Ceria-Based Dual-Phase Membranes for High-Temperature Carbon Dioxide Separation: Effect of Iron Doping and Pore Generation with MgO Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. SDC-FSDC/MgO/MC Membrane Preparation
2.2. Characterization Methods
2.3. Membrane Performance
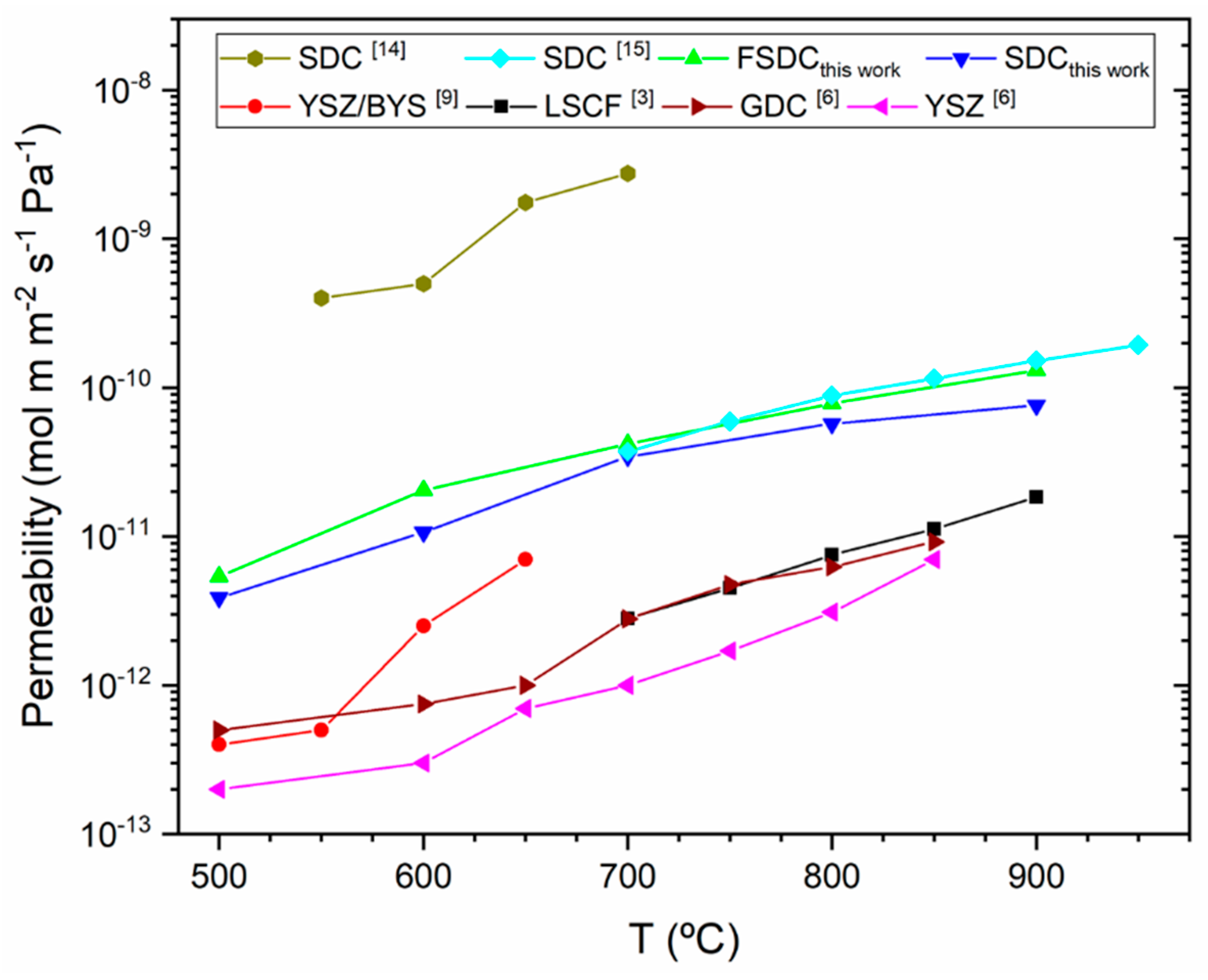
3. Results
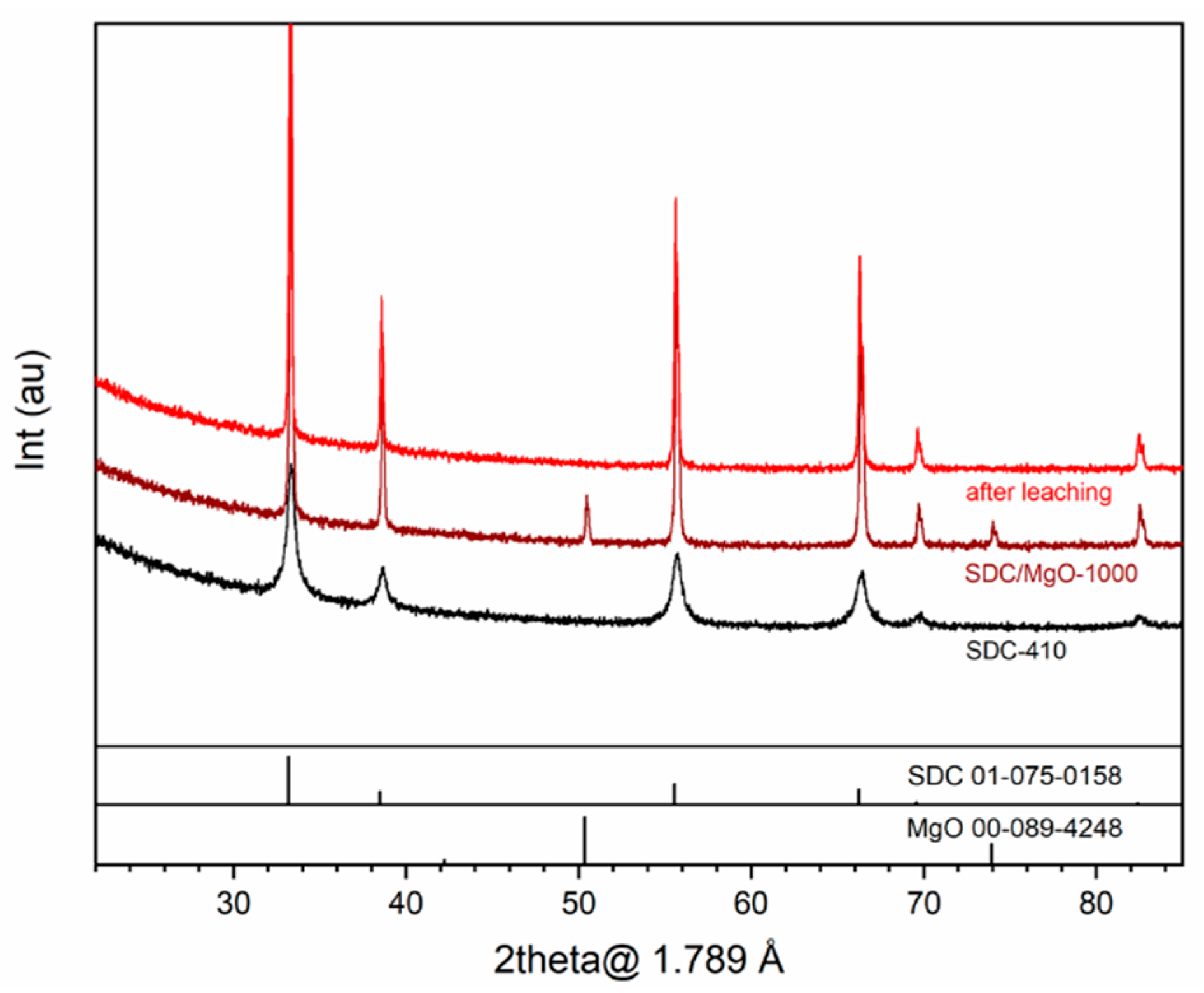
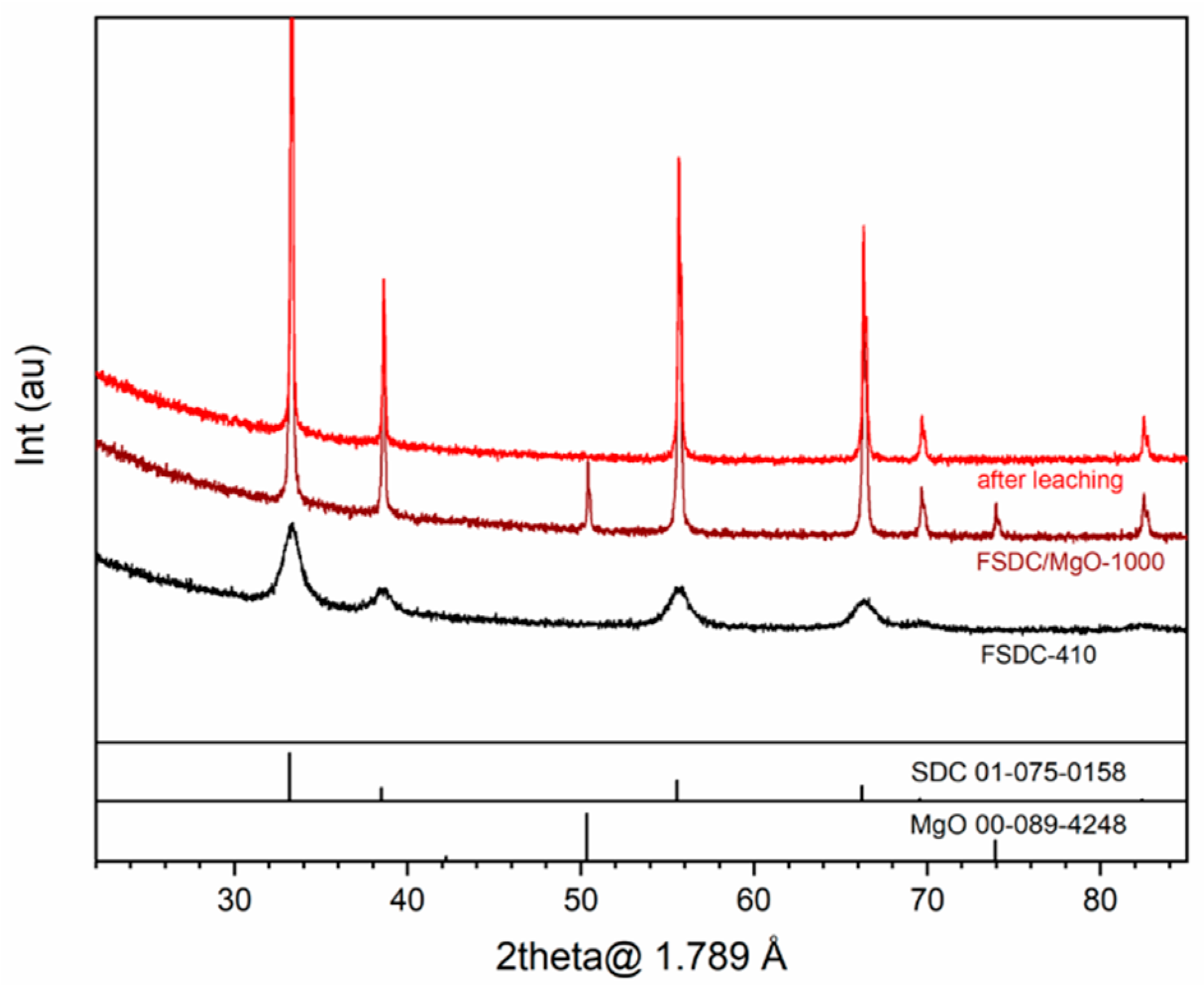
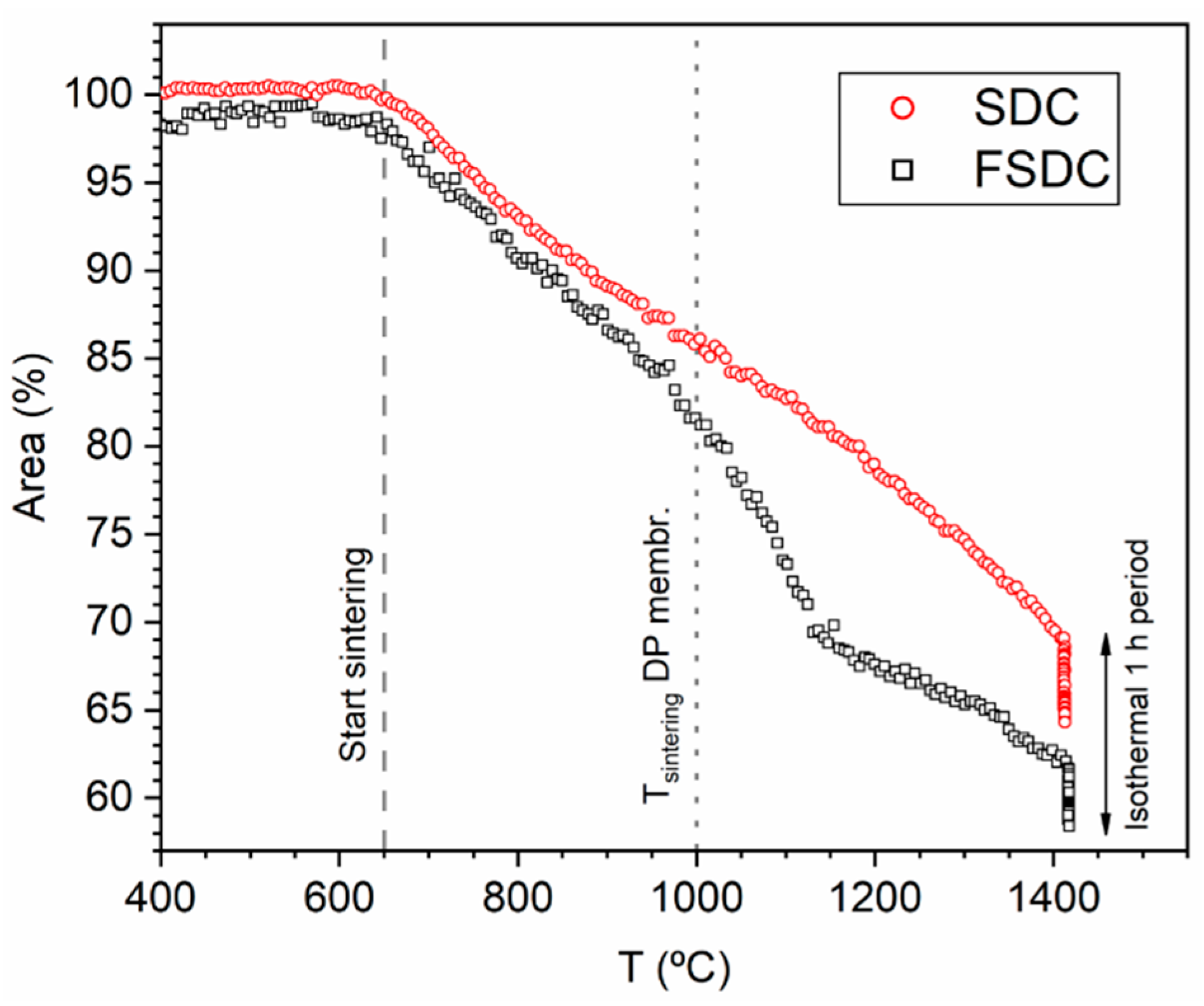
3.1. Elemental and Phase Composition and Sintering Behavior of Membrane Materials
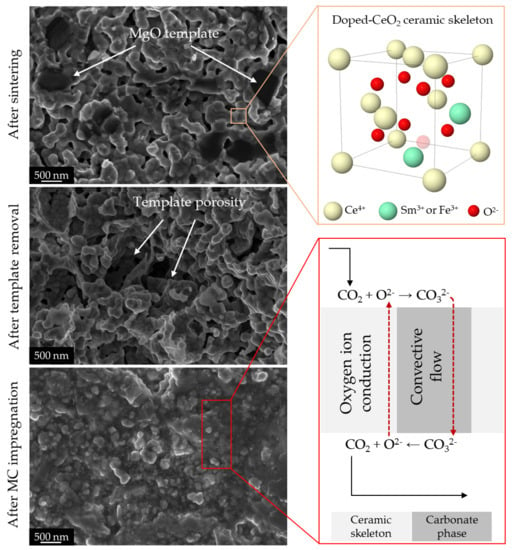
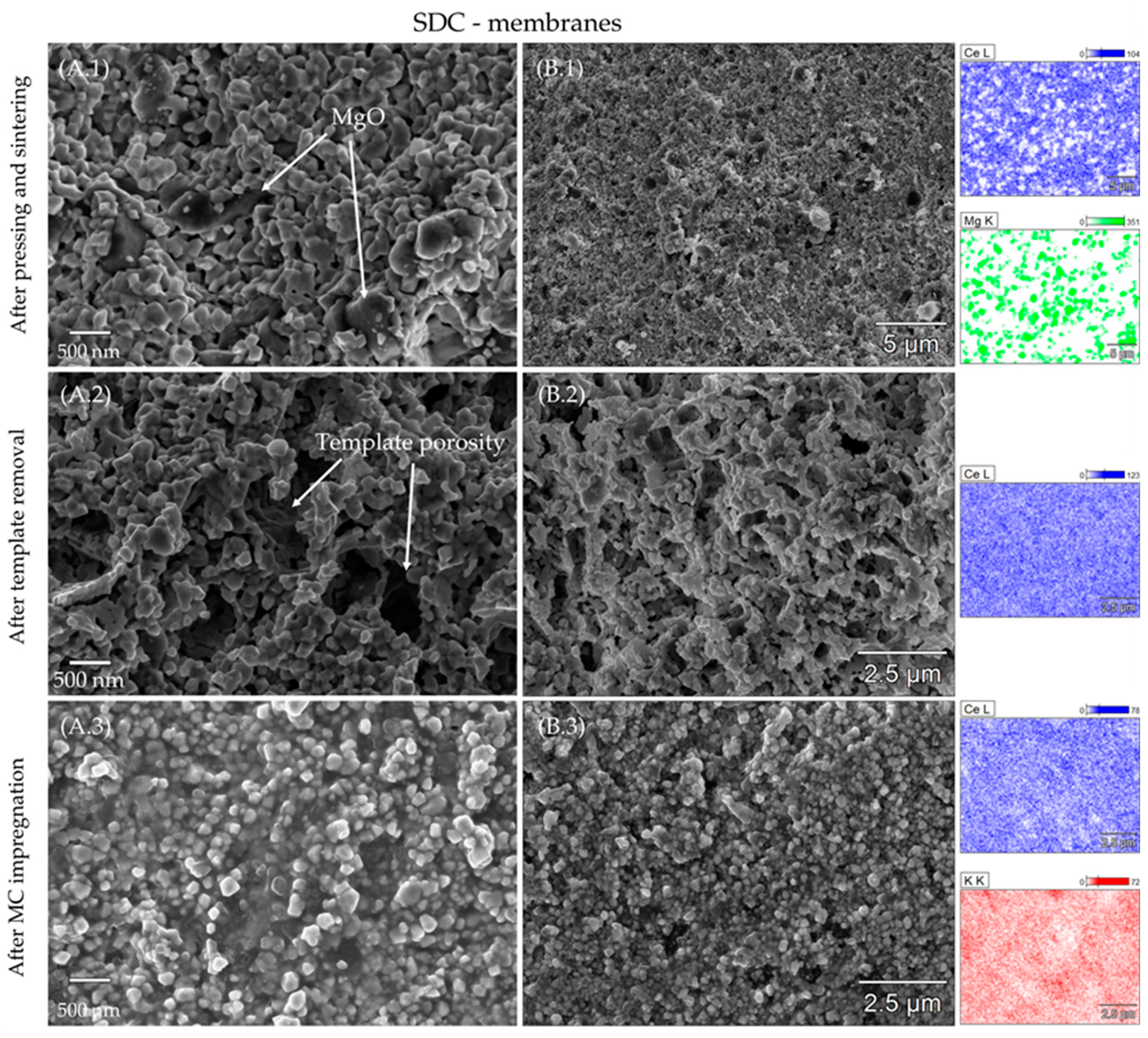
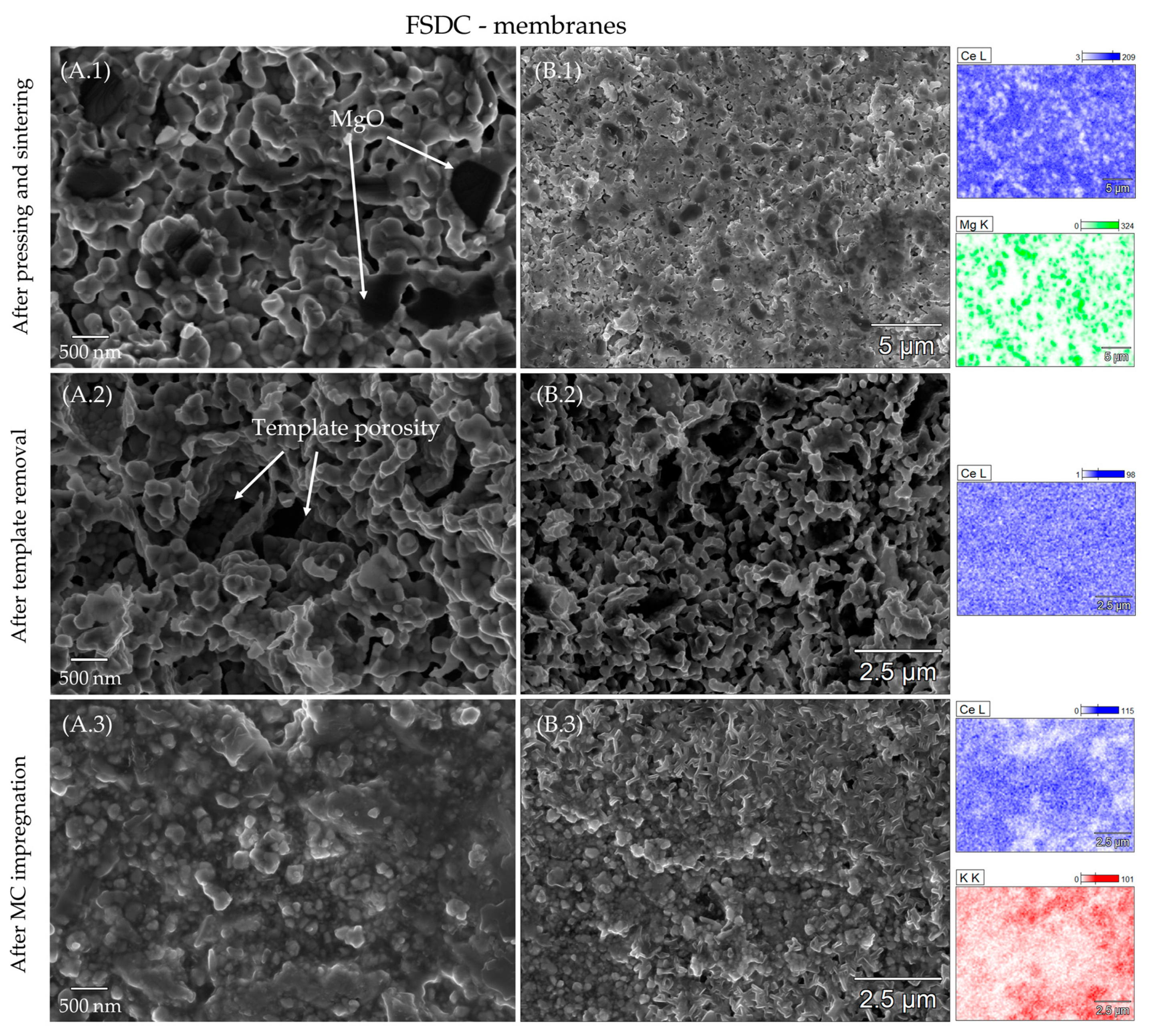
3.2. Porosity and Element Distribution of the Sintered Membranes
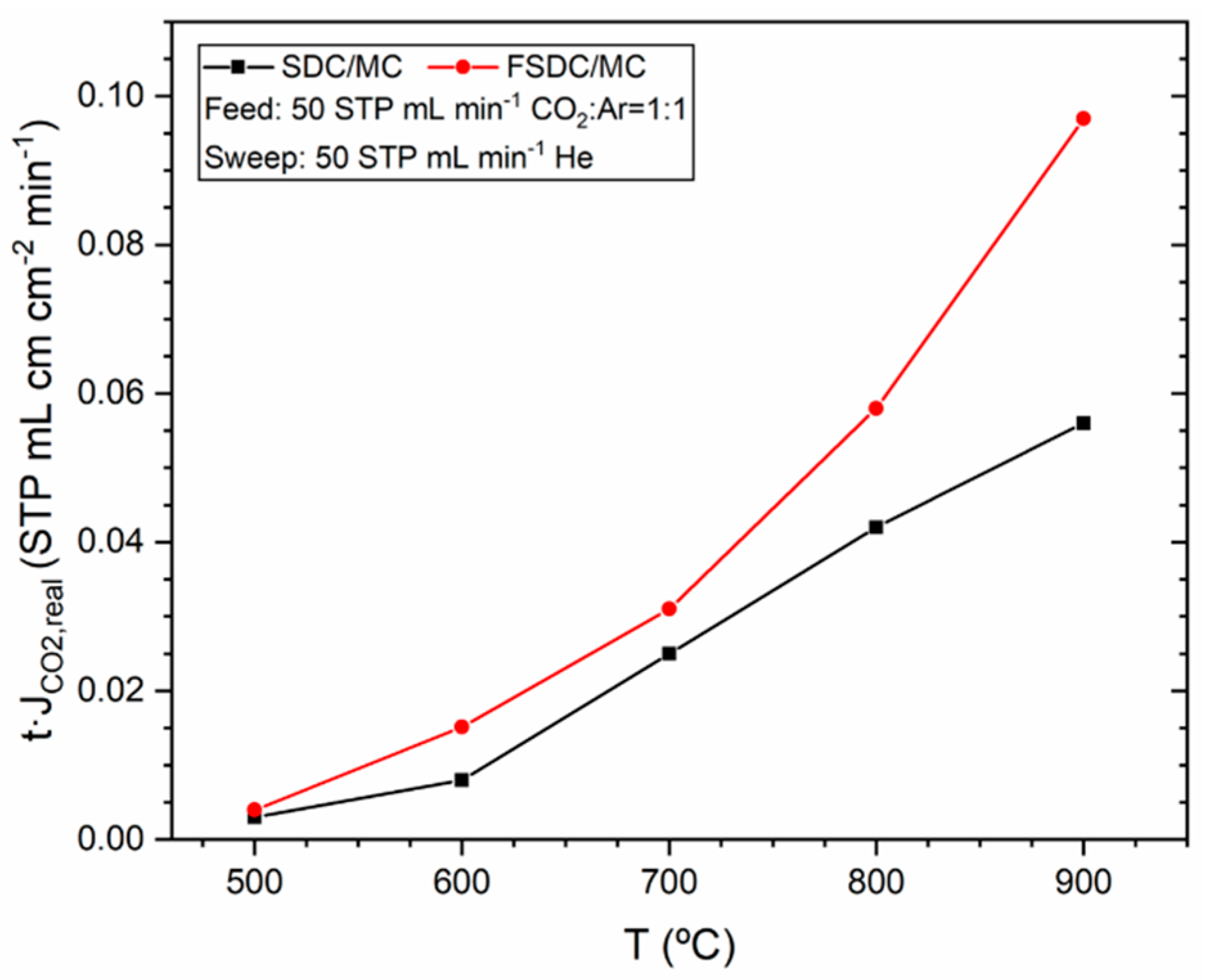
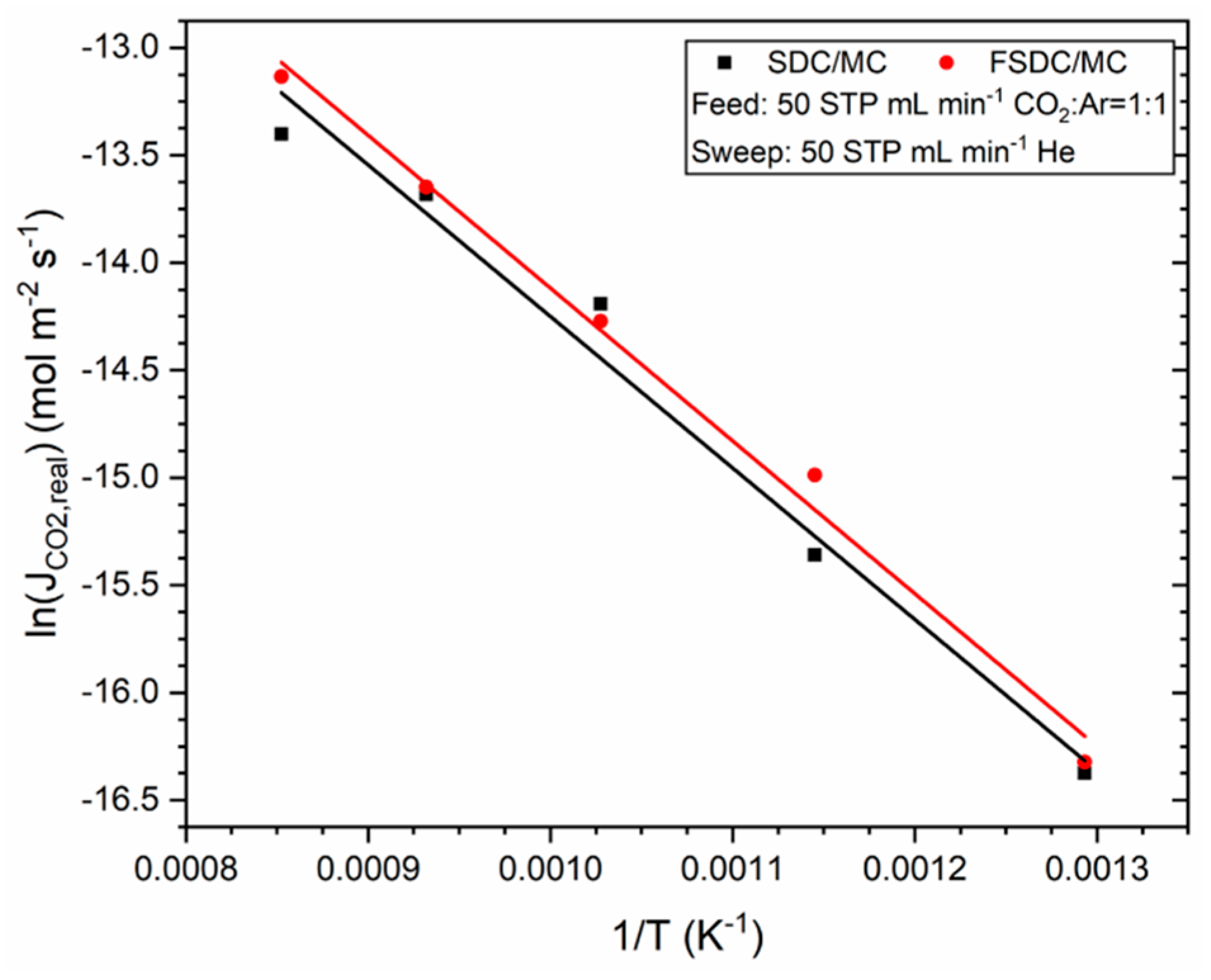
3.3. Membrane Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations and Symbols
| Symbol | Description | Units |
| Abbreviations | ||
| DP | Dual-phase | - |
| FSDC | Iron and samarium co-doped ceria | - |
| HSM | Hot stage microscopy | - |
| ICP-OES | Inductively coupled plasma optical emission spectrometry | - |
| MC | Molten carbonate | - |
| SDC | Samarium doped ceria | - |
| SEM/EDX | Scanning electron microscopy/energy-dispersive X-ray spectroscopy | - |
| XRD | X-ray diffraction | - |
| Symbols | ||
| A | Membrane effective area | cm−2 |
| a | Lattice parameter | cm or Å |
| J | Flux density | mL min−1 cm−2 |
| K | Membrane permeability | mol m m−2 s−1 Pa−1 |
| NA | Avogadro’s number | - |
| P | Pressure | Pa |
| Q | Volumetric flow rate | mL min−1 |
| t | Thickness | mm |
| X | Component fraction or ratio | - |
| Z | Formula units per unit cell | - |
| Greek letters | ||
| ρ | Density | g cm−3 |
| ϕ | Porosity | - |
| Subindex | ||
| a.l. | After leaching | - |
| b.l. | Before leaching | - |
| c | Ceramic phase (SDC or FSDC) | - |
| i | species, usually gas Ar, CO2 or He | - |
| inlet | Referring to the feed gas inlet in the membrane system | - |
| leak | Flux density through cracks and defective sealing | - |
| m | Membrane | - |
| MC | Molten carbonate | - |
| MgO | MgO template | - |
| permeate | Referring to the permeate out of the membrane system | - |
| real | Real gas flux density | - |
| sweep | Referring to the sweep gas inlet in the membrane system | - |
| theor | Theoretical mass of the leached membranes | - |
| total | Total flux density (real + leak) | - |
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Chung, S.J.; Park, J.H.; Li, D.; Ida, J.-I.; Kumakiri, I.; Lin, J.Y.S. Dual-Phase Metal−Carbonate Membrane for High-Temperature Carbon Dioxide Separation. Ind. Eng. Chem. Res. 2005, 44, 7999–8006. [Google Scholar] [CrossRef]
- Anderson, M.; Lin, Y.; Lin, J. Carbonate–ceramic dual-phase membrane for carbon dioxide separation. J. Membr. Sci. 2010, 357, 122–129. [Google Scholar] [CrossRef]
- Wade, J.L.; Lackner, K.S.; West, A.C. Transport model for a high temperature, mixed conductiong CO2 separation membrane. Solid State Ionics 2007, 178, 1530–1540. [Google Scholar] [CrossRef]
- Rui, Z.; Anderson, M.; Lin, Y.; Li, Y.; Lin, J. Modeling and analysis of carbon dioxide permeation through ceramic-carbonate dual-phase membranes. J. Membr. Sci. 2009, 345, 110–118. [Google Scholar] [CrossRef]
- Gude, U.; Baumann, S.; Meulenberg, W.A.; Müller, M. Towards the development of materials for chemically stable carbonate-ceramic membranes to be used for CO2 separation in water-gas-shift reactors. Sep. Purif. Technol. 2019, 215, 378–383. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, J.; Huang, K. Role of CO2 in Catalytic Ethane-to-Ethylene Conversion Using a High-Temperature CO2 Transport Membrane Reactor. ACS Sustain. Chem. Eng. 2019, 7, 6889–6897. [Google Scholar] [CrossRef]
- Wade, J.L.; Lee, C.; West, A.C.; Lackner, K.S. Composite electrolyte membranes for high temperature CO2 separation. J. Membr. Sci. 2011, 369, 20–29. [Google Scholar] [CrossRef]
- Norton, T.T.; Ortiz-Landeros, J.; Lin, Y.S. Stability of La–Sr–Co–Fe Oxide-Carbonate Dual-Phase Membranes for Carbon Dioxide Separation at High Temperatures. Ind. Eng. Chem. Res. 2014, 53, 2432–2440. [Google Scholar] [CrossRef]
- Norton, T.T.; Lin, J. Ceramic–carbonate dual-phase membrane with improved chemical stability for carbon dioxide separation at high temperature. Solid State Ionics 2014, 263, 172–179. [Google Scholar] [CrossRef]
- Lu, B.; Lin, J. Synthesis and characterization of thin ceramic-carbonate dual-phase membranes for carbon dioxide separation. J. Membr. Sci. 2013, 444, 402–411. [Google Scholar] [CrossRef]
- Zuo, M.; Zhuang, S.; Tan, X.; Meng, B.; Yang, N.; Liu, S. Ionic conducting ceramic–carbonate dual phase hollow fibre membranes for high temperature carbon dioxide separation. J. Membr. Sci. 2014, 458, 58–65. [Google Scholar] [CrossRef]
- Patrício, S.; Papaioannou, E.; Zhang, G.; Metcalfe, I.; Marques, F.; Patrício, S.; Marques, F. High performance composite CO2 separation membranes. J. Membr. Sci. 2014, 471, 211–218. [Google Scholar] [CrossRef]
- Patrício, S.G.; Soares, C.M.; Santos, C.F.; Figueiredo, F.M.; Marques, F.M. Ceria-based substrates for CO2 separation membranes. Solid State Ionics 2014, 262, 248–252. [Google Scholar] [CrossRef]
- Rui, Z.; Anderson, M.; Li, Y.; Lin, Y.; Lin, J. Ionic conducting ceramic and carbonate dual phase membranes for carbon dioxide separation. J. Membr. Sci. 2012, 417, 174–182. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, N.; Li, X.; Wang, S.; Huang, K.; Harris, W.H.; Chiu, W.K.S. High CO2 permeation flux enabled by highly interconnected three-dimension ionic channels in selective CO2 separation membranes. Energy Environ. Sci. 2012, 5, 8310–8317. [Google Scholar] [CrossRef]
- Norton, T.T.; Lu, B.; Lin, J. Carbon dioxide permeation properties and stability of samarium-doped-ceria carbonate dual-phase membranes. J. Membr. Sci. 2014, 467, 244–252. [Google Scholar] [CrossRef]
- Pandiyan, A.; Preethi, S.; Babu, K.S. Role of iron addition on grain boundary conductivity of pure and samarium doped cerium oxide. RSC Adv. 2014, 4, 44367–44376. [Google Scholar]
- Zhang, T.; Ma, J.; Kong, L.B.; Chan, S.; Hing, P.; Kilner, J. Iron oxide as an effective sintering aid and a grain boundary scavenger for ceria-based electrolytes. Solid State Ionics 2004, 167, 203–207. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, M.; Ge, L.; Li, S.; Chen, H.; Guo, L. Effect of Fe2O3 on Sm-doped ceria system solid electrolyte for IT-SOFCs. J. Alloys Compd. 2011, 509, 546–550. [Google Scholar] [CrossRef]
- TianShu, Z.; Hing, P.; Huang, H.; Kilner, J. Early-stage sintering mechanisms of Fe-doped CeO2. J. Mater. Sci. 2002, 37, 997–1003. [Google Scholar] [CrossRef]
- Mutch, G.A.; Qu, L.; Triantafyllou, G.; Xing, W.; Fontaine, M.-L.; Metcalfe, I.S. Supported molten-salt membranes for carbon dioxide permeation. J. Mater. Chem. A 2019, 7, 12951–12973. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Cheng, S.; Wan, B.-Z. Porous Ag–CGO cermets as anode materials for ITSOFC using CO fuel. Catal. Commun. 2008, 9, 1595–1599. [Google Scholar] [CrossRef]
- Yin, Q.; Lin, Y.; Lin, J. Beneficial effect of order-disorder phase transition on oxygen sorption properties of perovskite-type oxides. Solid State Ionics 2007, 178, 83–89. [Google Scholar] [CrossRef]
- Norton, T.T. Synthesis and Stability of Ceramic-Carbonate Dual-Phase Membrane for Carbon Dioxide Separation. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2013. [Google Scholar]
- An, X.; Cheng, J.; Zhang, P.; Tang, Z.; Wang, J. Determination and evaluation of the thermophysical properties of an alkali carboante eutectic molten salt. Faraday Discuss. 2016, 190, 327–338. [Google Scholar] [CrossRef]
- Shand, M.A. The Chemistry and Technology of Magnesia; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rodríguez-Carvajal, J. Recent developments of the program FullProf. Comission on powder diffraction (IUCr). Newsletter 2001, 26, 12–16. [Google Scholar]
- Finger, L.W.; Cox, D.E.; Jephcoat, A.P. A correction for powder diffraction peak asymmetry due to axial divergence. J. Appl. Crystallogr. 1994, 27, 892–900. [Google Scholar] [CrossRef]
- Bhakar, A.; Pandey, A.H.; Singh, M.N.; Upadhyay, A.; Sinha, A.K.; Gupta, S.M.; Ganguli, T.; Rai, S.K. Effect of processing parameters on microstructural properties of lead magnesium niobates. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 1095–1104. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley-Interscience: Hoboken, NJ, USA, 1974. [Google Scholar]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Huang, W. Properties of sol-gel prepared Ce1−xSmxO2−x/2 solid electrolytes. Solid State Ionics 1997, 100, 23–27. [Google Scholar] [CrossRef]
- Hos, J.P.; McCormick, P.G. Mechanochemical synthesis and characterisation of nanoparticulate samarium-doped cerium oxide. Scr. Mater. 2003, 48, 85–90. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Grünbacher, M.; Schlicker, L.; Gili, A.; Doran, A.; Epping, J.D.; Gurlo, A.; Klötzer, B.; Penner, S. On the structural stability of crystalline ceria phases in undoped and acceptor-doped ceria materials under in situ reduction conditions. CrystEngComm 2019, 38, 439. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image. J. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
| Sample | Ceatomic (-) | Smatomic (-) | Featomic (-) | Siwt% (wt%) | Formula |
|---|---|---|---|---|---|
| SDC | 0.810 (s = 0.001) | 0.190 (s = 0.005) | 0.000 (s = 0.000) | 0.088 (s = 0.139) | Ce0.81Sm0.19O2-δ |
| FSDC | 0.797 (s = 0.003) | 0.192 (s = 0.003) | 0.011 (s = 0.001) | 0.255 (s = 0.399) | Ce0.797Sm0.192Fe0.011O2-δ |
| Sample 1 | Crystallite Size (nm) | Lattice Parameter (Å) |
|---|---|---|
| SDC-410 | 12.1 (s = 0.2) | 5.4376 (s = 0.0005) |
| SDC-1000-b.l. | 80.5 (s = 1.5) | 5.4360 (s = 0.0002) |
| SDC-1000-a.l. | >100 | 5.4353 (s = 0.0001) |
| FSDC-410 | 5.6 (s = 0.1) | 5.4331 (s = 0.0009) |
| FSDC-1000-b.l. | 88.9 (s = 1.6) | 5.4343 (s = 0.0001) |
| FSDC-1000-a.l. | >100 | 5.4353 (s = 0.0001) |
| Material | Porosity after Sintering (%) | Porosity after Leaching (%) | Leached MgO 1 (-) | Impregnated Carbonate 2 (-) |
|---|---|---|---|---|
| SDC-1000 | 20.3 | 44.6 | 0.99 | 1 |
| FSDC-1000 | 23.6 | 47.0 | 0.99 | 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gili, A.; Bischoff, B.; Simon, U.; Schmidt, F.; Kober, D.; Görke, O.; Bekheet, M.F.; Gurlo, A. Ceria-Based Dual-Phase Membranes for High-Temperature Carbon Dioxide Separation: Effect of Iron Doping and Pore Generation with MgO Template. Membranes 2019, 9, 108. https://doi.org/10.3390/membranes9090108
Gili A, Bischoff B, Simon U, Schmidt F, Kober D, Görke O, Bekheet MF, Gurlo A. Ceria-Based Dual-Phase Membranes for High-Temperature Carbon Dioxide Separation: Effect of Iron Doping and Pore Generation with MgO Template. Membranes. 2019; 9(9):108. https://doi.org/10.3390/membranes9090108
Chicago/Turabian StyleGili, Albert, Benjamin Bischoff, Ulla Simon, Franziska Schmidt, Delf Kober, Oliver Görke, Maged F. Bekheet, and Aleksander Gurlo. 2019. "Ceria-Based Dual-Phase Membranes for High-Temperature Carbon Dioxide Separation: Effect of Iron Doping and Pore Generation with MgO Template" Membranes 9, no. 9: 108. https://doi.org/10.3390/membranes9090108
APA StyleGili, A., Bischoff, B., Simon, U., Schmidt, F., Kober, D., Görke, O., Bekheet, M. F., & Gurlo, A. (2019). Ceria-Based Dual-Phase Membranes for High-Temperature Carbon Dioxide Separation: Effect of Iron Doping and Pore Generation with MgO Template. Membranes, 9(9), 108. https://doi.org/10.3390/membranes9090108