Identification of Skin Lesions by Using Single-Step Multiframe Detector
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Ethical Statement
2.3. Clinical Features
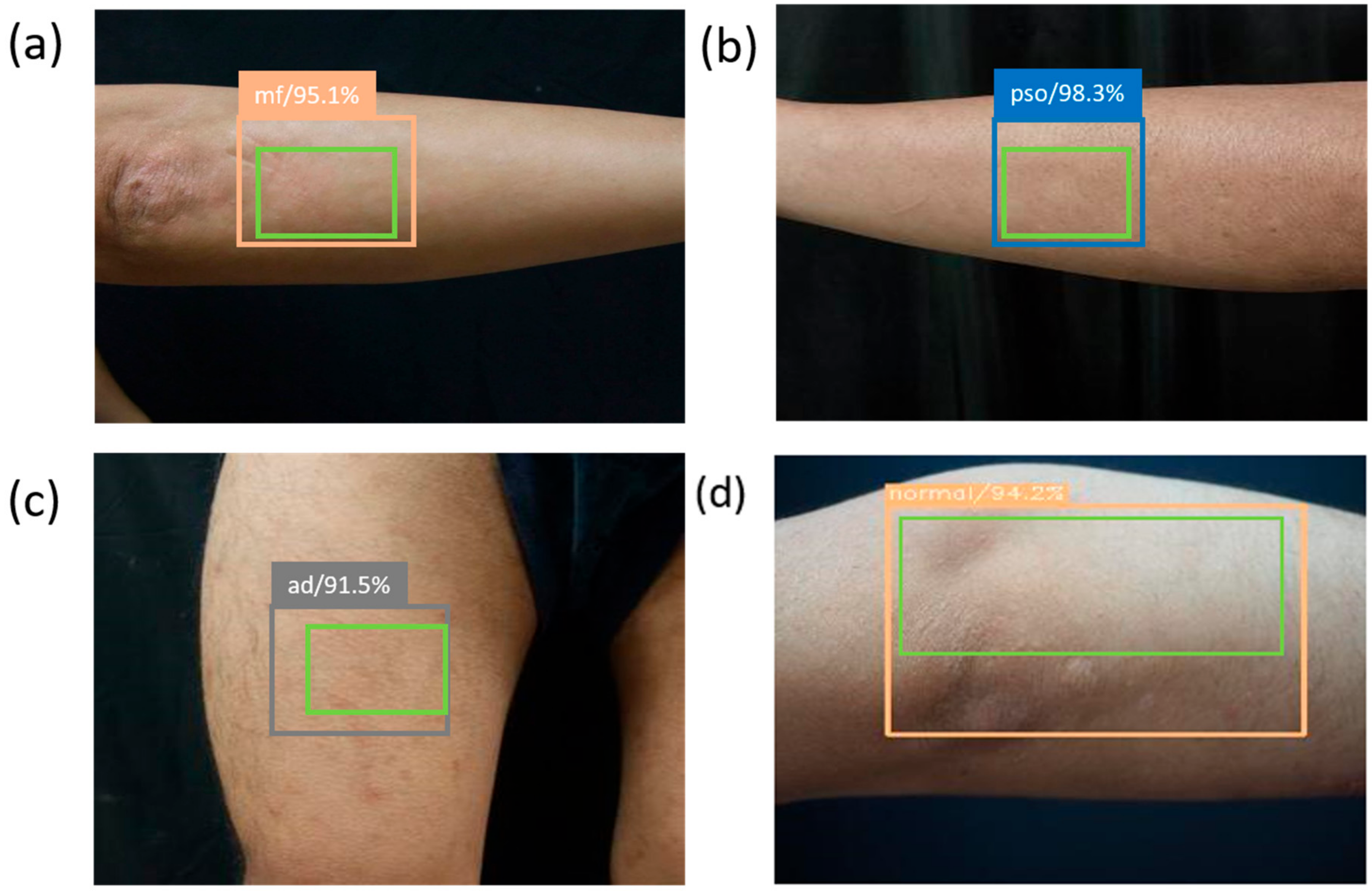
2.4. Single Shot Multibox Detector (SSD)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaidya, T.; Zulfiqar, H.; Badri, T. Mycosis Fungoides; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Mercier, T.; Wera, J.; Chai, L.Y.; Lagrou, K.; Maertens, J. A Mortality Prediction Rule for Hematology Patients with Invasive Aspergillosis Based on Serum Galactomannan Kinetics. J. Clin. Med. 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Savage, L.; Tinazzi, I.; Zabotti, A.; Laws, P.M.; Wittmann, M.; McGonagle, D. Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment. J. Clin. Med. 2020, 9, 3262. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, E.C.; Hebert, A.A. Diagnosis of atopic dermatitis: Mimics, overlaps, and complications. J. Clin. Med. 2015, 4, 884–917. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Dreyling, M. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v177–v180. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.-H.; Park, J.; Lee, S.Y. Analysis of the treatment results of localized conventional radiotherapy for early-and advanced-stage cutaneous T-cell lymphoma refractory to other skin-directed therapies. Dermatol. Sin. 2019, 37, 117. [Google Scholar]
- Hsiao, Y.-P.; Wang, H.-C.; Chen, S.-H.; Tsai, C.-H.; Yang, J.-H. Identified early stage mycosis fungoides from psoriasis and atopic dermatitis using non-invasive color contrast enhancement by LEDs lighting. Opt. Quantum Electron. 2015, 47, 1599–1611. [Google Scholar] [CrossRef]
- Hsiao, Y.-P.; Wang, H.-C.; Chen, S.-H.; Tsai, C.-H.; Yang, J.-H. Optical perception for detection of cutaneous T-cell lymphoma by multi-spectral imaging. J. Opt. 2014, 16, 125301. [Google Scholar] [CrossRef]
- Thijs, J.L.; Van Seggelen, W.; Bruijnzeel-Koomen, C.; Bruin-Weller, D.; Hijnen, D. New developments in biomarkers for atopic dermatitis. J. Clin. Med. 2015, 4, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Pomponiu, V.; Nejati, H.; Cheung, N.-M. Deepmole: Deep Neural Networks for Skin Mole Lesion Classification. In Proceedings of the 2016 IEEE International Conference on Image Processing (ICIP), Phoenix, AZ, USA, 25–28 September 2016; pp. 2623–2627. [Google Scholar]
- Brinker, T.J.; Hekler, A.; Utikal, J.S.; Grabe, N.; Schadendorf, D.; Klode, J.; Berking, C.; Steeb, T.; Enk, A.H.; von Kalle, C. Skin cancer classification using convolutional neural networks: Systematic review. J. Med. Internet Res. 2018, 20, e11936. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Haenssle, H.A.; Fink, C.; Schneiderbauer, R.; Toberer, F.; Buhl, T.; Blum, A.; Kalloo, A.; Hassen, A.B.; Thomas, L.; Enk, A.; et al. Man against machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann. Oncol. 2018, 29, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Tschandl, P.; Rosendahl, C.; Akay, B.N.; Argenziano, G.; Blum, A.; Braun, R.P.; Cabo, H.; Gourhant, J.-Y.; Kreusch, J.; Lallas, A. Expert-level diagnosis of nonpigmented skin cancer by combined convolutional neural networks. JAMA Dermatol. 2019, 155, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Hekler, A.; Utikal, J.S.; Enk, A.H.; Hauschild, A.; Weichenthal, M.; Maron, R.C.; Berking, C.; Haferkamp, S.; Klode, J.; Schadendorf, D. Superior skin cancer classification by the combination of human and artificial intelligence. Eur. J. Cancer 2019, 120, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Irtaza, A.; Nida, N.; Javed, A.; Malik, H.; Malik, K.M. Segmenting melanoma Lesion using Single Shot Detector (SSD) and Level Set Segmentation Technique. In Proceedings of the 2019 13th International Conference on Mathematics, Actuarial Science, Computer Science and Statistics (MACS), Karachi, Pakistan, 14–15 December 2019; pp. 1–5. [Google Scholar]
- Hubel, D.H.; Wiesel, T.N. Brain and Visual Perception: The Story of a 25-Year Collaboration; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Zeiler, M.D.; Fergus, R. Stochastic pooling for regularization of deep convolutional neural networks. arXiv 2013, arXiv:1301.3557. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster r-cnn: Towards real-time object detection with region proposal networks. In Proceedings of the Advances in Neural Information Processing Systems, Montreal, QC, Canada, 7–12 December 2015; pp. 91–99. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.-Y.; Berg, A.C. Ssd: Single shot multibox detector. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 8–16 October 2016; pp. 21–37. [Google Scholar]
- Uijlings, J.R.; Van De Sande, K.E.; Gevers, T.; Smeulders, A.W. Selective search for object recognition. Int. J. Comput. Vis. 2013, 104, 154–171. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
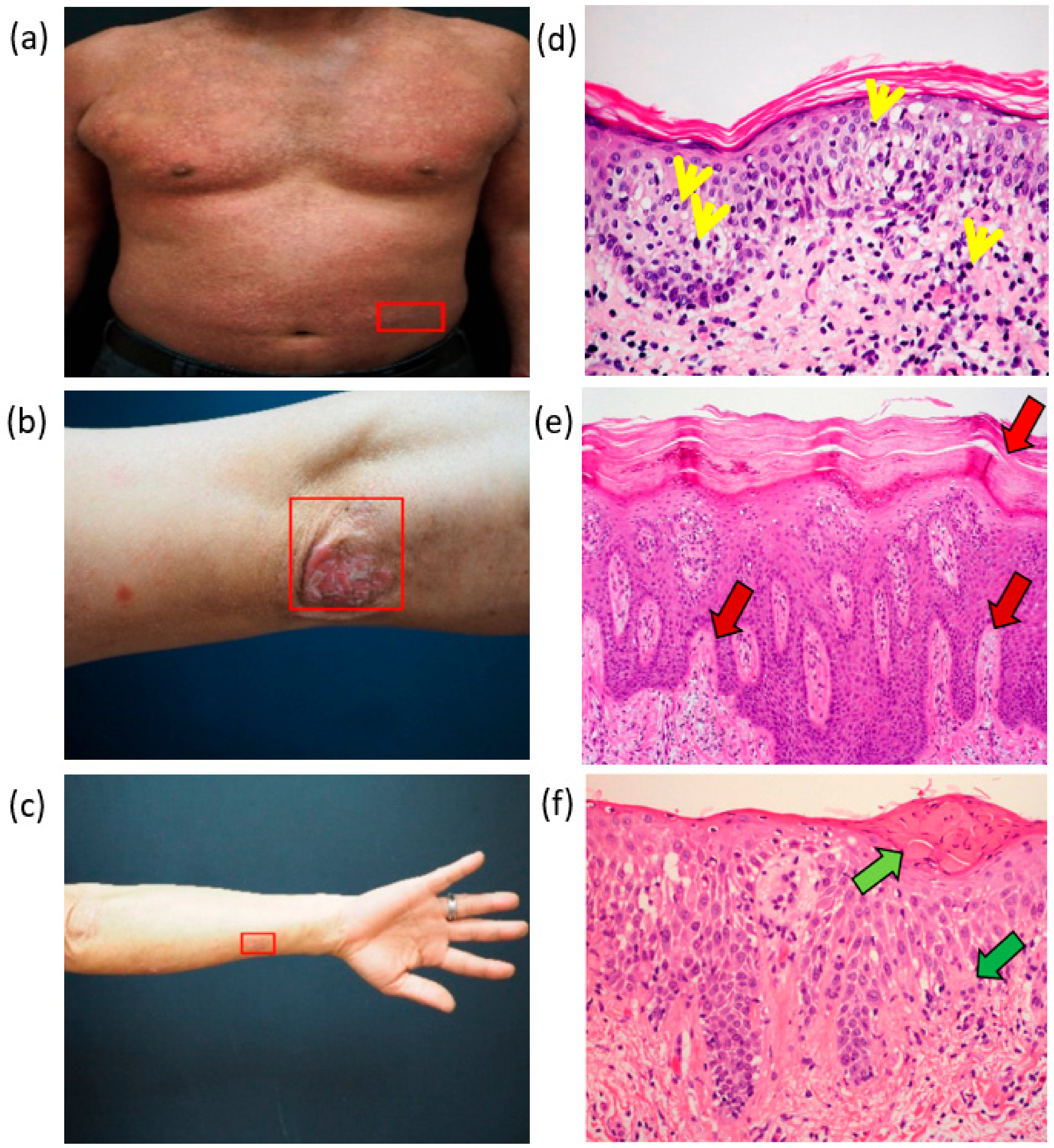

| Pathology | MF | PSO | AD |
|---|---|---|---|
| Stratum corneum | No change | Thickened | Thickened |
| Stratum granulosum | No change | Fewer | Increased |
| Keratinocyte | Cell edema | Cell edema | Cell edema |
| Dermis | Lymphocytes gathered at the border of the epidermis, fibrosis | Mastoid dermis, dilated blood vessels | Mastoid dermis, edema, fibrosis |
| Lymphocytes | As the number increased, abnormal lymphocytes rushed up into the epidermis | Abnormal lymphocytes rushed up into the epidermis | Uncertain |
| Skin Disease | Results of Prediction by SSD | |||
|---|---|---|---|---|
| Images | PSO | AD | MF | Normal |
| PSO | 134 | 3 | 0 | 2 |
| AD | 3 | 32 | 1 | 4 |
| MF | 1 | 1 | 47 | 1 |
| Normal | 2 | 1 | 0 | 60 |
| Sensitivity (%) | Precision (%) | F1-Score (%) | Accuracy (%) | |
|---|---|---|---|---|
| PSO | 96 | 96 | 96 | |
| AD | 80 | 86 | 83 | |
| MF | 94 | 98 | 96 | |
| Normal | 95 | 90 | 92 | |
| Total | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-P.; Chiu, C.-W.; Lu, C.-W.; Nguyen, H.T.; Tseng, Y.S.; Hsieh, S.-C.; Wang, H.-C. Identification of Skin Lesions by Using Single-Step Multiframe Detector. J. Clin. Med. 2021, 10, 144. https://doi.org/10.3390/jcm10010144
Hsiao Y-P, Chiu C-W, Lu C-W, Nguyen HT, Tseng YS, Hsieh S-C, Wang H-C. Identification of Skin Lesions by Using Single-Step Multiframe Detector. Journal of Clinical Medicine. 2021; 10(1):144. https://doi.org/10.3390/jcm10010144
Chicago/Turabian StyleHsiao, Yu-Ping, Chih-Wei Chiu, Chih-Wei Lu, Hong Thai Nguyen, Yu Sheng Tseng, Shang-Chin Hsieh, and Hsiang-Chen Wang. 2021. "Identification of Skin Lesions by Using Single-Step Multiframe Detector" Journal of Clinical Medicine 10, no. 1: 144. https://doi.org/10.3390/jcm10010144
APA StyleHsiao, Y.-P., Chiu, C.-W., Lu, C.-W., Nguyen, H. T., Tseng, Y. S., Hsieh, S.-C., & Wang, H.-C. (2021). Identification of Skin Lesions by Using Single-Step Multiframe Detector. Journal of Clinical Medicine, 10(1), 144. https://doi.org/10.3390/jcm10010144







