L.; Vijayan, K.V.; Bolton, E.; Bray, P.F. Human platelets contain a glycosylated estrogen receptor beta. Circ. Res. 2001, 88, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, M.; Flores, M.; Bratoeff, E.; de la Peña, A.; Mendez, E.; Ceballos, G. Intracellular Ca2+ stimulates the binding to androgen receptors in platelets. Steroids 2004, 69, 767–772. [Google Scholar] [CrossRef]
- Milewich, L.; Whisenant, M.G. Metabolism of androstenedione by human platelets: A source of potent androgens. J. Clin. Endocrinol. Metab. 1982, 54, 969–974. [Google Scholar] [CrossRef]
- Gnatenko, D.V.; Cupit, L.D.; Huang, E.C.; Dhundale, A.; Perrotta, P.L.; Bahou, W.F. Platelets express steroidogenic 17beta-hydroxysteroid dehydrogenases. Distinct profiles predict the essential thrombocythemic phenotype. Thromb. Haemost. 2005, 94, 412–421. [Google Scholar] [CrossRef]
- Garrido, A.; Munoz, Y.; Sierralta, W.; Valladares, L. Metabolism of dehydroepiandrosterone sulfate and estrone-sulfate by human platelets. Physiol. Res. 2012, 61, 381–388. [Google Scholar] [CrossRef]
- Zaslavsky, A.B.; Gloeckner-Kalousek, A.; Adams, M.; Putluri, N.; Venghatakrishnan, H.; Li, H.; Morgan, T.M.; Feng, F.Y.; Tewari, M.; Sreekumar, A.; et al. Platelet-Synthesized Testosterone in Men with Prostate Cancer Induces Androgen Receptor Signaling. Neoplasia 2015, 17, 490–496. [Google Scholar] [CrossRef]
- Ajayi, A.A.L.; Halushka, P.V. Castration reduces platelet thromboxane A 2 receptor density and aggregability. QJM 2005, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Rao, A.K.; Willis, J.; Colman, R.W. Inhibition of thromboxane A2 synthesis in human platelets by coagulation factor Xa. Proc. Natl. Acad. Sci. USA 1983, 80, 6086–6090. [Google Scholar] [CrossRef] [PubMed]
- Martina, V.; Benso, A.; Gigliardi, V.R.; Masha, A.; Origlia, C. Short-term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects. Clin. Endocrinol. 2006, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Karolczak, K.; Konieczna, L.; Kostka, T.; Witas, P.J.; Soltysik, B.; Baczek, T.; Watala, C. Testosterone and dihydrotestosterone reduce platelet activation and reactivity in older men and women. Aging 2018, 10, 902–929. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Feng, R.; Wang, M.; Zhang, J.; Jiang, H.; Jiang, Q.; Lu, J.; Liu, H.; Peng, J.; Hou, M.; et al. Articles Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: A multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2017, 3026. [Google Scholar] [CrossRef]
- Jaime-Pérez, J.C.; Colunga-Pedraza, P.R.; Gómez-Ramírez, C.D.; Gutiérrez-Aguirre, C.H.; Cantú-Rodríguez, O.G.; Tarín-Arzaga, L.C.; Gómez-Almaguer, D. Danazol as first-line therapy for aplastic anemia. Ann. Hematol. 2011, 90, 523–527. [Google Scholar] [CrossRef]
- Chan, G.; DiVenuti, G.; Miller, K. Danazol for the treatment of thrombocytopenia in patients with myelodysplastic syndrome. Am. J. Hematol. 2002, 71, 166–171. [Google Scholar] [CrossRef]
- Viniou, N.; Plata, E.; Terpos, E.; Variami, E.; Meletis, J.; Vaiopoulos, G.; Loukopoulos, D.; Chatzidimitriou, G.; Yataganas, X. Danazol therapy for thrombocytopenia in patients with myelodysplastic syndromes. Acta Haematol. 2002, 107, 234–236. [Google Scholar] [CrossRef]
- Urhausen, A.; Torsten, A.; Wilfried, K. Reversibility of the effects on blood cells, lipids, liver function and hormones in former anabolic-androgenic steroid abusers. J. Steroid Biochem. Mol. Biol. 2003, 84, 369–375. [Google Scholar] [CrossRef]
- Gaughan, W.J.; Liss, K.A.; Dunn, S.R.; Mangold, A.M.; Buhsmer, J.P.; Michael, B.; Burke, J.F. A 6-Month Study of Low-Dose Recombinant Human Erythropoietin Alone and in Combination with Androgens for the Treatment of Anemia in Chronic Hemodialysis Patients. Am. J. Kindy Dis. 1997, 30, 495–500. [Google Scholar] [CrossRef]
- Koiou, E.; Tziomalos, K.; Katsikis, I.; Kalaitzakis, E.; Kandaraki, E.A.; Tsourdi, E.A.; Delkos, D.; Papadakis, E.; Panidis, D. Circulating platelet-derived microparticles are elevated in women with polycystic ovary syndrome diagnosed with the 1990 criteria and correlate with serum testosterone levels. Eur. J. Endocrinol. 2011, 63–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, S. Critically ill Patients and Platelet-Derived Microparticles. J. Atheroscler. Thromb. 2015, 22, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Kebapcilar, L.; Taner, C.E.; Kebapcilar, A.G.; Sari, I. High mean platelet volume, low-grade systemic coagulation and fibrinolytic activation are associated with androgen and insulin levels in polycystic ovary syndrome. Arch. Gynecol. Obstet. 2009, 280, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.A.; Arduc, A.; Tuna, M.M.; Karakılıc, E.; Dagdelen, I.; Tutuncu, Y.; Berker, D.; Guler, S. Association of mean platelet volume with androgens and insulin resistance in nonobese patients with polycystic ovary syndrome. Int. J. Endocrinol. Metab. 2014, 12, e18642. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.; George, W.R.; Kadowitz, P.J.; Wilson, W.R. Androgen-induced enhancement of vascular reactivity. Can. J. Physiol. Pharmacol. 1974, 52, 14–22. [Google Scholar] [CrossRef]
- Morikawa, M.; Kojima, T.; Inoue, M.; Tsuboi, M. Sex difference in the effect of aspirin on intracellular Ca2+ mobilization and thromboxane A2 production in rat platelets. Jpn. J. Pharmacol. 1986, 40, 463–468. [Google Scholar] [CrossRef]
- Morinelli, T.A.; Oatis, J.E.J.; Okwu, A.K.; Mais, D.E.; Mayeux, P.R.; Masuda, A.; Knapp, D.R.; Halushka, P. V Characterization of an 125I-labeled thromboxane A2/prostaglandin H2 receptor agonist. J. Pharmacol. Exp. Ther. 1989, 251, 557–562. [Google Scholar] [PubMed]
- Vicencio, J.M.; Estrada, M.; Galvis, D.; Bravo, R.; Contreras, A.E.; Rotter, D.; Szabadkai, G.; Hill, J.A.; Rothermel, B.A.; Jaimovich, E.; et al. Anabolic Androgenic Steroids and Intracellular Calcium Signaling: A Mini Review on Mechanisms and Physiological Implications. Mini Rev. Med. Chem. 2011, 56, 390–398. [Google Scholar] [CrossRef]
- Nakao, J.; Change, W.C.; Murota, S.I.; Orimo, H. Testosterone inhibits prostacyclin production by rat aortic smooth muscle cells in culture. Atherosclerosis 1981, 39, 203–209. [Google Scholar] [CrossRef]
- Wakasugi, M.; Noguchi, T.; Kazama, Y.I.; Kanemaru, Y.; Onaya, T. The effects of sex hormones on the synthesis of prostacyclin (PGI2) by vascular tissues. Prostaglandins 1989, 37, 401–410. [Google Scholar] [CrossRef]
- Gonzales, R.J.; Ghaffari, A.A.; Duckles, S.P.; Krause, D.N. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H578–H585. [Google Scholar] [CrossRef] [PubMed]
| Types of Androgens | Structural Classification | Way of Administration | Androgenic: Anabolic Ratio |
|---|---|---|---|
| Stanozol | 17-α derivative | Oral, parenteral | 1:30 |
| Oxandrolone | 17-α derivative | oral | 1:10 |
| Nandrolone | 17-β derivative | parenteral | 1:10 |
| Oxymetholone | 17-α derivative | oral | 1:9 |
| Methandrostenolone | 17-α derivative | oral | 1:5→1:2 |
| Methyltestosterone | 17-α derivative | oral | 1 |
| Testosterone | 1 |
| Reference | Treated Subjects, Sex, Number of Subjects Per Group, Type of Experiment | Androgen Formulation | Design of the Study | Platelet Aggregation Variation (Agonist) | Outcome from Other Assays of Platelet Function | Platelet Count |
|---|---|---|---|---|---|---|
| Johnson M. 1977 et al. [144] | Rats, females, n = 8, ex vivo | Testosterone | 1 mg/kg, s.c, single administration | ↑(ADP-0.1–1 μg/mL) | ||
| Rats, males, n = 8, in vitro | Testosterone | 1 μg/mL, 30 min before adding ADP | ↑(ADP-0.1–1 μg/mL) | |||
| Skjaerlund JM. 1983 et al. [145] | Pigeons, females, n = 6, ex vivo | Testosterone Oxandrolone | 5 mg/kg, i.m, biweekly, for 2.5 weeks | Neutral (ADP-40 μM, collagen, epinephr-6 μM, arach. ac. -130 μM) | Neutral effect on platelet TxB2, PG F2α, PG E2 synthesis from [14C] arach. ac. (40 mCi/mM) | |
| Rosenblum WI. 1987 et al. [96] | Mice, n = 10, ex vivo | Testosterone | Pellets, 1.0 mg, s.c, 8–19 days before the experiment | ↑(arach. ac, 0.25 mM -male mice) Neutral (arach.ac, 0.4 mM -female mice) | ||
| Mice, n = 11, ex vivo | DHT | Pellets, 0.1 mg, s.c, 8–19 days before the experiment | Neutral (arach. ac, ADP -0.25 μM) -male and female mice | |||
| Muguruma K. 1993 et al. [146] | Guinea pigs, castrated males, ex vivo | Testosterone propionate | 1 mg/day, i.m, for 1 week | ↑(PAF solution, 3 × 10−11 M, 5 × 10−11 M, 7 × 10−11 M -prepared from seminal vesicles of guinea pig added to washed rabbit platelets) | ||
| Matsuda K. 1994 et al. [147] | Rats, males, n = 7, ex vivo | Testosterone cypionate | 10 mg/kg, i.m, twice weekly, for 2 weeks | ↑(TC for I-BOP in A vs. C group: 0.07 + 0.01 nM vs. 0.45 + 0.16 nM) | ||
| Rats, males, n = 15, ex vivo | Testosterone cypionate | 10 mg/kg, i.m, twice weekly, for 2 weeks | ↑Bmax (80 μL of [125I]BOP) | |||
| Bednarek-Tupikowska G. 2000 et al. [148] | Rabbits, male, n = 11, ex vivo | DHEA | 0.5% of DHEA added to diet (0.125 g/kg/day), for 12 weeks | Neutral (ADP-5 μmol/mL, collagen-2 μmol/mL) | ||
| Aydilek N. 2005 et al. [149] | Rabbits, males, n = 8, ex vivo | Testosterone propionate | 10 mg, s.c, every other day, for 6 weeks | ↑ | ||
| Sullivan PS. 1995 et al. [150] | Mice, castrated males, ex vivo | Testosterone propionate | Maintenance doses, for several days | ↑ | ||
| Alhawiti NM. 2018 et al. [151] | Rats, males, n = 10, ex vivo | Testosterone propionate | 0.5 mg/kg, three times per week, for 12 weeks | Neutral (ADP-10 μM) | Neutral | |
| Roşca A. 2013 et al. [152] | Rats, males, n = 10, ex vivo | DECA | 10 mg/kg, i.m, weekly, for 3 months | ↑(ADP-2.5 µM) | ||
| Roşca A. 2013 et al. [153] | Rats, males, n = 10, ex vivo | DECA | 10 mg/kg, i.m, weekly, for 3 months | ↑Maximal clot strength and stability |
| Reference | Human subjects, Number of Individuals Per Group | Androgen Formulation | Design of the Study | Platelet Aggregation Variation (Agonist) | Outcome from Other Assays of Platelet Function, or from Tests Involving Cellular Lineage (Agonist) | Platelet Count |
|---|---|---|---|---|---|---|
| In vitro | ||||||
| Johnson M. 1975 et al. [160] | Healthy men and women | Testosterone | 1 μg/mL, 30 min before adding agonist | ↑(ADP-1 μg/mL, adrenaline-10 μM, collagen-30 μL, arach. ac-1 mM) | ||
| Pilo R. 1981 et al. [161] | Healthy subjects | Testosterone | Variable doses | ↑(ionophore)-in a dose and time dependent manner | ↑platelet TxA2 synthesis (and other PG products) | |
| Togna GI. 2003 et al. [162] | Healthy volunteers | Testosterone and cocaine | - Testosterone 0.75 μM and cocaine 50 or 100 μM, 10 min - Testosterone 1.5 μM and cocaine 100 μM, 10 min | - Neutral (collagen-1–3 µg/mL), ↑(arach. ac-150–200 µM) - ↑(collagen-1–5 µg/mL, arach. ac-150–400 µg/mL) | - Neutral on platelet TxB2 production (collagen-1–3 µg/mL); ↑ platelet TxB2 production (arach. ac-150–200 µM) - ↑platelet TxB2 production (collagen-1–5 µg/mL, arach. ac-150–400 µg/mL) | |
| Banerjee D. 2014 et al. [163] | - Healthy male volunteers - Healthy female volunteers | - Testosterone - Testosterone | - 40 nM, 40 min - 40 nM, 40 min | - ↑(ADP-2 μM) - Neutral (ADP-2 μM) | - ↓ platelet NO, ↑ platelet TxA2 synthesis (ADP-2 μM); - Neutral on platelet NO, and TxA2 synthesis (ADP-2 μM) | |
| Lee SJ. 2012 et al. [164] | Human megakaryocytic DAMI cell line | Testosterone | 50 nM, 150 nM, 450 nM, for 36 h. | ↑P2Y12 mRNA and P2Y12 protein level in a dose-dependent manner | ||
| Matsuda K. 1993 et al. [165], Halushka PV. 1994 et al. [166] | Human erythroleukemia cells | - Testosterone - DHT | - 200 nM, for 24 h - 75, 100, 200 nM, for 24 h | ↑Bmax ([125I]BOP-50 pM) following T and DHT administration; ↑[Ca2+]i ([125I]BOP-100 nM, or U-46619) for T administration | ||
| Zucker TP. 1996 et al. [167] | Human erythroleukemia cells | - Testosterone; - Androstenedione | - 150 nM, for 48 h; - 250, 500 or 750 nM, for 48 h | ↑Bmax ([125I]BOP-60 pM) | ||
| Jesse RL. 1995 et al. [168] | Healthy donors | DHEAS | 0.075, 0.15, or 0.3 mM, for 1 min | ↓(arachidonic acid) | ↓TxB2 synthesis (arachidonic acid) | |
| Bertoni A. 2012 et al. [169] | Healthy donors | DHEAS | - 0.068 × 10−4 M, for 1 min; - 3 × 10−4 M, for 1 min | - ↓(thrombin-0.05 U/mL, 0.025 U/mL, 0.02 U/mL) - ↓(collagen-2 × 10−6 g/mL, thrombin- 0.05 U/mL, U46619–1 × 10−6 M) | Activation of platelet NOS/cGMP/PKG pathway (DHEAS at 3 × 10−4 M) | |
| Munoz YC. 2012 et al. [170] | Postmenopausal women, type II diabetes mellitus | DHEA | 100 nmol/L, for 20 min | ↓(ADP-10 μmol/L) | Activation of platelet PKCδ/eNOS/NO/cGMP pathway | |
| Ex vivo | ||||||
| Ferenchick G. 1992b et al. [29] | -Weightlifters, n = 24 for A users; -Weightlifters, n = 13 for A users, stratified by age | Various type AAS intake (an average of three separate AAS/each user) | Various doses and length of AAS use | - Neutral [TC in AU vs. N group: 2.50 ± 0.21 µM/mL vs. 2.90 ± 1.10 µM/mL (for ADP); 2.50 ± 0.38 µg/mL vs. 1.96 ± 1.11 µg/mL (for collagen)]; - ↑(age subgroup analysis, TC for collagen in AU > 22 yo vs. AU ≤ 22 yo group: 1.47 µg/mL vs. 3.35 µg/mL) | Neutral | |
| Ajayi AA. 1995 et al. [83] | Healthy men, n = 9 | Testosterone cypionate | 200 mg, i.m, given twice, 2 weeks apart | - ↑(TxA2 analog I-BOP-0.25 to 100 nmol/L) - Neutral (thrombin-0.00625 to 0.1 U/mL) | ↑Bmax ([125I]BOP-80 µl); | |
| Kahn NN. 2006 et al. [171] | Healthy subjects, n = 14 | Oxandrolone | 10 mg, twice daily, for 2 weeks | Neutral (ADP) | ||
| Jesse RL. 1995 et al. [168] | Healthy men, n = 5 | DHEA | 300 mg, p.o, 3 times daily, for 14 days | ↓(arachidonic acid) | ||
| Liu W. 2016 et al. [172] | Patients with ITP, n = 103 | Danazol | 200 or 300 mg daily, for a median duration of 7 months | ↑ | ||
| Colunga-Pedraza PR. 2018 et al. [173] | Patients with MDS, n = 42 | Danazol | Median dose of 400 mg/day, median follow-up of 12 months | ↑ | ||
| Bodybuilders and powerlifters, n = 17 (AAS abusers); n = 15 (AAS ex-abusers) | Heterogenous AAS intake | Mean dosage of 750 mg/week, 33 weeks per year, over 8 years (AAS abusers); mean dosage 700 mg/week, for 26 weeks per year, over 9 years (AAS ex-abusers) | ↑ | |||
| Ansell JE. 1993 et al. [174] | Bodybuilders, n = 11 | Various type of AAS intake, 3 different AAS/each user (average) | Various doses and length of AAS intake | ↑ | ||
| Severo CB. 2013 et al. [175] | Weightlifters, n = 10 | Various type of AAS intake, 3 different AAS/each user (average) | Various doses and length of AAS intake | ↑ | ||
| Zitzmann M. 2002 et al. [176] | Healthy men, n = 14 | Testosterone undecanoate | 1000 mg, i.m, in study weeks 0, 6, 12, and 18 | Neutral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roşca, A.E.; Vlădăreanu, A.-M.; Mititelu, A.; Popescu, B.O.; Badiu, C.; Căruntu, C.; Voiculescu, S.E.; Onisâi, M.; Gologan, Ş.; Mirica, R.; et al. Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review. J. Clin. Med. 2021, 10, 147. https://doi.org/10.3390/jcm10010147
Roşca AE, Vlădăreanu A-M, Mititelu A, Popescu BO, Badiu C, Căruntu C, Voiculescu SE, Onisâi M, Gologan Ş, Mirica R, et al. Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review. Journal of Clinical Medicine. 2021; 10(1):147. https://doi.org/10.3390/jcm10010147
Chicago/Turabian StyleRoşca, Adrian Eugen, Ana-Maria Vlădăreanu, Alina Mititelu, Bogdan Ovidiu Popescu, Corin Badiu, Constantin Căruntu, Suzana Elena Voiculescu, Minodora Onisâi, Şerban Gologan, Radu Mirica, and et al. 2021. "Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review" Journal of Clinical Medicine 10, no. 1: 147. https://doi.org/10.3390/jcm10010147
APA StyleRoşca, A. E., Vlădăreanu, A.-M., Mititelu, A., Popescu, B. O., Badiu, C., Căruntu, C., Voiculescu, S. E., Onisâi, M., Gologan, Ş., Mirica, R., & Zăgrean, L. (2021). Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review. Journal of Clinical Medicine, 10(1), 147. https://doi.org/10.3390/jcm10010147







