Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review
Abstract
1. Introduction
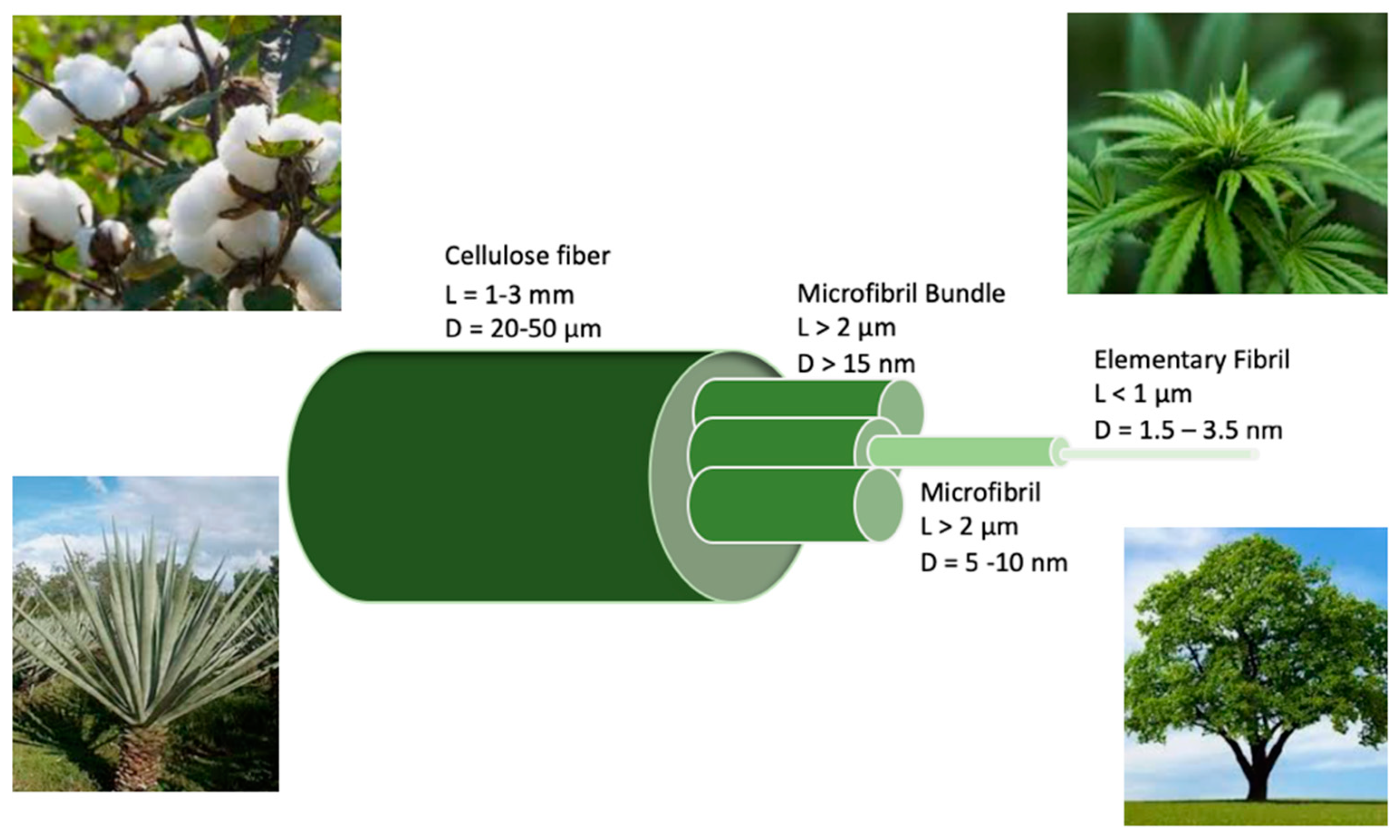
2. Isolation of Nanocellulose from Natural Plants
2.1. Cellulose in Plants
2.2. Decortication
2.3. Mechanical Methods for Nanocellulose Isolation
2.4. Chemical Methods for Nanocelllulose Isolation
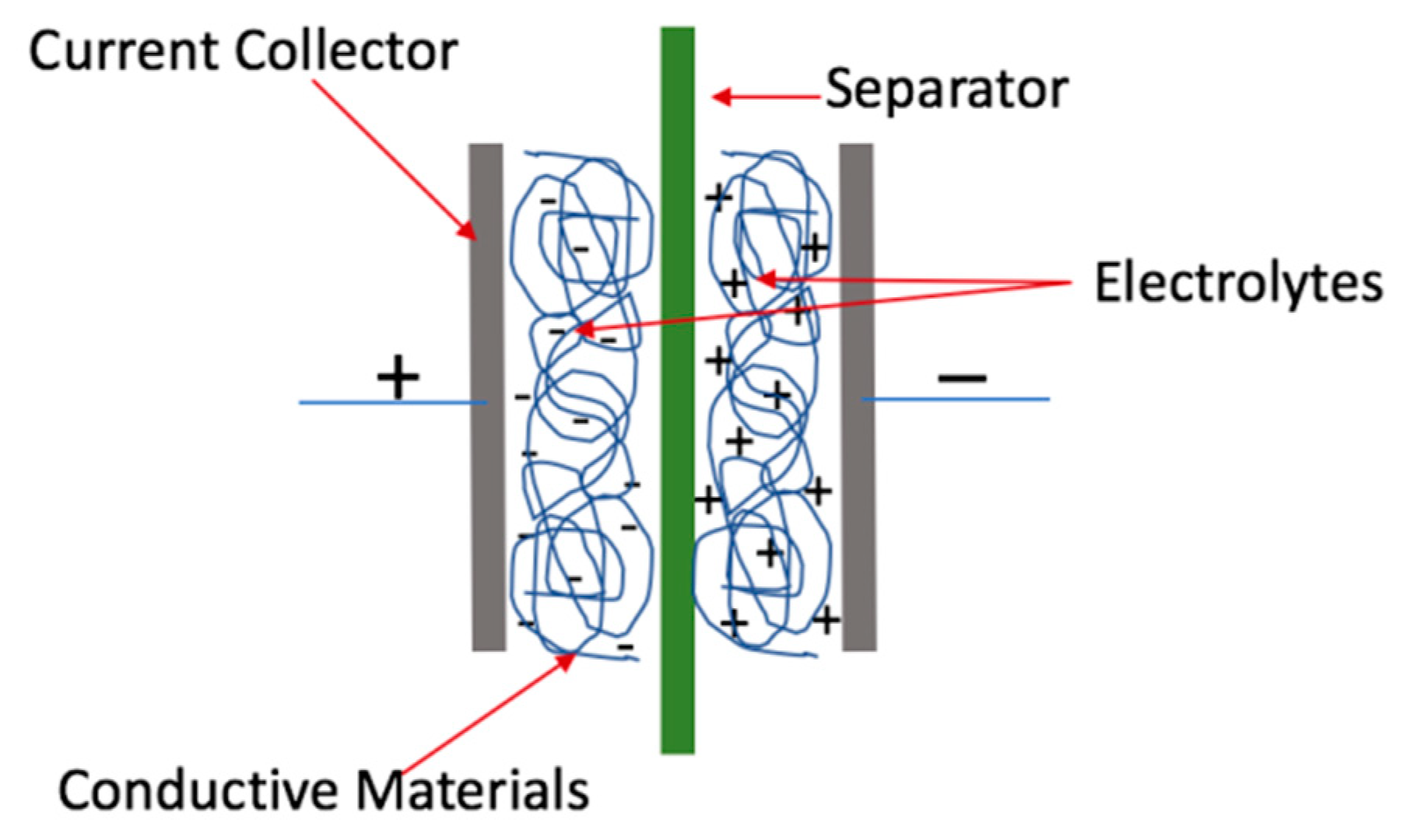
3. Nanocellulose-Based Supercapacitors
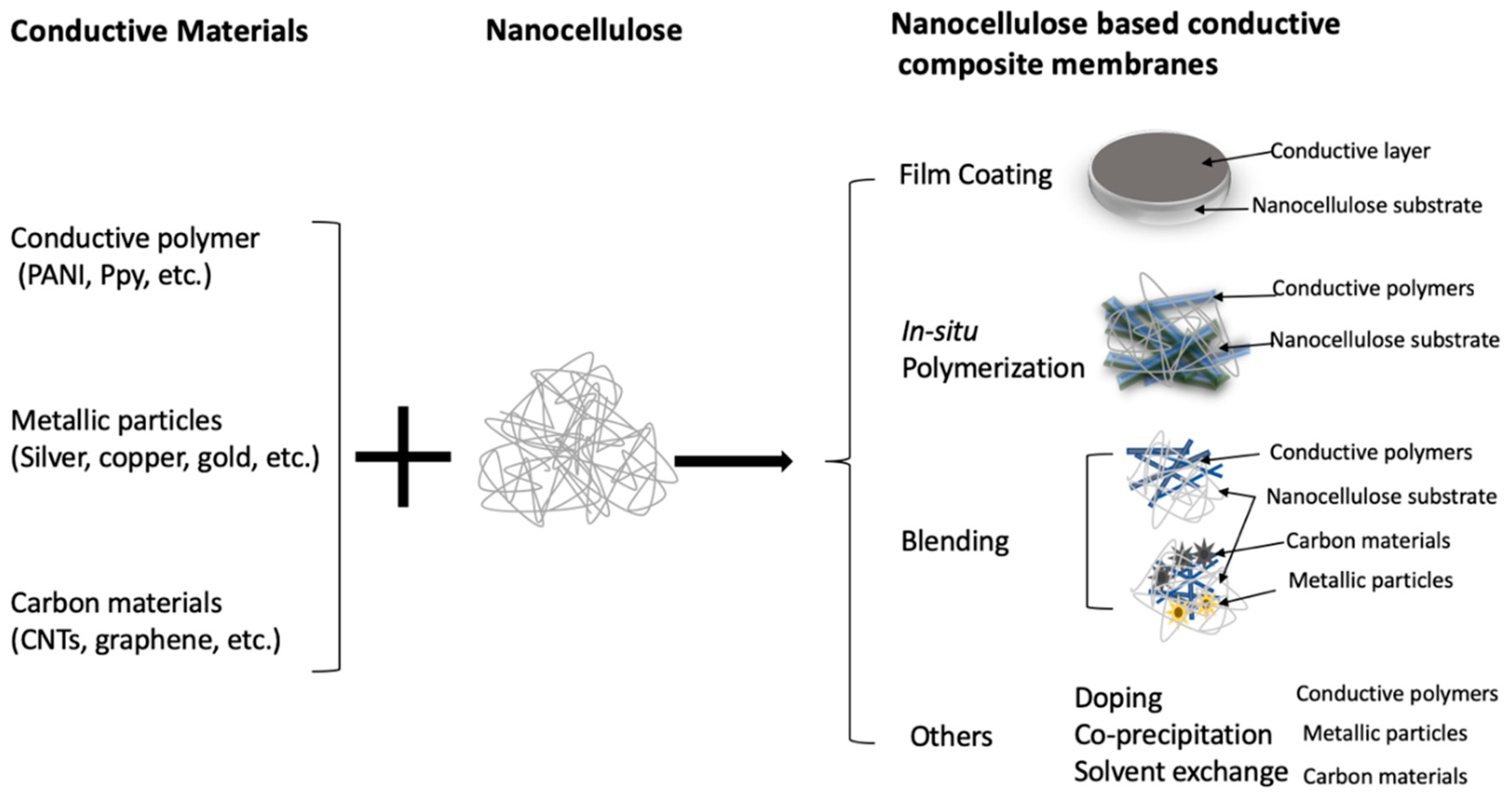
3.1. Nanocellulose Supercapacitors Loaded with Metallic Particles
3.2. Nanocellulose Supercapacitors Loaded with Conductive Polymers
3.3. Nanocellulose Supercapacitors Loaded with Conductive Carbon Materials
3.4. Cellulose Membranes Loaded with Multiple Conductive Components
| Source of Nanocellulose | Conductive Materials | Methods | Highlights of Studies | Applications | References |
|---|---|---|---|---|---|
| Plants | SWCNTs | Inkjet printing | Ultra-strong, transparent, conductive, flexible, and printable nanocomposites | Supercapacitor | [107] |
| Plants | PANI/SWCNT | Solution deposition | Outstanding redox reaction, high energy density, and high power density | Supercapacitor | [62] |
| Plants | PANI | Coating | Flexible electrodes without binder | Supercapacitor | [108] |
| Plants | CNT/PANI | Lay-by-lay coating | High flexibility and good cycling stability, high-loading and high-energy capacitance | Wearable portable devices | [60] |
| Plants/Bacterial | PPy/GO | Coating/in situ polymerization | Free standing, light weight, low cost, high conductive performance and capacitance | Paper electrodes | [73,86,87] |
| Plants | PPy/GO | In situ polymerization, blending | Flexible, very high volumetric capacitance | Paper electrodes | [95] |
| Plants | Indium oxide, CNT, Silver nanowires | N-Doped | Highly transparent, improved optical property | Solar cell, touch screen, interactive paper | [57] |
| Plants | Nickel-phosphorus (Ni-P) | Coating | Feasible to control the crystallite size and hollow cavity pore size | Electrodes | [88] |
| Plants | PANI | In situ polymerization | Improved capacitance, good electrochemical performance after 1000 cycles | All-solid-state supercapacitor | [65] |
| Plants | Graphite foil, graphene, nanographite, | Coating | Low-cost and eco-friendly aqueous supercapacitor | Supercapacitor, water-purification carbons | [93] |
| Wood | GO/graphene | Hybrid/coating/blending | Large contact area | Li-S batteries | [96] |
| Softwood | Silver nanoparticle ink | Inkjet printing | High optical transparency, sufficiently high performance, low coefficient of thermal expansion | Paper device | [55] |
| Paper | Active carbon/CNT, Ag Nanowires | Inkjet printing | User-customized control of cell, free-standing flexible supercapacitor | Supercapacitor | [100] |
| Filter paper | Ni/active carbon, Ni/MnO2 | Electroless plating, Electrodeposition | A free-standing flexible asymmetrically supercapacitor, large volume density, and superior flexibility | Supercapacitor | [109] |
| Paper/PVA | PANI/Au | In situ depositing | Flexible self-powered piezoelectric generator | Supercapacitor | [82] |
| Cellulose | MWCNT | Doping | Excellent tensile test result, high Young’s modulus | Supercapacitor | [98] |
| Cellulose | rGO/PANI | Coating, in situ polymerization | Highly flexible/foldable, high electrochemical performance | Supercapacitor | [110] |
| Bacterial cellulose | PANI | In situ depositing | New method of peeling off conductive film | Supercapacitor | [111] |
| Bacterial cellulose | PPy/copper oxide | Coating | Free-standing, flexible | Supercapacitor | [48] |
| Bacterial cellulose | CNT/GO/RGO | In situ polymerization | Excellent capability, cycling stability | Electrodes | [73,99] |
| Bacterial cellulose | SiNPs/PANI | Binding/coating | Stable, flexible, less stress dissipation | Electrodes | [97] |
| Bacterial cellulose | PPy | In situ polymerization. coating | Flexible, high electrical/electrochemical performance | Electroactive membrane | [94] |
4. Evaluation of Electrical Performance of Nanocellulose-Based Supercapacitors
5. Applications of Nanocellulose-Based Electronic Devices
6. Challenges of Nanocellulose-Based Supercapacitors
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Wegner, T.H.; Jones, P.E. Advancing cellulose-based nanotechnology. Cellulose 2006, 13, 115–118. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; De Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Misra, M.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276, 1–24. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yu, D.S.; Qian, Q.H.; Wei, L.; Jiang, W.C.; Goh, K.L.; Wei, J.; Zhang, J.; Chen, Y. Emergence of fiber supercapacitors. Chem. Soc. Rev. 2015, 44, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Wang, Z.H.; Tammela, P.; Stromme, M.; Nyholm, L. Cellulose-based Supercapacitors: Material and Performance Considerations. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Conway, B.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sour. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Supercapacitors, E. Scientific Fundamentals and Technological Applications; Conway, B.E., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Zheng, W.; Lv, R.; Na, B.; Liu, H.; Jin, T.; Yuan, D. Nanocellulose-mediated hybrid polyaniline electrodes for high performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 12969–12976. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Wang, H.; Shao, H.; Fang, J.; He, J.; Xiong, H.; Ma, C.; Lin, T. High-performance supercapacitor electrode from cellulose-derived, inter-bonded carbon nanofibers. J. Power Sour. 2016, 324, 302–308. [Google Scholar] [CrossRef]
- Islam, N.; Li, S.; Ren, G.; Zu, Y.; Warzywoda, J.; Wang, S.; Fan, Z. High-frequency electrochemical capacitors based on plasma pyrolyzed bacterial cellulose aerogel for current ripple filtering and pulse energy storage. Nano Energy 2017, 40, 107–114. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Jiang, K.; Thundat, T. Carbonized nanocellulose sustainably boosts the performance of activated carbon in ionic liquid supercapacitors. Nano Energy 2016, 25, 161–169. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Wang, Z.; Lindh, J.; Strømme, M. Carbonized cellulose beads for efficient capacitive energy storage. Cellulose 2018, 25, 3545–3556. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Cui, B.; Yin, P.; Zhang, C. Design and preparation of biomass-derived carbon materials for supercapacitors: A review. C 2018, 4, 53. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.; Mubarak, N.; Baloch, H.A.; Mazari, S.A.; Tunio, M.; Griffin, G.; Srinivasan, M.; Tanksale, A.; Riaz, S. Advanced nanomaterials synthesis from pyrolysis and hydrothermal carbonization: A review. Curr. Org. Chem. 2018, 22, 446–461. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Behzad, T.; Ahmadi, M. Nanofibers. In Nanofiber Research-Reaching New Heights; InTech: Temse, Belgium, 2016. [Google Scholar]
- Islam, M.T.; Alam, M.M.; Zoccola, M. Review on modification of nanocellulose for application in composites. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 5444–5451. [Google Scholar]
- Wang, B.; Sain, M.; Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl. Compos. Mater. 2007, 14, 89. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Timell, T.E. Wood hemicelluloses: Part I. Adv. Carbohydr. Chem. 1964, 19, 247–302. [Google Scholar] [PubMed]
- Chirayil, C.J.; Mathew, L.; Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28. [Google Scholar]
- Harada, H.; Goto, T. The structure of cellulose microfibrils in Valonia. In Cellulose and Other Natural Polymer Systems; Springer: Berlin, Germany, 1982; pp. 383–401. [Google Scholar]
- Ray, D.P.; Banerjee, P.; Satya, P.; Mitra, S.; Ghosh, R.K.; Mondal, S.B. Degumming of decorticated ramie fibre through novel chemical process. Indian J. Nat. Fibers 2014, 1, 125–129. [Google Scholar]
- Münder, F.; Fürll, C.; Hempel, H. Advanced decortication technology for unretted bast fibres. J. Nat. Fibers 2004, 1, 49–65. [Google Scholar] [CrossRef]
- Paridah, M.T.; Basher, A.B.; SaifulAzry, S.; Ahmed, Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources 2011, 6, 5260–5281. [Google Scholar]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Shah, D.U. Developing plant fibre composites for structural applications by optimising composite parameters: A critical review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Kenny, J.M. A review on natural fibre-based composites-part I: Structure, processing and properties of vegetable fibres. J. Nat. Fibers 2004, 1, 37–68. [Google Scholar] [CrossRef]
- Mwaikambo, L. Review of the history, properties and application of plant fibres. Afr. J. Sci. Technol. 2006, 7, 121. [Google Scholar]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sain, M.; Kortschot, M. Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing. Holzforschung 2005, 59, 102–107. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Dispersion of soybean stock-based nanofiber in a plastic matrix. Polym. Int. 2007, 56, 538–546. [Google Scholar] [CrossRef]
- Joonobi, M.; Harun, J.; Tahir, P.M.; Zaini, L.H.; SaifulAzry, S.; Makinejad, M.D. Characteristic of nanofibers extracted from kenaf core. BioResources 2010, 5, 2556–2566. [Google Scholar]
- Habibi, Y.; Vignon, M.R. Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 2008, 15, 177–185. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Alila, S.; Besbes, I.; Vilar, M.R.; Mutjé, P.; Boufi, S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crop. Prod. 2013, 41, 250–259. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils–an enzymatic approach. Bioresources 2007, 1, 176–188. [Google Scholar]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Miloh, T.; Spivak, B.; Yarin, A. Needleless electrospinning: Electrically driven instability and multiple jetting from the free surface of a spherical liquid layer. J. Appl. Phys. 2009, 106, 114910. [Google Scholar] [CrossRef]
- Takagi, H.; Nakagaito, A.; Nishimura, K.; Matsui, T. Mechanical characterisation of nanocellulose composites after structural modification. High Perform. Optim. Des. Struct. Mater. II 2016, 166, 335–341. [Google Scholar]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Peng, S.; Fan, L.; Rao, W.; Bai, Z.; Xu, W.; Xu, J. Bacterial cellulose membranes coated by polypyrrole/copper oxide as flexible supercapacitor electrodes. J. Mater. Sci. 2017, 52, 1930–1942. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Schmidt, O.G.; Yan, C. Engineered nanomembranes for smart energy storage devices. Chem. Soc. Rev. 2016, 45, 1308–1330. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, D.; Wang, H.; Guo, L. Smart Electrochemical Energy Storage Devices with Self-Protection and Self-Adaptation Abilities. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional energy storage and conversion devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Maitra, A.; Karan, S.K.; Paria, S.; Das, A.K.; Bera, R.; Halder, L.; Si, S.K.; Bera, A.; Khatua, B.B. Fast charging self-powered wearable and flexible asymmetric supercapacitor power cell with fish swim bladder as an efficient natural bio-piezoelectric separator. Nano Energy 2017, 40, 633–645. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Zhang, Y.F.; Kong, L.B.; Kang, L.; Ran, F. Nano-Au@PANI core-shell nanoparticles via in-situ polymerization as electrode for supercapacitor. J. Alloy. Compd. 2017, 722, 1–7. [Google Scholar] [CrossRef]
- Tan, Y.T.; Liu, Y.S.; Kong, L.B.; Kang, L.; Xu, C.G.; Ran, F. In Situ doping of PANI nanocomposites by gold nanoparticles for high-performance electrochemical energy storage. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Yagyu, H.; Saito, T.; Isogai, A.; Koga, H.; Nogi, M. Chemical modification of cellulose nanofibers for the production of highly thermal resistant and optically transparent nanopaper for paper devices. ACS Appl. Mater. Interfaces 2015, 7, 22012–22017. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.W.; Li, Y.; Wen, Z.W.; Gao, F.X.; Liang, C.Y.; Che, R.C. Ultrathin beta-Ni(OH)(2) Nanoplates Vertically Grown on Nickel-Coated Carbon Nanotubes as High-Performance Pseudocapacitor Electrode Materials. ACS Appl. Mater. Interfaces 2015, 7, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Zheng, G.Y.; Yao, J.; Liu, N.A.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.C.; Fan, S.H.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar] [CrossRef]
- Eftekhari, A. Nanostructured Conductive Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dong, L.B.; Liang, G.M.; Xu, C.J.; Ren, D.Y.; Wang, J.J.; Pan, Z.Z.; Li, B.H.; Kang, F.Y.; Yang, Q.H. Stacking up layers of polyaniline/carbon nanotube networks inside papers as highly flexible electrodes with large areal capacitance and superior rate capability. J. Mater. Chem. A 2017, 5, 19934–19942. [Google Scholar] [CrossRef]
- Fan, Z.M.; Cheng, Z.J.; Feng, J.Y.; Xie, Z.M.; Liu, Y.Y.; Wang, Y.S. Ultrahigh volumetric performance of a freestanding compact N-doped holey graphene/PANI slice for supercapacitors. J. Mater. Chem. A 2017, 5, 16689–16701. [Google Scholar] [CrossRef]
- Jiang, Q.S.; Kacica, C.; Soundappan, T.; Liu, K.K.; Tadepalli, S.; Biswas, P.; Singamaneni, S. An in situ grown bacterial nanocellulose/graphene oxide composite for flexible supercapacitors. J. Mater. Chem. A 2017, 5, 13976–13982. [Google Scholar] [CrossRef]
- Lv, X.D.; Li, G.H.; Li, D.W.; Huang, F.L.; Liu, W.T.; Wei, Q.F. A new method to prepare no-binder, integral electrodes-separator, asymmetric all-solid-state flexible supercapacitor derived from bacterial cellulose. J. Phys. Chem. Solids 2017, 110, 202–210. [Google Scholar] [CrossRef]
- Feng, E.K.; Peng, H.; Zhang, Z.G.; Li, J.D.; Lei, Z.Q. Polyaniline-based carbon nanospheres and redox mediator doped robust gel films lead to high performance foldable solid-state supercapacitors. New J. Chem. 2017, 41, 9024–9032. [Google Scholar] [CrossRef]
- Liu, F.W.; Luo, S.J.; Liu, D.; Chen, W.; Huang, Y.; Dong, L.; Wang, L. Facile Processing of Free-Standing Polyaniline/SWCNT Film as an Integrated Electrode for Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 33791–33801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Lu, C.; Zhou, Z. Dialysis-free and in situ doping synthesis of polypyrrole@ cellulose nanowhiskers nanohybrid for preparation of conductive nanocomposites with enhanced properties. ACS Sustain. Chem. Eng. 2015, 3, 675–682. [Google Scholar] [CrossRef]
- Khosrozadeh, A.; Darabi, M.A.; Xing, M.; Wang, Q. Flexible Cellulose-Based Films of Polyaniline–Graphene–Silver Nanowire for High-Performance Supercapacitors. J. Nanotechnol. Eng. Med. 2015, 6, 011005. [Google Scholar] [CrossRef]
- Khosrozadeh, A.; Darabi, M.A.; Xing, M.; Wang, Q. Flexible electrode design: Fabrication of freestanding polyaniline-based composite films for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 11379–11389. [Google Scholar] [CrossRef] [PubMed]
- Khosrozadeh, A.; Xing, M.; Wang, Q. A high-capacitance solid-state supercapacitor based on free-standing film of polyaniline and carbon particles. Appl. Energy 2015, 153, 87–93. [Google Scholar] [CrossRef]
- Hsu, H.H.; Khosrozadeh, A.; Li, B.; Luo, G.; Xing, M.M.; Zhong, W. An Eco-friendly, Nanocellulose/RGO/in-situ Formed Polyaniline for Flexible and Free-standing Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 4766–4776. [Google Scholar] [CrossRef]
- Liang, H.-W.; Guan, Q.-F.; Song, L.-T.; Yao, H.-B.; Lei, X.; Yu, S.-H. Highly conductive and stretchable conductors fabricated from bacterial cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef]
- Luan, D.X.; Zhang, X.W.; Yu, Y.; Chen, Y.H.; Ma, Y.; Bi, C.L.; Zhao, D.Y. Fabrication and electrochemical properties of graphene/copper-nickel solid solution reinforced polyaniline composite. J. Mater. Sci.-Mater. Electron. 2017, 28, 14738–14746. [Google Scholar] [CrossRef]
- Kang, Z.P.; Jiao, K.L.; Xu, X.P.; Peng, R.Y.; Jiao, S.Q.; Hu, Z.Q. Graphene oxide-supported carbon nanofiber-like network derived from polyaniline: A novel composite for enhanced glucose oxidase bioelectrode performance. Biosens. Bioelectron. 2017, 96, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, P.; Lazzaroni, R.; Riga, J.; Verbist, J.; Gonbeau, D. A photoelectron spectroscopic study of the electrochemical processes in polyaniline. J. Chem. Phys. 1990, 92, 2187–2193. [Google Scholar] [CrossRef]
- Manoj, M.; Anilkumar, K.M.; Jinisha, B.; Jayalekshmi, S. Polyaniline-Graphene Oxide based ordered nanocomposite electrodes for high-performance supercapacitor applications. J. Mater. Sci.-Mater. Electron. 2017, 28, 14323–14330. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Zahedi, F. Electrochemical reduced graphene oxide-polyaniline as effective nanocomposite film for high-performance supercapacitor applications. Electrochim. Acta 2017, 245, 575–586. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Metals 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Kulkarni, V.G.; Campbell, L.D.; Mathew, W.R. Thermal stability of polyaniline. Synth. Metals 1989, 30, 321–325. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-Based Supercapacitors for Self-Powered Nanosystems. Angew. Chem. 2012, 124, 5018–5022. [Google Scholar] [CrossRef]
- Aytug, T.; Rager, M.S.; Higgins, W.; Brown, F.G.; Veith, G.M.; Rouleau, C.M.; Wang, H.; Hood, Z.D.; Mahurin, S.M.; Mayes, R.T. Vacuum-Assisted Low-temperature Synthesis of Reduced Graphene Oxide Thin Film Electrodes for High Performance Transparent and Flexible All-Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 11008–11017. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, J.; Zhu, G.; Zheng, J. Fabrication of flexible free-standing reduced graphene oxide/polyaniline nanocomposite film for all-solid-state flexible supercapacitor. Electrochim. Acta 2018, 261, 151–159. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Deng, W.; Zhang, D.; Li, N.; Wu, Q.; He, C. In-situ growth of high-performance all-solid-state electrode for flexible supercapacitors based on carbon woven fabric/polyaniline/graphene composite. J. Power Sour. 2018, 384, 278–286. [Google Scholar] [CrossRef]
- Carlsson, D.O.; Sjodin, M.; Nyholm, L.; Stromme, M. A Comparative Study of the Effects of Rinsing and Aging of Polypyrrole/Nanocellulose Composites on Their Electrochemical Properties. J. Phys. Chem. B 2013, 117, 3900–3910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Tammela, P.; Zhang, P.; Stromme, M.; Nyholm, L. Efficient high active mass paper-based energy-storage devices containing free-standing additive-less polypyrrole-nanocellulose electrodes. J. Mater. Chem. A 2014, 2, 7711–7716. [Google Scholar] [CrossRef]
- Pan, Y.F.; Guo, Z.Q.; Guo, T.C.; Wang, X.; Huang, J.T. The preparation, characterization, and influence of multiple electroless nickel-phosphorus (Ni-P) hollow composite coatings on micro-nano cellulose fibers. Surf. Coat. Technol. 2016, 298, 33–38. [Google Scholar] [CrossRef]
- Du, P.C.; Lin, L.; Wang, H.X.; Liu, D.; Wei, W.L.; Li, J.G.; Liu, P. Fabrication of porous polyaniline modified MWNTs core-shell structure for high performance supercapacitors with high rate capability. Mater. Des. 2017, 127, 76–83. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Nagaraju, D.H.; Balakrishna, R.G.; Surareungchai, W.; Ramakrishnappa, T.; Shivanandareddy, A.B. Hydrogels of polyaniline with graphene oxide for highly sensitive electrochemical determination of lead ions. Anal. Chim. Acta 2017, 990, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, X.; Liu, Q.Z.; Chen, J.H.; Jiang, H.Q.; Wang, Y.D.; Liu, K.; Li, M.F.; Wang, D. Three-dimensional non-woven poly(vinyl alcohol-co-ethylene) nanofiber based polyaniline flexible electrode for high performance supercapacitor. J. Alloy. Compd. 2017, 715, 137–145. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, D.; Du, P.C.; Wei, W.L.; Wang, Q.; Liu, P. Comparative study on polyvinyl chloride film as flexible substrate for preparing free-standing polyaniline-based composite electrodes for supercapacitors. J. Coll. Interface Sci. 2017, 506, 572–581. [Google Scholar] [CrossRef]
- Blomquist, N.; Wells, T.; Andres, B.; Backstrom, J.; Forsberg, S.; Olin, H. Metal-free supercapacitor with aqueous electrolyte and low-cost carbon materials. Sci. Rep. 2017, 7, 39836. [Google Scholar] [CrossRef]
- Lay, M.; Gonzalez, I.; Tarres, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.H.; Tammela, P.; Stromme, M.; Nyholm, L. Nanocellulose coupled flexible polypyrrole@graphene oxide composite paper electrodes with high volumetric capacitance. Nanoscale 2015, 7, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.U.M.; Luong, N.D.; Seppala, J.; Tchernychova, E.; Dominko, R. Low surface area graphene/cellulose composite as a host matrix for lithium sulphur batteries. J. Power Sour. 2014, 254, 55–61. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Shin, S.; Kim, H.J.; Hyun, J. Flexible conductive nanocellulose combined with silicon nanoparticles and polyaniline. Carbohydr. Polym. 2016, 140, 43–50. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Chen, B.; Sharma, S.; Wong, C.-p.; Zhang, W.; Deng, Y. Solid-state, flexible, high strength paper-based supercapacitors. J. Mater. Chem. A 2013, 1, 5835–5839. [Google Scholar] [CrossRef]
- Chang, Y.H.; Zhou, L.; Xiao, Z.C.; Liang, J.X.; Kong, D.B.; Li, Z.H.; Zhang, X.H.; Li, X.L.; Zhi, L.J. Embedding Reduced Graphene Oxide in Bacterial Cellulose-Derived Carbon Nanofibril Networks for Supercapacitors. Chemelectrochem 2017, 4, 2448–2452. [Google Scholar] [CrossRef]
- Choi, K.-H.; Yoo, J.; Lee, C.K.; Lee, S.-Y. All-inkjet-printed, solid-state flexible supercapacitors on paper. Energy Environ. Sci. 2016, 9, 2812–2821. [Google Scholar] [CrossRef]
- Koga, H.; Tonomura, H.; Nogi, M.; Suganuma, K.; Nishina, Y. Fast, scalable, and eco-friendly fabrication of an energy storage paper electrode. Green Chem. 2016, 18, 1117–1124. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Herrasti, P.; Dıaz, L.; Ocón, P.; Ibáñez, A.; Fatas, E. Electrochemical and mechanical properties of polypyrrole coatings on steel. Electrochim. Acta 2004, 49, 3693–3699. [Google Scholar] [CrossRef]
- Marcilla, R.; Ochoteco, E.; Pozo-Gonzalo, C.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. New organic dispersions of conducting polymers using polymeric ionic liquids as stabilizers. Macromol. Rapid Commun. 2005, 26, 1122–1126. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Rannou, P.; Planès, J.; Proń, A.; Nechtschein, M. Preparation of low density polyethylene-based polyaniline conducting polymer composites with low percolation threshold via extrusion. Synth. Metals 1998, 93, 169–173. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Yang, C.; Si, L.L.; Si, G.L. Flexible self-assembled membrane electrodes based on eco-friendly bamboo fibers for supercapacitors. J. Mater. Sci.-Mater. Electron. 2017, 28, 15338–15344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, P.; Zhou, F.; Zeng, W.; Su, H.; Li, G.; Gao, J.; Sun, R.; Wong, C.-P. Flexible asymmetrical solid-state supercapacitors based on laboratory filter paper. ACS Nano 2015, 10, 1273–1282. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Zhang, L.; Zhou, W.; Chen, X.; Xie, S. Nanostructured graphene composite papers for highly flexible and foldable supercapacitors. Adv. Mater. 2014, 26, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Zoski, C.G. Ultramicroelectrodes: Design, fabrication, and characterization. Electroanalysis 2002, 14, 1041–1051. [Google Scholar] [CrossRef]
- Li, B.; Zheng, M.B.; Xue, H.G.; Pang, H. High performance electrochemical capacitor materials focusing on nickel based materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J. Materials science and materials chemistry for large scale electrochemical energy storage: From transportation to electrical grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Burke, A.; Miller, M. Testing of electrochemical capacitors: Capacitance, resistance, energy density, and power capability. Electrochim. Acta 2010, 55, 7538–7548. [Google Scholar] [CrossRef]
- Linzen, D.; Buller, S.; Karden, E.; De Doncker, R.W. Analysis and evaluation of charge-balancing circuits on performance, reliability, and lifetime of supercapacitor systems. IEEE Trans. Ind. Appl. 2005, 41, 1135–1141. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Tang, J.; Tang, W. Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high-performance supercapacitors. Chem. Mater. 2015, 27, 7034–7041. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Zhang, P.; Strømme, M.; Nyholm, L. High areal and volumetric capacity sustainable all-polymer paper-based supercapacitors. J. Mater. Chem. A 2014, 2, 16761–16769. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C.m. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, Z. Iridescent chiral nematic cellulose nanocrystal/polyvinylpyrrolidone nanocomposite films for distinguishing similar organic solvents. ACS Sustain. Chem. Eng. 2018, 6, 6192–6202. [Google Scholar] [CrossRef]
- Lombardo, S.; Eyley, S.; Schütz, C.; Van Gorp, H.; Rosenfeldt, S.; Van den Mooter, G.; Thielemans, W. Thermodynamic study of the interaction of bovine serum albumin and amino acids with cellulose nanocrystals. Langmuir 2017, 33, 5473–5481. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Benítez-Martínez, S.; Soriano, M.L.; Valcárcel, M. Fluorescent nanocellulosic hydrogels based on graphene quantum dots for sensing laccase. Anal. Chim. Acta 2017, 974, 93–99. [Google Scholar] [CrossRef]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Kämpf, M.M.; Zimmermann, T.; Maniura-Weber, K.; Faccio, G. A Protein-Nanocellulose Paper for Sensing Copper Ions at the Nano-to Micromolar Level. Adv. Funct. Mater. 2017, 27, 1604291. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Mabrouk, S.; Wu, F.; Chen, K.; Yang, S.; Qiao, Q. A strategic review on processing routes towards highly efficient perovskite solar cells. J. Mater. Chem. A 2018, 6, 2406–2431. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, J.; Shi, Z.; Xiang, S.; Xiong, C. Recent progress of nanocellulose-based electroconductive materials and their applications as electronic devices. J. For. Eng. 2018, 3, 1–11. [Google Scholar]
- Gao, L.; Chao, L.; Hou, M.; Liang, J.; Chen, Y.; Yu, H.-D.; Huang, W. Flexible, transparent nanocellulose paper-based perovskite solar cells. Npj Flex. Electron. 2019, 3, 4. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Arazi, R.; Mirjalili, B.B.F.; Azad, S. Synthesis and application of Fe3O4@ nanocellulose/TiCl as a nanofiller for high performance of quasisolid-based dye-sensitized solar cells. Int. J. Energy Res. 2019. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, D.; Lee, Y.-H.; Chen, W.; Lee, S.-Y. Nanocellulose for Energy Storage Systems: Beyond the Limits of Synthetic Materials. Adv. Mater. 2019, 31, e1804826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, R.; Sun, R.; Edstrom, K.; Strømme, M.; Nyholm, L. Nanocellulose structured paper-based lithium metal batteries. ACS Appl. Energy Mater. 2018, 1, 4341–4350. [Google Scholar] [CrossRef]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose modified polyethylene separators for lithium metal batteries. Small 2018, 14, e1704371. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Edberg, J.; Zhao, D.; Puzinas, S.; Khan, Z.U.; Mäkie, P.; Naderi, A.; Lindström, T.; Odén, M.; Engquist, I. Nanofibrillated Cellulose-Based Electrolyte and Electrode for Paper-Based Supercapacitors. Adv. Sustain. Syst. 2018, 2, 1700121. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible Asymmetric Micro-Supercapacitors Based on Bi2O3 and MnO2 Nanoflowers: Larger Areal Mass Promises Higher Energy Density. Adv. Energy Mater. 2015, 5, 1401882. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Zhang, P.; Huo, J.; Ericson, F.; Strømme, M.; Nyholm, L. Freestanding nanocellulose-composite fibre reinforced 3D polypyrrole electrodes for energy storage applications. Nanoscale 2014, 6, 13068–13075. [Google Scholar] [CrossRef] [PubMed]

| Fiber | Density (g/cm3) | Diameter (µm) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| Hemp | 1.47 | 25–50 | 690 | 70 | 1.6 |
| Kenaf | 1.49 | 20–60 | 930 | 53 | 1.6 |
| Sisal | 1.5 | 30–50 | 467–700 | 9.4–22 | 3–7 |
| Cotton | 1.5 | 12–38 | 287–800 | 5.5–12.6 | 7–8 |
| Oil Palm | 0.7–1.55 | 15–50 | 248 | 3.2 | 25 |
| Carbon | 1.78 | 5–7 | 3400–4800 | 240 | 1.4–1.8 |
| E-glass | 2.55 | <17 | 3400 | 73 | 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.H.; Zhong, W. Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review. Membranes 2019, 9, 74. https://doi.org/10.3390/membranes9060074
Hsu HH, Zhong W. Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review. Membranes. 2019; 9(6):74. https://doi.org/10.3390/membranes9060074
Chicago/Turabian StyleHsu, Helen H., and Wen Zhong. 2019. "Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review" Membranes 9, no. 6: 74. https://doi.org/10.3390/membranes9060074
APA StyleHsu, H. H., & Zhong, W. (2019). Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review. Membranes, 9(6), 74. https://doi.org/10.3390/membranes9060074




