The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health
Abstract
1. Introduction
2. Experimental Section
3. Results, Figures and Tables
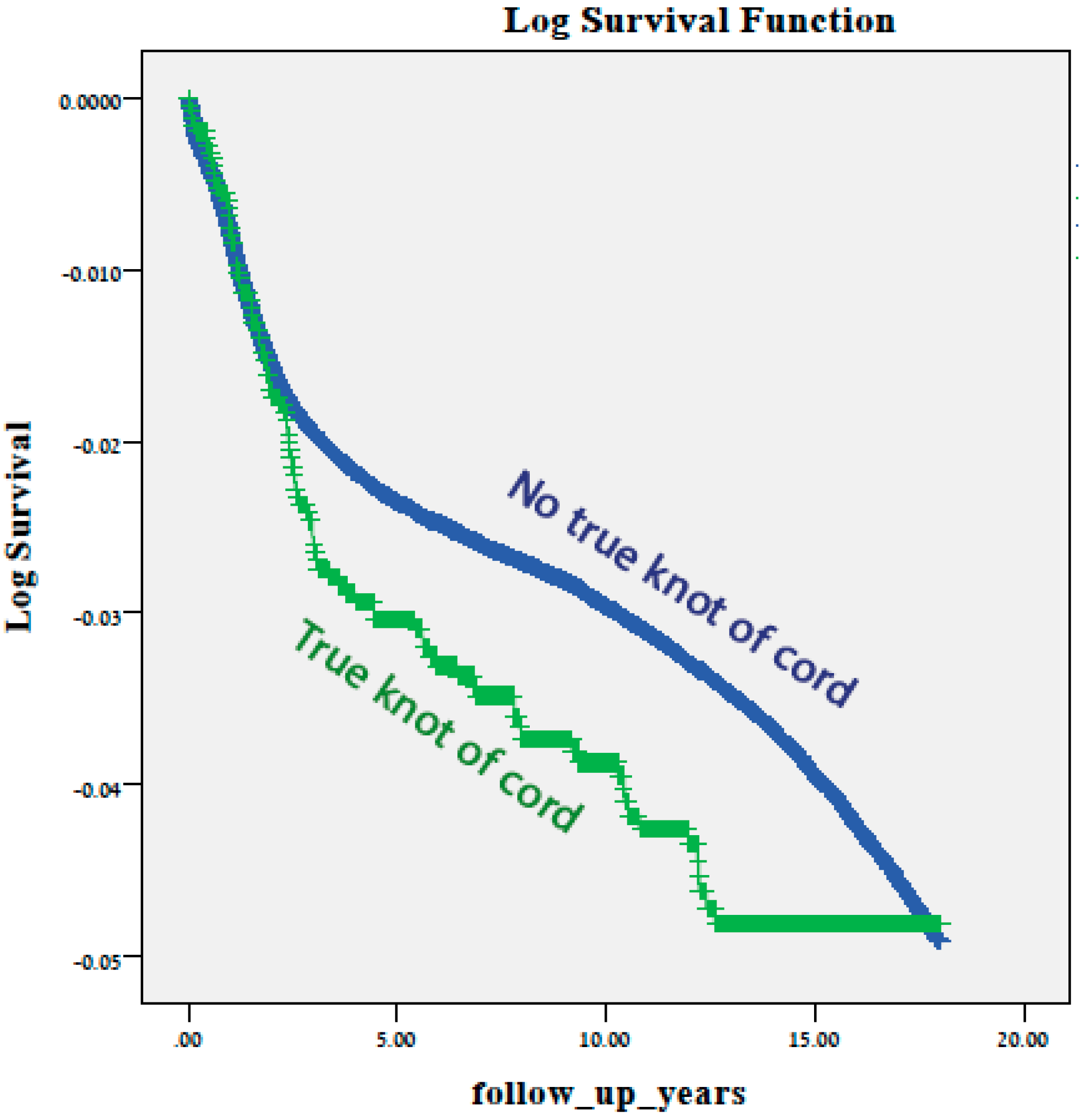
Results
4. Discussion
- Although several confounders were controlled for and an independent association was found with IUFD, it is possible due to the retrospective nature of the study, that some confounders were not accounted for.
- Most childhood neurological morbidities, especially on the “lighter” side of the spectrum, are cared for in an ambulatory setting and were not accounted for in this long-term analysis. This can lead to under reporting of some diagnosis due to the fact that some diagnosed children are not hospitalized. Furthermore, for several of the outcomes (like autistic spectrum disorders), diagnosis typically only comes through specialized screening, which is a potential for selection bias (of children who suffer from the condition but were not screened for it). Nevertheless, some of the conditions included in the study are significant morbidities, and therefore are likely to necessitate hospitalization at some point. There is a possibility that the study groups were underpowered to detect neurological-related hospitalizations in the offspring.
- Hospitalization at a different, distant, medical center, although unlikely, is possible. SUMC is the only tertiary center in the Negev region, it is reasonable to assume that this is the only place for children to be hospitalized in case of morbidity; however, there can be no guarantee of that. Therefore, ascertainment bias potentially exists. There seems to be no reason, however, for either of those phenomenon to be more common in either of the compared groups.
- It was assumed that children that did not visit our hospital were healthy (which might be a biased assumption). This possibility as well is probably just as likely in both the exposed and unexposed groups.
- A heterogeneous group of neurological outcomes was used rather than a specific neurological diagnosis. The purpose of this work was to search for an association between different groups of neurological morbidities and true knot of cord upon birth. We did not look for specific diagnoses since no specific associations were mentioned in the literature nor were part of our hypothesis. Additionally, these types of diagnoses are quite rare and looking for specific diagnoses (rather than groups of diagnoses) would have diminished the power of our results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Group | Subgroup | Code | Diagnosis Description |
|---|---|---|---|
| Neurology | Autistic spectrum disorders | 2990 | Autistic Disorder |
| 2990 | Infantile Autism | ||
| 29,900 | Autistic Disorder, Current or Active State | ||
| 29,901 | Autistic Disorder, Residual State | ||
| 29,910 | Childhood Disintegrative Disorder, Current or Active State | ||
| 2998 | Other Specified Pervasive Developmental Disorders | ||
| 29,981 | Other Specified Pervasive Developmental Disorders, Residula State | ||
| 29,990 | Unspecif. Pervasive Developmental Disorder, Current or Active State | ||
| Eating disorders | 3071 | Anorexia Nervosa | |
| 3075 | Other and Unspecified Disorders of Eating | ||
| 30,750 | Eating Disorder, Unspecified | ||
| 30,751 | Bulimia Nervosa | ||
| 30,753 | Rumination Disorder | ||
| 30,759 | Other Disorders of Eating | ||
| V691 | Inappropriate Diet & Eating Habits | ||
| Sleeping disorders | 3073 | Stereotypic Movement Disorder | |
| 30,746 | Sleep Arousal Disorder | ||
| 30,746 | Somnambulism or Night Terrors | ||
| 30,747 | Other Dysfunctions of Sleep Stages or Arousal from Sleep | ||
| 32,727 | Central Sleep Apnea in Conditions Classified Elsewhere | ||
| 32,730 | Circadian Rhythm Sleep Disorder, Unspecified | ||
| 32,732 | Circadian Rhythm Sleep Disorder, Advanced Sleep Phase Type | ||
| 34,700 | Narcolepsy without Cataplexy | ||
| 34,701 | Narcolepsy with Cataplexy | ||
| 7805 | Sleep Disturbances | ||
| 78,050 | Unspecified Sleep Disturbance | ||
| 78,051 | Insomnia with Sleep Apnea | ||
| 78,051 | Insomnia with Sleep Apnea, Unspecified | ||
| 78,052 | Insomnia, Unspecified | ||
| 78,052 | Other Insomnia | ||
| 78,054 | Hypersomnia, Unspecified | ||
| 78,056 | Dysfunctions Associated with Sleep Stages or Arousal from Sleep | ||
| 78,059 | Other Sleep Disturbances | ||
| V694 | Lack of Adequate Sleep | ||
| Movement disorders | 3331 | Essential and Other Specified Forms of Tremor | |
| 3332 | Myoclonus | ||
| 3335 | Other Choreas | ||
| 3336 | Genetic Torsion Dystonia | ||
| 3336 | Idiopathic Torsion Dystonia | ||
| 33,390 | Unsp. Extrapyramidal Disease + Abnormal Movement Disorder | ||
| 33,399 | Other Extrapyramidal Diseases and Abnormal Movement Disorders | ||
| 3343 | Other Cerebellar Ataxia | ||
| Epilepsy | 3450 | Generalized Nonconvulsive Epilepsy | |
| 34,500 | Generalized Nonconvulsive Epilepsy without Intractable Epilepsy | ||
| 34,501 | Generalized Nonconvulsive Epilepsy with Intractable Epilepsy | ||
| 34,510 | Generalized Convulsive Epilepsy without Intractable Epilepsy | ||
| 34,511 | Generalized Convulsive Epilepsy with Intractable Epilepsy | ||
| 3452 | Petit Mal Status, Epileptic | ||
| 3453 | Grand Mal Status, Epileptic | ||
| 34,540 | Partial Epilepsy + Impairment of Consciousness without Intractable Epilepsy | ||
| 3455 | Partial Epilepsy, without Impairment of Consciousness | ||
| 34,550 | Partial Epilepsy without Impairment of Consciousness without Intr Actabel Epilepsy | ||
| 3456 | Infantile Spasms | ||
| 34,560 | Infantile Spasms without Intractable Epilepsy | ||
| 3459 | Epilepsy, Unspecified | ||
| 34,590 | Epilepsy, Nusp. without Intractabel Epilepsy | ||
| 34,590 | Epilepsy, Unsp. without Intractable Epilepsy | ||
| 34,591 | Epilepsy Unsp. With Intractable Epilepsy | ||
| 78,039 | Other Convulsions | ||
| 7810 | Abnormal Involuntary Movements | ||
| 7812 | Abnormality of Gait | ||
| 7813 | Lack of Coordination | ||
| Cerebral palsy | 3341 | Hereditary Spastic Paraplegia | |
| 3421 | Spastic Hemiplegia | ||
| 34,210 | Spastic Hemiplegia Affecting Unsp. Side | ||
| 3429 | Hemiplegia, Unspecified | ||
| 34,290 | Hemiplegia, Unsp., Affecting Unsp. Side | ||
| 34,291 | Hemiplegia, Unsp., Affecting Dominant Side | ||
| 34,292 | Hemiplegia, Unsp., Affecting Nondominant Side | ||
| 3430 | Congenital Diplegia | ||
| 3431 | Congenital Hemiplegia | ||
| 3432 | Congenital Quadriplegia | ||
| 3439 | Infantile Cerebral Palsy, Unspecified | ||
| 34,400 | Quadriplegia, Unspecified | ||
| 3441 | Paraplegia | ||
| 3442 | Diplegia Of Upper Limbs | ||
| 34,430 | Monoplegia of Lower Limb, Affecting Unsp. Side | ||
| 34,440 | Monoplegia of Upper Limb, Affecting Unsp. Side | ||
| 34,489 | Other Specified Paralytic Syndrome | ||
| 3449 | Paralysis, Unspecified | ||
| 3481 | Anoxic Brain Damage | ||
| 3526 | Multiple Cranial Nerve Palsies | ||
| 43,811 | Aphasia | ||
| 43,820 | Hemiplegia Affecting Unsp. Side | ||
| 7814 | Transient Paralysis of Limb | ||
| Psychiatric disorders | 2930 | Acute Delirium | |
| 2930 | Delirium Due to Conditions Classified Elsewhere | ||
| 29,384 | Anxiety Disorder in Conditions Classified Elsewhere | ||
| 2940 | Amnestic Disorder in Conditions Classified Elsewhere | ||
| 2949 | Unspecified Persistent Mental Disorders Due to Cond. Class. Elsewh. | ||
| 29,530 | Paranoid Type Schizophrenia, Unspecified State | ||
| 29,570 | Schizoaffective Disorder Schizophrenia, Unspecified State | ||
| 29,580 | Other Specified Types of Schizophrenia, Unspecified State | ||
| 29,590 | Unspecified Type Schizophrenia, Unspecified State | ||
| 29,600 | Bipolar I Disorder, Single Manic Episode, Unspecified Degree | ||
| 29,620 | Major Depressive Affective Disorder, Single Episode, Unsp. Degree | ||
| 29,680 | Bipolar Disorder, Unspecified | ||
| 29,690 | Unspecified Episodic Mood Disorder | ||
| 29,699 | Other Specified Affective Psychoses | ||
| 2971 | Delusional Disorder | ||
| 2979 | Unspecified Paranoid State | ||
| 2981 | Excitative Type Psychosis | ||
| 2983 | Acute Paranoid Reaction | ||
| 2989 | Unspecified Psychosis | ||
| 30,000 | Anxiety State, Unspecified | ||
| 30,001 | Panic Disorder without Agoraphobia | ||
| 30,009 | Other Anxiety States | ||
| 30,010 | Hysteria, Unspecified | ||
| 30,011 | Conversion Disorder | ||
| 30,029 | Other Isolated or Simple Phobias | ||
| 3003 | Obsessive-Compulsive Disorders | ||
| 3004 | Dysthymic Disorder | ||
| 3004 | Neurotic Depression | ||
| 3009 | Unspecified Nonpsychotic Mental Disorder | ||
| 30,183 | Borderline Personality | ||
| 30,183 | Borderline Personality Disorder | ||
| 3019 | Unspecified Personality Disorder | ||
| 3026 | Disorders of Psychosexual Identity | ||
| 30,302 | Ac. Alcoholic Intoxic. in Alcoholism, Episodic Drinking Behavior | ||
| 30,400 | Opioid Type Dependence, Unspecified Use | ||
| 30,430 | Cannabis Dependence, Unspecified Use | ||
| 30,432 | Cannabis Dependence, Episodic Use | ||
| 30,500 | Alcohol Abuse, Unspecified Drinking Behavior | ||
| 30,501 | Alcohol Abuse, Continuous Drinking Behavior | ||
| 30,502 | Alcohol Abuse, Episodic Drinking Behavior | ||
| 3051 | Tobacco Use Disorder (Tobacco Dependence) | ||
| 30,591 | Other, Mixed, Or Unspecified Drug Abuse, Continuous Use | ||
| 3061 | Respiratory Malfunction Arising from Mental Factors | ||
| 3062 | Cardiovascular Malfunction Arising from Mental Factors | ||
| 3068 | Other Specified Psychophysiological Malfunction | ||
| 3069 | Unspecified Psychophysiological Malfunction | ||
| 3070 | Adult Onset Fluency Disorder | ||
| 3070 | Stammering and Stuttering | ||
| 3070 | Stuttering | ||
| 30,720 | Tic Disorder, Unspecified | ||
| 30,722 | Chronic Motor or Vocal Tic Disorder | ||
| 30,723 | Tourette’s Disorder | ||
| 30,752 | Pica | ||
| 3080 | Predominant Disturbance of Emotions | ||
| 3089 | Unspecified Acute Reaction to Stress | ||
| 309 | Adjustment Reaction | ||
| 3090 | Adjustment Disorder with Depressed Mood | ||
| 30,924 | Adjustment Disorder with Anxiety | ||
| 3094 | Adjustment Disor. with Mixed Disturb. of Emotions and Conduct | ||
| 30,981 | Posttraumatic Stress Disorder | ||
| 3099 | Unspecified Adjustment Reaction | ||
| 311 | Depressive Disorder, Not Elsewhere Classified | ||
| 31,210 | Undersocialized Conduct Disorder, Unaggressive Type, Unspecified | ||
| 31,239 | Other Disorders of Impulse Control | ||
| 3129 | Unspecified Disturbance of Conduct | ||
| 31,389 | Other Emotional Disturbances of Childhood or Adolescence | ||
| 3139 | Unspecified Emotional Disturbance of Childhood or Adolescence | ||
| 316 | Psychic Factors Associated with Diseases Classified Elsewhere | ||
| 7801 | Hallucinations | ||
| 7803 | Convulsions | ||
| 7992 | Nervousness | ||
| 79,921 | Nervousness | ||
| 79,922 | Irritability | ||
| 79,925 | Demoralization and Apathy | ||
| 79,929 | Other Signs and Symptoms Involving Emotional State | ||
| 7993 | Debility, Unspecified | ||
| V6284 | Suicidal Ideation | ||
| Attention deficit disorders | 31,400 | Attention Deficit Disorder without Hyperactivity | |
| 31,401 | Attention Deficit Disorder with Hyperactivity | ||
| 3142 | Hyperkinetic Conduct Disorder of Childhood | ||
| 3149 | Unspecified Hyperkinetic Syndrome of Childhood | ||
| V400 | Mental and Behavioral Problems with Learning | ||
| V409 | Unspecified Mental or Behavioral Problem | ||
| Developmental disorders | 3152 | Other Specific Developmental Learning Difficulties | |
| 31,531 | Expressive Language Disorder | ||
| 31,534 | Speech and Language Developmental Delay Due to Hearing Loss | ||
| 31,539 | Other Developmental Speech Disorder | ||
| 3154 | Developmental Coordination Disorder | ||
| 3158 | Other Specified Delays in Development | ||
| 3159 | Unspecified Delay in Development | ||
| 317 | Mild Intellecutal Disabilities | ||
| 317 | Mild Mental Retardation | ||
| 319 | Unspecified Intellectual Disabilities | ||
| 319 | Unspecified Mental Retardation | ||
| 33,183 | Mild Cognitive Impairment, So Stated | ||
| 7834 | Lack of Expected Normal Physiological Development | ||
| 7834 | Lack of Expected Normal Physiological Development in Childhood | ||
| 78,340 | Lack of Normal Physiological Development, Unspecified | ||
| Degenerative disorders | 330 | Cerebral Degenerations Usually Manifest in Childhood | |
| 3300 | Leukodystrophy | ||
| 3308 | Other Specified Cerebral Degenerations in Childhood | ||
| 3313 | Communicating Hydrocephalus | ||
| 33,132 | Post Hemorrhagic Hydrocephalus | ||
| 3314 | Obstructive Hydrocephalus | ||
| 33,189 | Other Cerebral Degeneration | ||
| 3319 | Cerebral Degeneration, Unspecified | ||
| 3348 | Other Spinocerebellar Diseases | ||
| 335 | Anterior Horn Cell Disease | ||
| 3350 | Werdnig-Hoffmann Disease | ||
| 33,510 | Spinal Muscular Atrophy, Unspecified | ||
| 33,522 | Progressive Bulbar Palsy | ||
| 33,523 | Pseudobulbar Palsy | ||
| 3360 | Syringomyelia And Syringobulbia | ||
| 340 | Multiple Sclerosis | ||
| 3410 | Neuromyelitis Optica | ||
| 3411 | Schilder’s Disease | ||
| 34,120 | Acute (Transverse) Myelitis Nos | ||
| 3419 | Demyelinating Disease of Central Nervous System, Unspecified | ||
| 3480 | Cerebral Cysts | ||
| 348,891 | Cerebral Calcification | ||
| 3590 | Congenital Hereditary Muscular Dystrophy | ||
| 3591 | Hereditary Progressive Muscular Dystrophy | ||
| Headache | 30,781 | Tension Headache | |
| 34,600 | Migraine With Aura without Mention Of Intractable Migraine, Without Mention Of Status Migrainosus | ||
| 34,601 | Migraine with Aura, So Stated, without Mention of Statu. Migrainosus | ||
| 34,620 | Variants of Migraine, without Intractable Migraine | ||
| 34,630 | Hemiplegic Migraine without Mention of Intractable Migraine, With Out Mention of Status Migrainosus | ||
| 34,670 | Chronic Migraine without Aura without Mention of Intractable Migr Aine, without Mention of Status Migrainosus | ||
| 3469 | Migraine, Unspecified | ||
| 34,690 | Migraine, Unspecified, without Intractabel Migraine | ||
| 34,690 | Migraine, Unspecified, without Mention of Intractable Migraine Wi Thout Mention of Status Migrainosus | ||
| Myopathy | 3556 | Lesion of Plantar Nerve | |
| 33,709 | Other Idiopathic Peripheral Autonomic Neuropathy | ||
| 33,720 | Reflex Sympathetic Dystrophy, Unspecified | ||
| 33,721 | Reflex Sympathetic Dystrophy of Upper Limb | ||
| 33,722 | Reflex Sympathetic Dystrophy of Lower Limb | ||
| 3379 | Unspecified Disorder of Autonomic Nervous System | ||
| 3510 | Bell’s Palsy | ||
| 3518 | Other Facial Nerve Disorders | ||
| 3519 | Facial Nerve Disorder, Unspecified | ||
| 352 | Disorders of Other Cranial Nerves | ||
| 3539 | Unspecified Nerve Root and Plexus Disorder | ||
| 3542 | Lesion of Ulnar Nerve | ||
| 3548 | Other Mononeuritis of Upper Limb | ||
| 3549 | Mononeuritis of Upper Limb, Unspecified | ||
| 3553 | Lesion of Lateral Popliteal Nerve | ||
| 3558 | Mononeuritis of Lower Limb, Unspecified | ||
| 3559 | Mononeuritis of Unspecified Site | ||
| 3562 | Hereditary Sensory Neuropathy | ||
| 3564 | Idiopathic Progressive Polyneuropathy | ||
| 3568 | Other Specified Idiopathic Peripheral Neuropathy | ||
| 3569 | Unspecified Idiopathic Peripheral Neuropathy | ||
| 3570 | Acute Infective Polyneuritis | ||
| 3571 | Polyneuropathy in Collagen Vascular Disease | ||
| 3572 | Polyneuropathy in Diabetes | ||
| 3577 | Polyneuropathy Due to Other Toxic Agents | ||
| 35,781 | Chronic Inflammatory Demyelinating Polyneuritis | ||
| 35,800 | Myasthenia Gravis without (Acute) Exacerbation | ||
| 3588 | Other Specified Myoneural Disorders | ||
| 3589 | Myoneural Disorders, Unspecified | ||
| 3592 | Myotonic Disorders | ||
| 3599 | Myopathy, Unspecified | ||
| Others | 30,789 | Other Psychalgia | |
| 33,381 | Blepharospasm | ||
| 3384 | Chronic Pain Syndrome | ||
| 33,903 | Episodic Paroxysmal Hemicrania | ||
| 3482 | Benign Intracranial Hypertension | ||
| 3483 | Encephalopathy, Unspecified | ||
| 3483 | Encephalopathy, not Elsewhere Classified | ||
| 34,830 | Encephalopathy, Unspecified | ||
| 34,831 | Metabolic Encephalopathy | ||
| 34,881 | Cerebral Calcification | ||
| 34,881 | Temporal Sclerosis | ||
| 34,889 | Other Conditions of Brain | ||
| 3490 | Reaction to Spinal or Lumbar Puncture | ||
| 3492 | Disorders of Meninges, not Elsewhere Classified | ||
| 34,981 | Cerebrospinal Fluid Rhinorrhea | ||
| 34,989 | Other Specified Disorders of Nervous System | ||
| 3499 | Unspecified Disorders of Nervous System | ||
| 3561 | Peroneal Muscular Atrophy | ||
| 7802 | Syncope and Collapse | ||
| 78,093 | Memory Loss | ||
| 7843 | Aphasia | ||
| 99,701 | Central Nervous System Complication | ||
| 99,709 | Other Nervous System Complications |
References
- Spellacy, W.N.; Gravem, H.; Fisch, R.O. The umbilical cord complications of true knots, nuchal coils, and cords around the body. Report from the collaborative study of cerebral palsy. Am. J. Obstet. Gynecol. 1966, 94, 1136–1142. [Google Scholar] [CrossRef]
- Sepulveda, W.; Shennan, A.H.; Bower, S.; Nicolaidis, P.; Fisk, N.M. True knot of the umbilical cord: A difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet. Gynecol. 1995, 5, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Sheiner, E.; Bashiri, A.; Shoham-Vardi, I.; Mazor, M. Is there a higher prevalence of pregnancy complications in a live-birth preceding the appearance of recurrent abortions? Arch. Gynecol. Obstet. 2005, 271, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, R.; Silberstein, T.; Sheiner, E.; Shoham-Vardi, I.; Holcberg, G.; Katz, M.; Mazor, M. Risk factors associated with true knots of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 36–39. [Google Scholar] [CrossRef]
- Maymon, E.; Ghezzi, F.; Shoham-Vardi, I.; Franchi, M.; Silberstein, T.; Wiznitzer, A.; Mazor, M. Isolated hydramnios at term gestation and the occurrence of peripartum complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 77, 157–161. [Google Scholar] [CrossRef]
- Blickstein, I.; Shoham-Schwartz, Z.; Lancet, M. Predisposing factors in the formation of true knots of the umbilical cord—Analysis of morphometric and perinatal data. Int. J. Gynaecol. Obstet. 1987, 25, 395–398. [Google Scholar] [CrossRef]
- Didion, S.P.; Kinzenbaw, D.A.; Schrader, L.I.; Chu, Y.; Faraci, F.M. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 2009, 54, 619–624. [Google Scholar] [CrossRef]
- Maher, J.T.; Conti, J.A. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet. Gynecol. 1996, 88, 863–866. [Google Scholar] [CrossRef]
- Jauniaux, E.; Campbell, S.; Vyas, S. The use of color Doppler imaging for prenatal diagnosis of umbilical cord anomalies: Report of three cases. Am. J. Obstet. Gynecol. 1989, 161, 1195–1197. [Google Scholar] [CrossRef]
- Gutvirtz, G.; Walfisch, A.; Beharier, O.; Sheiner, E. Isolated single umbilical artery is an independent risk factor for perinatal mortality and adverse outcomes in term neonates. Arch. Gynecol. Obstet. 2016, 294, 931–935. [Google Scholar] [CrossRef]
- Airas, U.; Heinonen, S. Clinical Significance of True Umbilical Knots: A Population-Based Analysis. Am. J. Perinatol. 2002, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ohana, O.; Holcberg, G.; Sergienko, R.; Sheiner, E. Risk factors for intrauterine fetal death (1988–2009). J. Matern. Neonatal Med. 2011, 24, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Matorras, R.; Diez, J.; Pereira, J.G.; Montoya, F.; de Terán, G.G.; Aranguren, G.; Rodriguez-Escudero, F.J. True knots in the umbilical cord: Clinical findings and fetal consequences. J. Obstet. Gynaecol. 1990, 10, 383–386. [Google Scholar] [CrossRef]
- McLennan, H.; Price, E.; Urbanska, M.; Craig, N.; Fraser, M. Umbilical cord knots and encirclements. Aust. N. Z. J. Obstet. Gynaecol. 1988, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.; Georgiadis, L.; Harju, M.; Keski-Nisula, L.; Heinonen, S. True umbilical cord knot and obstetric outcome. Int. J. Gynecol. Obstet. 2013, 122, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Lackman, F.; Capewell, V.; Gagnon, R.; Richardson, B. Fetal umbilical cord oxygen values and birth to placental weight ratio in relation to size at birth. Am. J. Obstet. Gynecol. 2001, 185, 674–682. [Google Scholar] [CrossRef]
- Chasnoff, I.J.; Fletcher, M.A. True knot of the umbilical cord. Am. J. Obstet. Gynecol. 1977, 127, 425–427. [Google Scholar] [CrossRef]
- Estis-Deaton, A.; Sheiner, E.; Wainstock, T.; Landau, D.; Walfisch, A. The association between inadequate prenatal care and future healthcare use among offspring in the Bedouin population. Int. J. Gynaecol. Obstet. 2017, 139, 284–289. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Sheiner, E.; Sherf, M.; Wiznitzer, A.; Sergienko, R.; Shoham-Vardi, I. Lack of prenatal care in a traditional community: Trends and perinatal outcomes. Arch. Gynecol. Obstet. 2012, 285, 1237–1242. [Google Scholar] [CrossRef]
- Sheiner, E.; Shoham-Vardi, I.; Weitzman, D.; Gohar, J.; Carmi, R. Decisions regarding pregnancy termination among Bedouin couples referred to third level ultrasound clinic. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 76, 141–146. [Google Scholar] [CrossRef]
- Sheiner, E.; Shoham-Vardi, I.; Ohana, E.; Segal, D.; Mazor, M.; Katz, M. Characteristics of parturients who choose to deliver without analgesia. J. Psychosom. Obstet. Gynaecol. 1999, 20, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Amir, H.; Abokaf, H.; Levy, Y.A.; Azem, F.; Sheiner, E. Bedouin Women’s Gender Preferences When Choosing Obstetricians and Gynecologists. J. Immigr. Minority Health 2018, 20, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Treister-Goltzman, Y.; Peleg, R. Health and Morbidity Among Bedouin Women in Southern Israel: A Descriptive Literature Review of the Past Two Decades. Journal of Community Health; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2014; Volume 39, pp. 819–825. [Google Scholar]
- Lewando-Hundt, G.; Shoham-Vardi, I.; Beckerleg, S.; Belmaker, I.; Kassem, F.; Jaafar, A.A. Knowledge, action and resistance: The selective use of pre-natal screening among Bedouin women of the Negev, Israel. Soc. Sci. Med. 2001, 52, 561–569. [Google Scholar] [CrossRef]
- Pariente, G.; Wainstock, T.; Walfisch, A.; Landau, D.; Sheiner, E. Placental abruption and long-term neurological hospitalisations in the offspring. Paediatr. Perinat. Epidemiol. 2019, 33, 215–222. [Google Scholar] [CrossRef]
- Weiner, E.; Fainstein, N.; Schreiber, L.; Sagiv, R.; Bar, J.; Kovo, M. The association between umbilical cord abnormalities and the development of non-reassuring fetal heart rate leading to emergent cesarean deliveries. J. Perinatol. 2015, 35, 919–923. [Google Scholar] [CrossRef]
- Lai, S.; Flatley, C.; Kumar, S. Perinatal risk factors for low and moderate five-minute Apgar scores at term. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 251–256. [Google Scholar] [CrossRef]
| Perinatal Outcomes | True Knot of Cord % (n = 2606) * | No True Knot of Cord % (n = 241,076) | Odds Ratio (Confidence Interval) | p-Value | ||
|---|---|---|---|---|---|---|
| Ethnicity | Jewish | 60 (1572) | 47.2 (113,782) | <0.001 | ||
| Bedouin | 39.7 (1034) | 52.8 (127,294) | <0.001 | |||
| Mean maternal age (years, mean ± SD) | 30.2 ± 5.9 | 28.1 ± 5.8 | <0.001 | |||
| Parity | 1 | 15.5 (405) | 23.7 (57,100) | <0.001 | ||
| 2–4 | 54.8 (1428) | 51.1 (123,086) | ||||
| ≥5 | 29.7 (773) | 25.2 (60,837) | ||||
| Maternal Diabetes | 8.4 (220) | 5 (11,939) | 1.77 (1.539–2.034) | <0.001 | ||
| Maternal Hypertension | 7.4 (192) | 5 (12,055) | 1.511 (1.303–1.752) | <0.001 | ||
| Mean gestational age (weeks, mean ± SD **) | 38.8 ± 2.4 | 39.1 ± 1.09 | <0.001 | |||
| Preterm delivery (<37 0/7 weeks of gestation) | 10.5 (274) | 6.8 (16,446) | 1.605 (1.415–1.821) | <0.001 | ||
| Induced labor | 28.7 (747) | 26.1 (62,897) | 1.138 (1.045–1.24) | 0.003 | ||
| Cesarean delivery | 17.4 (453) | 13.5 (32,573) | 1.347 (1.216–1.491) | <0.001 | ||
| Placental abruption | 0.8 (21) | 0.6 (1338) | 1.456 (0.944–2.244) | 0.087 | ||
| Meconium stained amniotic fluid | 18.9 (493) | 14.7 (35,399) | 1.356 (1.228–1.496) | <0.001 | ||
| Low (<7) 1 min Apgar score | 7.3 (190) | 5.3 (12,800) | 1.403 (1.209–1.627) | <0.001 | ||
| Low (<7) 5 min Apgar score | 2.9 (76) | 2.3 (5433) | 1.303 (1.035–1.639) | 0.024 | ||
| Perinatal mortality | Total perinatal mortality | 1.8 (48) | 0.5 (1292) | 3.483 (2.604–4.658) | <0.001 | |
| Intra uterine | 1.5 (39) | 0.3 (713) | 5.122 (3.702–7.086) | <0.001 | ||
| Intra-partum | 0.1 (2) | 0.024 (60) | 3.085 (0.754–12.629) | 0.099 | ||
| Immediately post-partum | 0.3 (7) | 0.2 (519) | 1.248 (0.592–2.634) | 0.560 | ||
| Mean birth weight (grams, mean ± SD) | 3209 ± 564 | 3205 ± 510 | 0.73 | |||
| Low birth weight (<2500 g) | 9.2 (239) | 6.7 (16,165) | 1.405 (1.229–1.606) | <0.001 | ||
| Male gender | 61.6 (1604) | 50.7 (122,273) | 1.555 (1.437–1.684) | <0.001 | ||
| Female gender | 38.4 (1002) | 49.3 (118,803) | 1.555 (1.437–1.684) | <0.001 | ||
| Adjusted Odds Ratio (Confidence Interval) | p-Value | |
|---|---|---|
| True knot of cord | 3.606 (2.685–4.841) | <0.001 |
| Ethnicity (Jewish compared to Bedouin) | 0.595 (0.529–0.668) | <0.001 |
| Smoking | 1.52 (0.909–2.54) | 0.11 |
| Maternal diabetes | 0.628 (0.463–0.852) | 0.003 |
| Maternal Hypertension | 2.089 (1.733–2.518) | <0.001 |
| Birth year | 0.937 (0.929–0.946) | <0.001 |
| Neurological Morbidity | True Knot of Cord % (n = 2558) | No Knot of Cord % (n = 239,784) | p-Value |
|---|---|---|---|
| Autistic spectrum disorders | 0.0003 (1) | 0.0001 (27) | 0.193 |
| Eating disorders | 0.2 (6) | 0.2 (429) | 0.508 |
| Sleeping disorders | 0.0003 (1) | 0.0001 (47) | 0.486 |
| Movement disorders | 2.2 (56) | 1.8 (4416) | 0.194 |
| Cerebral palsy | 0.1 (2) | 0.1 (199) | 0.933 |
| Psychiatric emotional | 0.5 (12) | 0.5 (1183) | 0.862 |
| Attention deficit disorders | 0.2 (4) | 0.1 (139) | 0.041 |
| Developmental disorders | 0.2 (5) | 0.1 (234) | 0.117 |
| Degenerative, demyelination | 0.03 (1) | 0.1 (180) | 0.508 |
| Headache | 0 (0) | 0.0002 (54) | 0.448 |
| Myopathy | 0.1 (2) | 0.1 (136) | 0.651 |
| Other | 0.4 (10) | 0.4 (907) | 0.917 |
| Total Neurological hospitalizations | 3.7 (95) | 3.1 (7448) | 0.078 |
| Adjusted Hazard Ratio (Confidence Interval) | p Value | |
|---|---|---|
| True knot of cord | 1.236 (0.728–2.1) | 0.432 |
| Diabetes | 1.143 (0.87–1.501) | 0.337 |
| Hypertension | 1.249 (1.017–1.534) | 0.034 |
| Maternal age (at birth) | 0.993 (0.981–1.005) | 0.265 |
| Child birth year | 1.092 (1.076–1.109) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtman, Y.; Wainstock, T.; Walfisch, A.; Sheiner, E. The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health. J. Clin. Med. 2021, 10, 123. https://doi.org/10.3390/jcm10010123
Lichtman Y, Wainstock T, Walfisch A, Sheiner E. The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health. Journal of Clinical Medicine. 2021; 10(1):123. https://doi.org/10.3390/jcm10010123
Chicago/Turabian StyleLichtman, Yael, Tamar Wainstock, Asnat Walfisch, and Eyal Sheiner. 2021. "The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health" Journal of Clinical Medicine 10, no. 1: 123. https://doi.org/10.3390/jcm10010123
APA StyleLichtman, Y., Wainstock, T., Walfisch, A., & Sheiner, E. (2021). The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health. Journal of Clinical Medicine, 10(1), 123. https://doi.org/10.3390/jcm10010123







