Development of Mass Production Technology of Highly Permeable Nano-Porous Supports for Silica-Based Separation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Structural Characterization
2.3. Gas Permeation Test
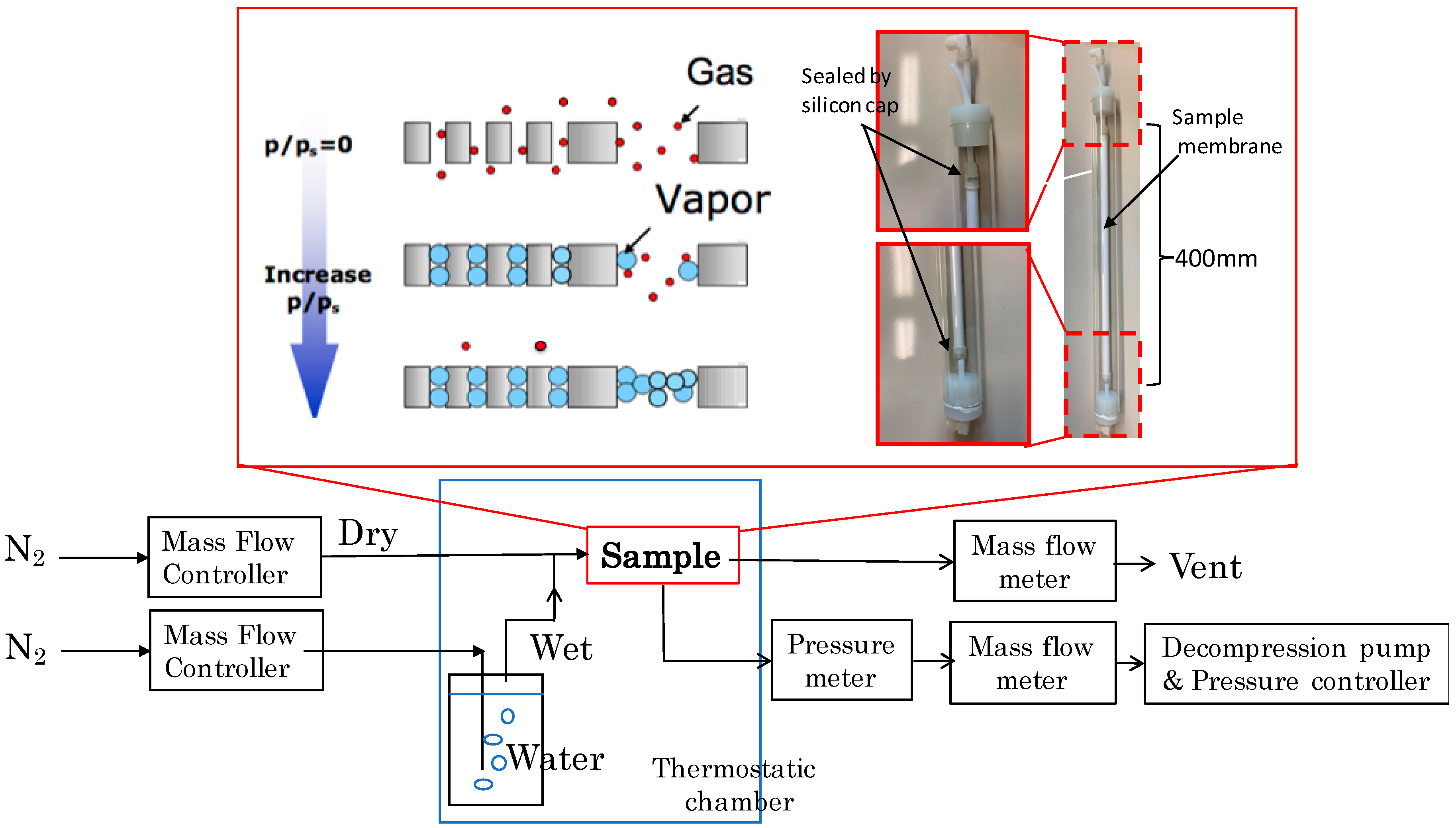
2.3.1. Nano-Permporometory
2.3.2. Single-Gas Permeation Test
3. Results
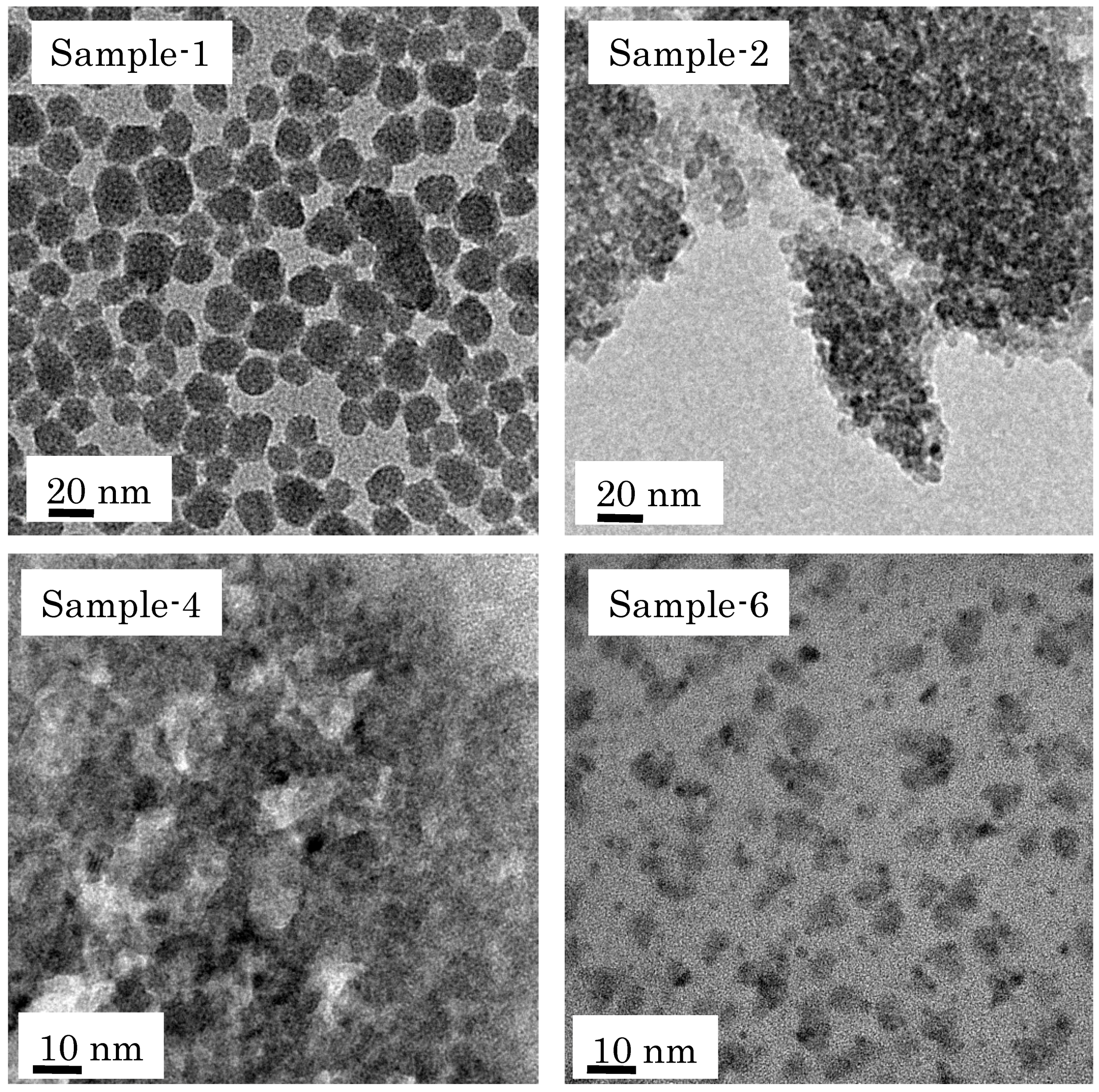
3.1. Characterization of Coating Nano-Particles
3.2. Characterization of Synthesized Silica-Based Nano-Porous Supports
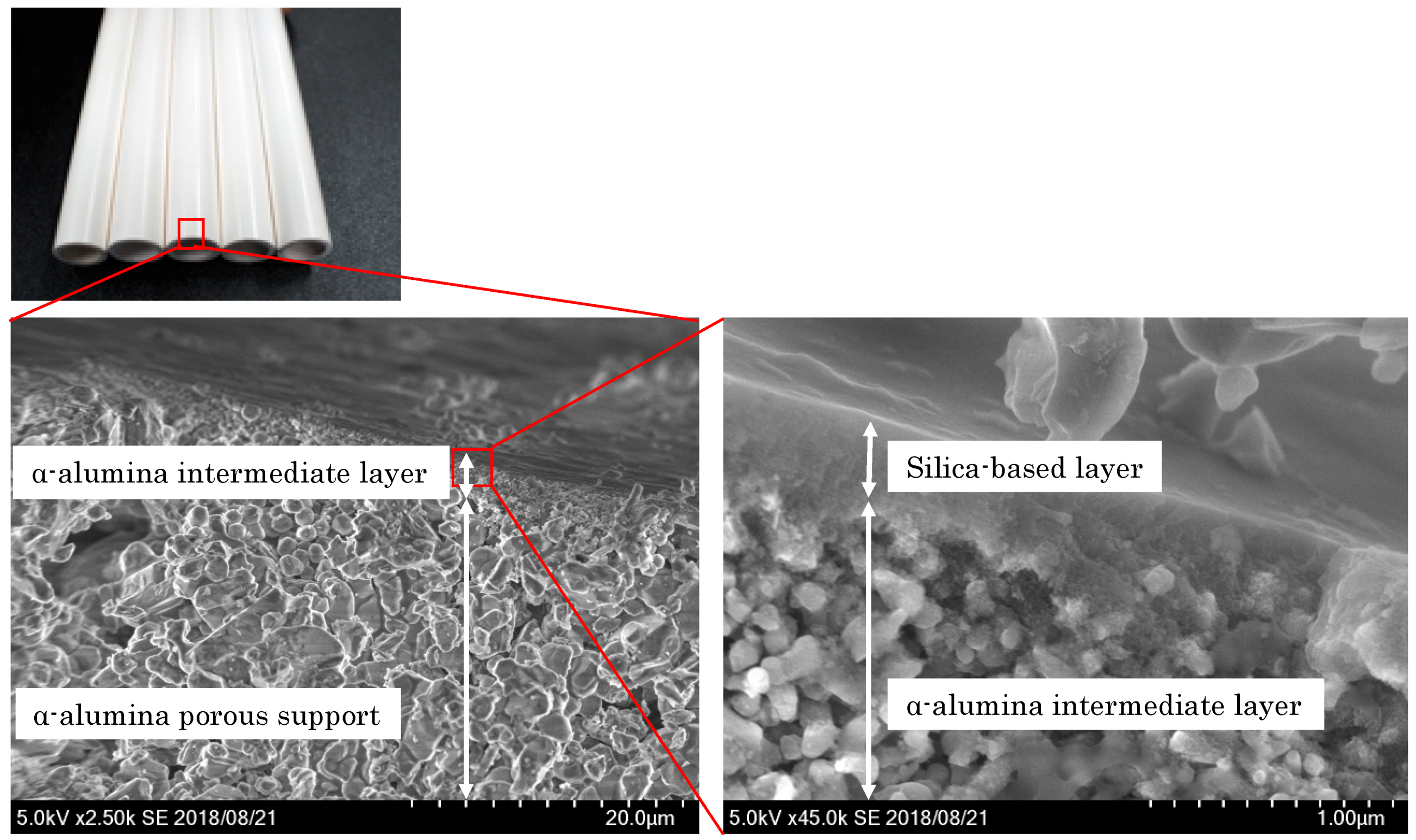
3.2.1. General Structure
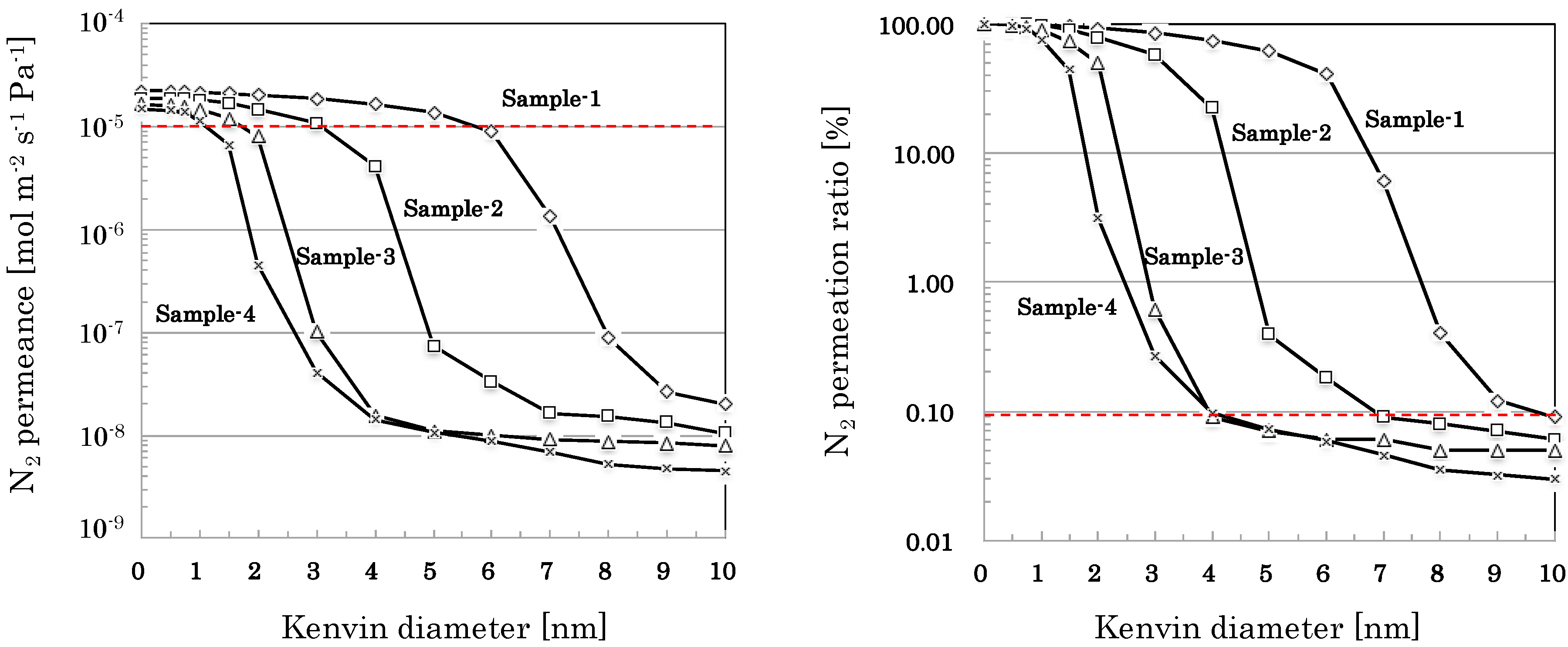
3.2.2. Pore Size Distribution of SiO2-Based Nano-Porous Supports
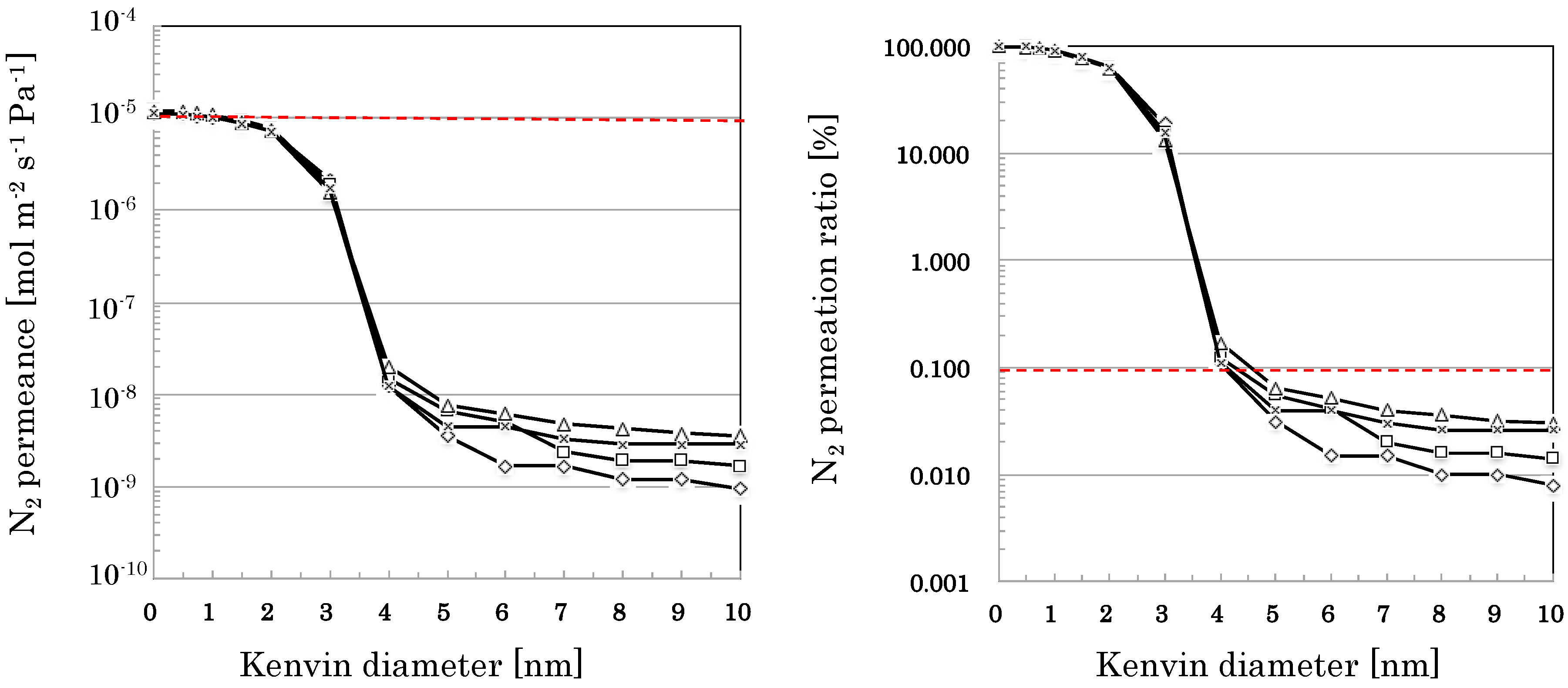
3.2.3. Reproducibility in the Flow-Type Production
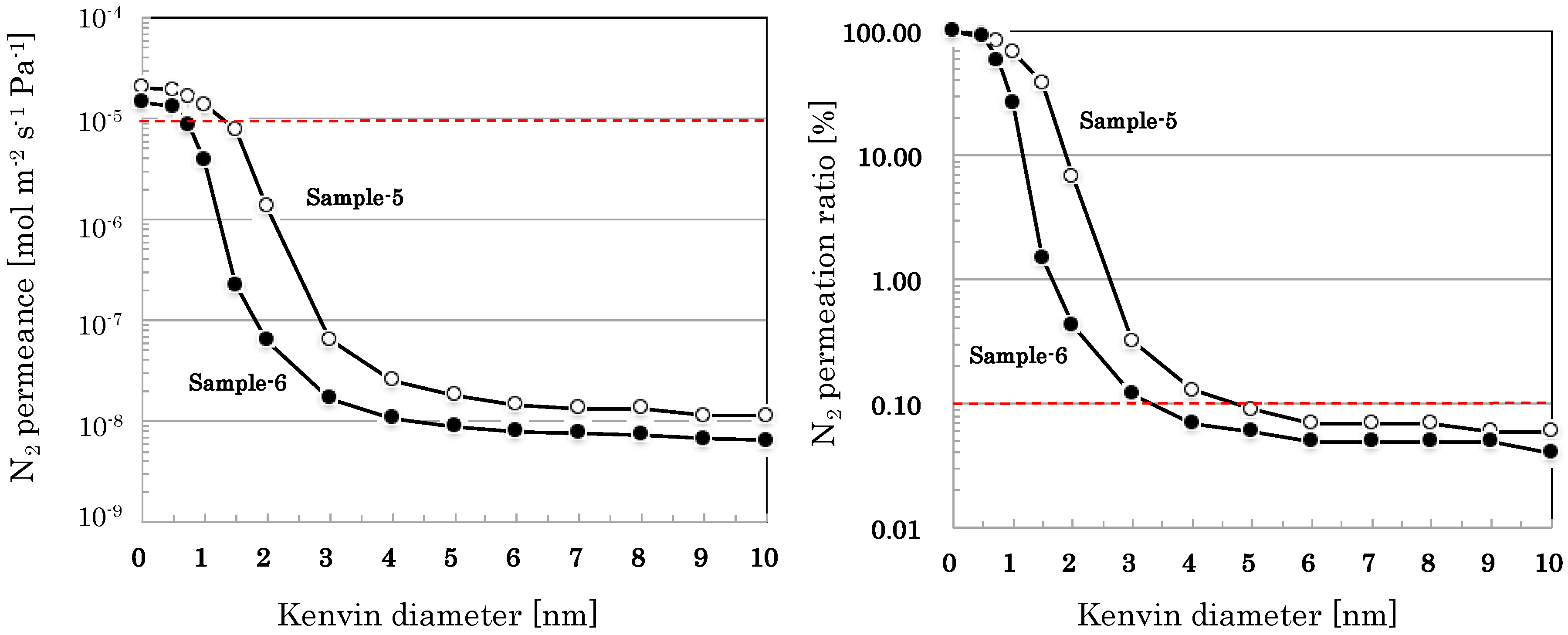
3.2.4. Pore Size Distribution of SiO2–ZrO2-Based Nano-Porous Supports
3.3. Characterization of Synthesized Organo-Silica Membranes
4. Discussion
4.1. Correlations Among Synthesis Conditions, Structural Properties, and Gas Permeation Properties
4.2. Quality of Nano-Porous Supports and Membranes
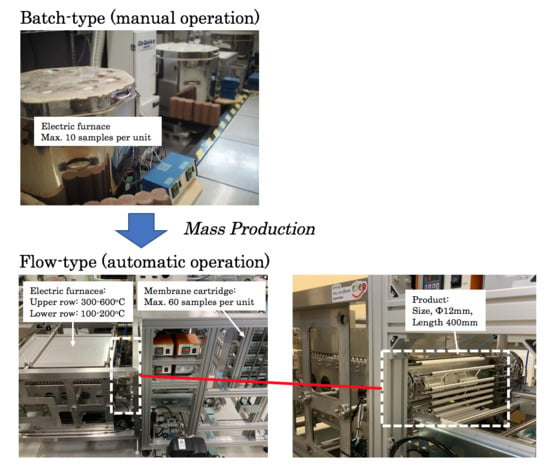
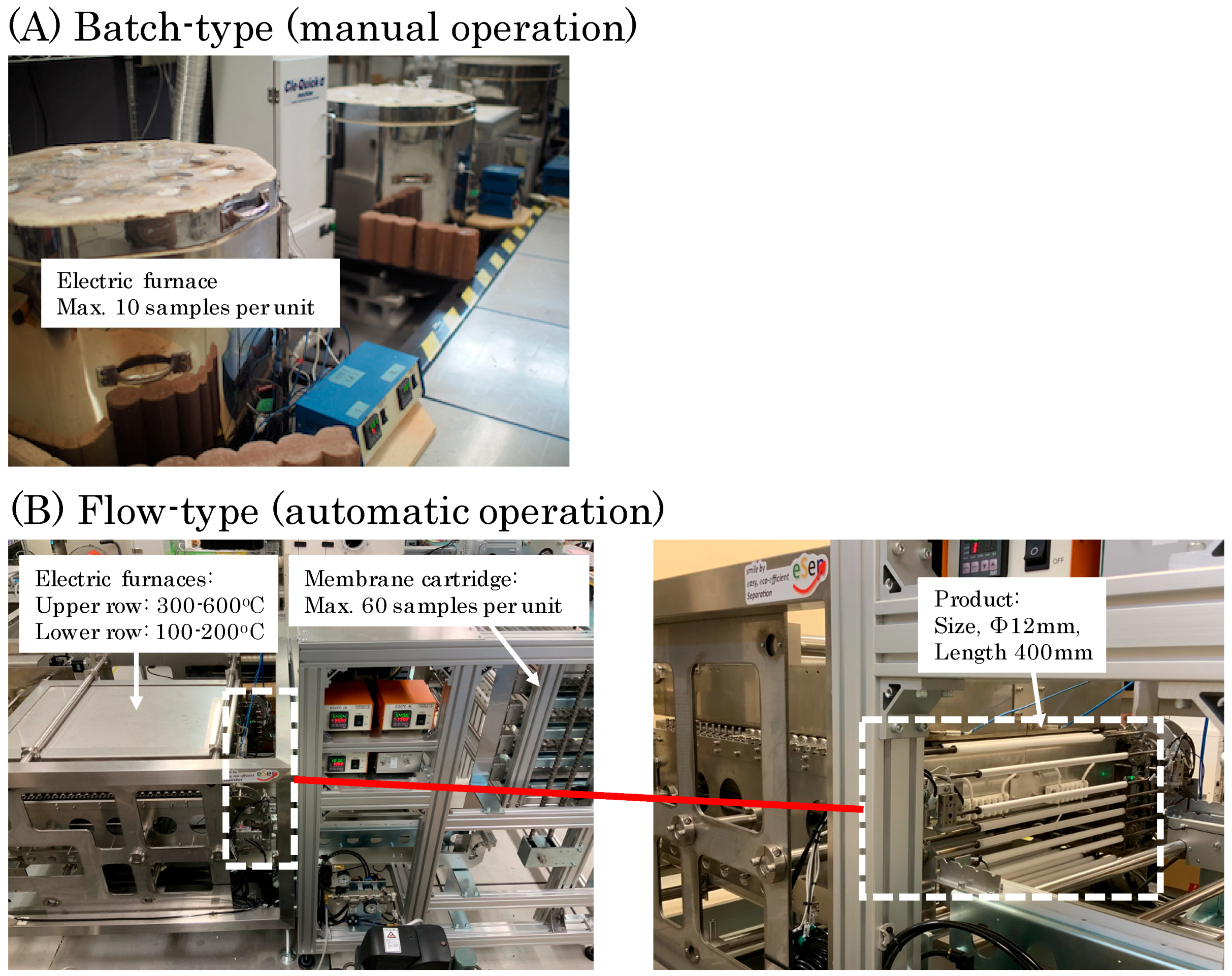
4.3. Mass Producibility
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asaeda, M.; Ishida, M.; Waki, T. Pervaporation of Aqueous Organic Acid Solutions by Porous Ceramic Membranes. J. Chem. Eng. Jpn. 2005, 38, 336–343. [Google Scholar] [CrossRef]
- Asaeda, M.; Yang, J.; Sakou, Y. Porous silica-zirconia (Zr, 50%) membranes for oervaporation of iso-propyl alcohol (IPA)/water mixtures. J. Chem. Eng. Jpn. 2002, 35, 365–371. [Google Scholar] [CrossRef]
- Tsuru, T.; Shibata, T.; Wang, J.; Lee, H.R.; Kanezashi, M.; Yoshioka, T. Pervaporation of Acetic Acid Aqueous Solutions by Organosilica Membranes. J. Membr. Sci. 2012, 421–422, 25–31. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Design of silica networks for development of highly permeable hydrogen separation membranes with hydrothermal stability. J. Am. Chem. Soc. 2009, 131, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic-inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- Tsuru, T.; Hino, T.; Yoshioka, T.; Asaeda, M. Permporometry characterization of microporous ceramic membrane. J. Membr. Sci. 2001, 186, 257–265. [Google Scholar] [CrossRef]
- Tsuru, T.; Takata, Y.; Kondo, H.; Hirano, F.; Yoshioka, T.; Asaeda, M. Characterization of sol-gel derived membranes and zeolite membranes by nanopermporometry. Sep. Purif. Technol. 2003, 32, 23–27. [Google Scholar] [CrossRef]
- Chang, K.-S.; Yoshioka, T.; Kanezashi, M.; Tsuru, T.; Tung, K.-L. Molecular simulation of micro-structures and gas diffusion behavior of organic-inorganic hybrid amorphous silica membranes. J. Membr. Sci. 2011, 381, 90–101. [Google Scholar] [CrossRef]
- Tsuru, T.; Shintani, H.; Yoshioka, T.; Asaeda, M. A bomodal catalytic membrane having a hydrogen-permselective silica layer on bimodal catalytic support: Preparation and application to the steam reforming of methane. Appl. Catal. Gen. 2006, 302, 78–85. [Google Scholar] [CrossRef]
- Kanezashi, M.; Lin, Y.S. Gas permeation and diffusion characteristics of MFI-type zeolite membranes at high temperatures. J. Phys. Chem. C 2009, 113, 3767–3774. [Google Scholar] [CrossRef]
- Kanezashi, M.; Lin, Y.S.; Suzuki, K.; O’Brien-Abraham, J.; O’Brien-Abraham, J. Gas permeation through DDR-type zeolite membranes at high temperatures. AIChE J. 2008, 54, 1478–1486. [Google Scholar] [CrossRef]
- ten Hove, M.; Luiten-Olieman, M.W.; Huiskes, C.; Nijmeijer, A.; Winnubst, L. Influence of the intermediate layer on the hydrothermal stability of sol-gel derived hybrid silica membranes. J. Eur. Ceram. Soc. 2017, 37, 3435–3441. [Google Scholar] [CrossRef]
- ten Hove, M.; Luiten-Olieman, M.W.; Huiskes, C.; Nijmeijer, A.; Winnubst, L. Hydrothermal stability of silica, hybrid silica and Zr-doped hybrid silica membranes. Sep. Purif. Technol. 2017, 189, 48–53. [Google Scholar] [CrossRef]
- Agirre, I.; Arias, P.L.; Castricum, H.L.; Creatore, M.; Johan, E.; Paradis, G.G.; Ngamou, P.H.; van Veen, H.M.; Vente, J.F. Hybrid organosilica membranes and prpcess: Status and outlook. Sep. Purif. Technol. 2014, 121, 2–12. [Google Scholar] [CrossRef]
- Nagasawa, H.; Matsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Pervaporation and vapor permeation characteristics of BTESE-derived organosilica membranes and their long-term stability in a high-water-content IPA/water mixture. J. Membr. Sci. 2016, 498, 336–344. [Google Scholar] [CrossRef]
- Yu, X.; Meng, L.; Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Network engineering of BTESE membrane for improved gas performance via a novel pH-swing method. J. Membr. Sci. 2016, 511, 219–227. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Silica membranes for hydrogen separation prepared by chemical vapor deposition (CVD). Sep. Purif. Technol. 2013, 111, 20–42. [Google Scholar] [CrossRef]
| Step | P/Ps [-] | Kenvin Diameter [nm] | N2 Flow [L(STP) min−1] | |
|---|---|---|---|---|
| Dry | Wet | |||
| 1 | 0 | 0 | 5.00 | 0.00 |
| 2 | 0.015 | 0.50 | 4.92 | 0.08 |
| 3 | 0.062 | 0.75 | 4.69 | 0.31 |
| 4 | 0.124 | 1.0 | 4.38 | 0.62 |
| 5 | 0.248 | 1.5 | 3.76 | 1.24 |
| 6 | 0.352 | 2.0 | 3.24 | 1.76 |
| 7 | 0.498 | 3.0 | 2.51 | 2.49 |
| 8 | 0.593 | 4.0 | 2.04 | 2.97 |
| 9 | 0.658 | 5.0 | 1.71 | 3.29 |
| 10 | 0.706 | 6.0 | 1.47 | 3.53 |
| 11 | 0.742 | 7.0 | 1.29 | 3.71 |
| 12 | 0.770 | 8.0 | 1.15 | 3.85 |
| 13 | 0.793 | 9.0 | 1.04 | 3.97 |
| 14 | 0.811 | 10.0 | 0.95 | 4.06 |
| Nano-Sol | Material | Preparation Conditions | Method | Estimaed Particle Size [nm] |
|---|---|---|---|---|
| Sample-1 | SiO2 | 2 wt % in water, aging at 40 °C for 30 day | DLS, TEM | 10–25 |
| Sample-2 | SiO2 | 1 wt % in water, aging at 40 °C for 30 day | DLS, TEM | 5–15 |
| Sample-3 | SiO2 | 1 wt % in water, aging at 25 °C for 30 day | DLS | 3–10 |
| Sample-4 | SiO2 | 0.5 wt % in water, aging at 25 °C for 7 day | DLS, TEM | 2–10 |
| Sample-5 | SiO2-ZrO2 | 1 wt % in water, agin at 25 °C for 7 day | DLS | 2–10 |
| Sample-6 | SiO2-ZrO2 | 0.5 wt % in water, aging at 25 °C for 7 day | DLS, TEM | 2–5 |
| Sample-7 | Organo-silica (BTESE) | 0.5 wt % in ethanol, aging at 25 °C for 1 day | DLS | 1–2 |
| Permeation Path r [nm] | Permeation Ratio [%] | |||
|---|---|---|---|---|
| Sample-1 | Sample-2 | Sample-3 | Sample-4 | |
| 0 < r ≦ 1 | 2.9 | 3.0 | 10.6 | 23.9 |
| 1 < r ≦ 2 | 5.4 | 18.3 | 40.1 | 73.0 |
| 2 < r ≦ 3 | 7.2 | 20.8 | 48.7 | 2.8 |
| 3 < r ≦ 4 | 10.1 | 35.7 | 0.53 | 0.17 |
| 4 < r ≦ 5 | 13.0 | 21.7 | 0.02 | 0.02 |
| 5 < r ≦ 6 | 20.9 | 0.21 | 0.01 | 0.01 |
| 6 < r ≦ 7 | 34.5 | 0.09 | 0.00 | 0.01 |
| 7 < r ≦ 8 | 5.72 | 0.01 | 0.01 | 0.01 |
| 8 < r ≦ 9 | 0.28 | 0.01 | 0.00 | 0.00 |
| 9 < r ≦ 10 | 0.03 | 0.01 | 0.00 | 0.00 |
| 10 < r | 0.09 | 0.06 | 0.05 | 0.03 |
| Permeation Path r [nm] | Permeation Ratio [%] | |||
|---|---|---|---|---|
| Sample-3 (F1) | Sample-3 (F2) | Sample-3 (F3) | Sample-3 (F4) | |
| 0 < r ≦ 1 | 10.5 | 10.5 | 9.3 | 8.5 |
| 1 < r ≦ 2 | 25.9 | 25.4 | 28.7 | 27.7 |
| 2 < r ≦ 3 | 44.7 | 48.4 | 48.7 | 48.2 |
| 3 < r ≦ 4 | 18.8 | 15.6 | 13.2 | 15.5 |
| 4 < r ≦ 5 | 0.08 | 0.07 | 0.11 | 0.07 |
| 5 < r ≦ 6 | 0.02 | 0.01 | 0.01 | 0.00 |
| 6 < r ≦ 7 | 0.00 | 0.02 | 0.01 | 0.01 |
| 7 < r ≦ 8 | 0.01 | 0.00 | 0.00 | 0.00 |
| 8 < r ≦ 9 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 < r ≦ 10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 < r | 0.01 | 0.01 | 0.03 | 0.03 |
| Permeation Path r [nm] | Permeation Ratio [%] | |
|---|---|---|
| Sample-5 | Sample-6 | |
| 0 < r ≦ 1 | 31.8 | 73.4 |
| 1 < r ≦ 2 | 61.5 | 26.2 |
| 2 < r ≦ 3 | 6.4 | 0.31 |
| 3 < r ≦ 4 | 0.19 | 0.05 |
| 4 < r ≦ 5 | 0.04 | 0.01 |
| 5 < r ≦ 6 | 0.02 | 0.01 |
| 6 < r ≦ 7 | 0.00 | 0.00 |
| 7 < r ≦ 8 | 0.00 | 0.00 |
| 8 < r ≦ 9 | 0.01 | 0.00 |
| 9 < r ≦ 10 | 0.00 | 0.01 |
| 10 < r | 0.06 | 0.04 |
| Membrane | H2 Permeance 10−6 [mol m−2 s−1 Pa−1] | Ratio of Permeance [ - ] | |
|---|---|---|---|
| H2/CH4 | H2/SF6 | ||
| Membrane 1 | 5.21 | 10.6 | >2000 |
| Membrane 2 | 4.85 | 19.7 | >2000 |
| Membrane 3 | 4.05 | 23.7 | >2000 |
| Membrane 4 | 5.60 | 14.0 | >2000 |
| Membrane 5 | 4.44 | 21.4 | >2000 |
| Membrane 6 | 5.56 | 8.2 | 273 |
| Intermediate Layer | After BTESE-Derived Silica Coated | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Material | N2 Permeance [10−6 mol m−2 s−1 Pa−1] | Average Pore Size [nm] | H2 Permeance [10−6 mol m−2 s−1 Pa−1] | Selectivity | Measurement Temperature [°C] | |||
| H2/N2 | H2/CH4 | H2/SF6 | ||||||
| γ-alumina | 0.1–1 | 1.7–5 | 0.2–0.5 | 7–20 | 7–20 | - | 200 | ten Hove et al. [12,13] |
| γ-alumina | - | 4 | 0.25 | 20–30 | - | - | 200 | Agirre et al. [14] |
| SiO2-ZrO2 | 4 | 0.65 | 1 | up to 100 | - | - | 200 | Nagasawa et al. [15] |
| SiO2-ZrO2 | - | several | 1–4 | 10–100 | - | 100–10,000 | 100–200 | Yu et al. [16] |
| SiO2-ZrO2 | - | several | 2–10 | 9–23 | - | 1000–25,500 | 100–300 | Kanezashi et al. [5] |
| SiO2-ZrO2 | 15 | 0.8 | 4.4–5.6 | - | 10–23 | >2000 | 100 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawamura, K.-i.; Okamoto, S.; Todokoro, Y. Development of Mass Production Technology of Highly Permeable Nano-Porous Supports for Silica-Based Separation Membranes. Membranes 2019, 9, 103. https://doi.org/10.3390/membranes9080103
Sawamura K-i, Okamoto S, Todokoro Y. Development of Mass Production Technology of Highly Permeable Nano-Porous Supports for Silica-Based Separation Membranes. Membranes. 2019; 9(8):103. https://doi.org/10.3390/membranes9080103
Chicago/Turabian StyleSawamura, Ken-ichi, Shigeru Okamoto, and Yoshihiro Todokoro. 2019. "Development of Mass Production Technology of Highly Permeable Nano-Porous Supports for Silica-Based Separation Membranes" Membranes 9, no. 8: 103. https://doi.org/10.3390/membranes9080103
APA StyleSawamura, K.-i., Okamoto, S., & Todokoro, Y. (2019). Development of Mass Production Technology of Highly Permeable Nano-Porous Supports for Silica-Based Separation Membranes. Membranes, 9(8), 103. https://doi.org/10.3390/membranes9080103