Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices
Abstract
1. Introduction
2. Experimental
3. Results
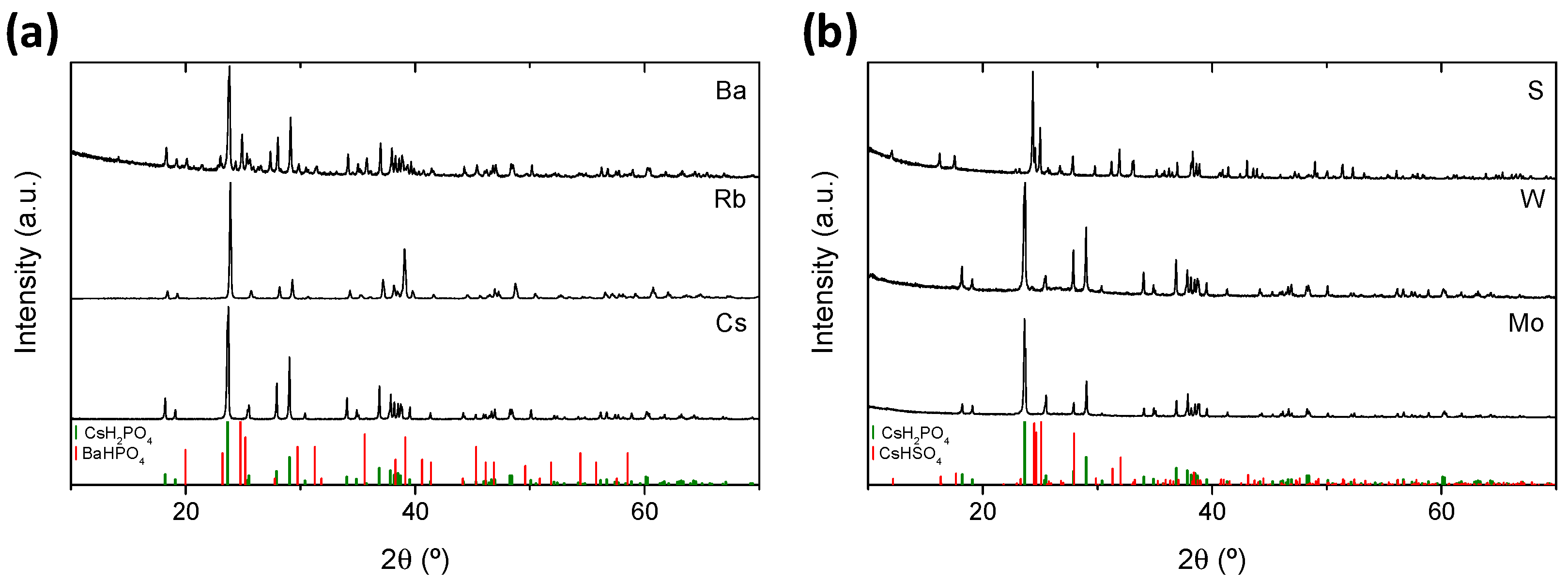
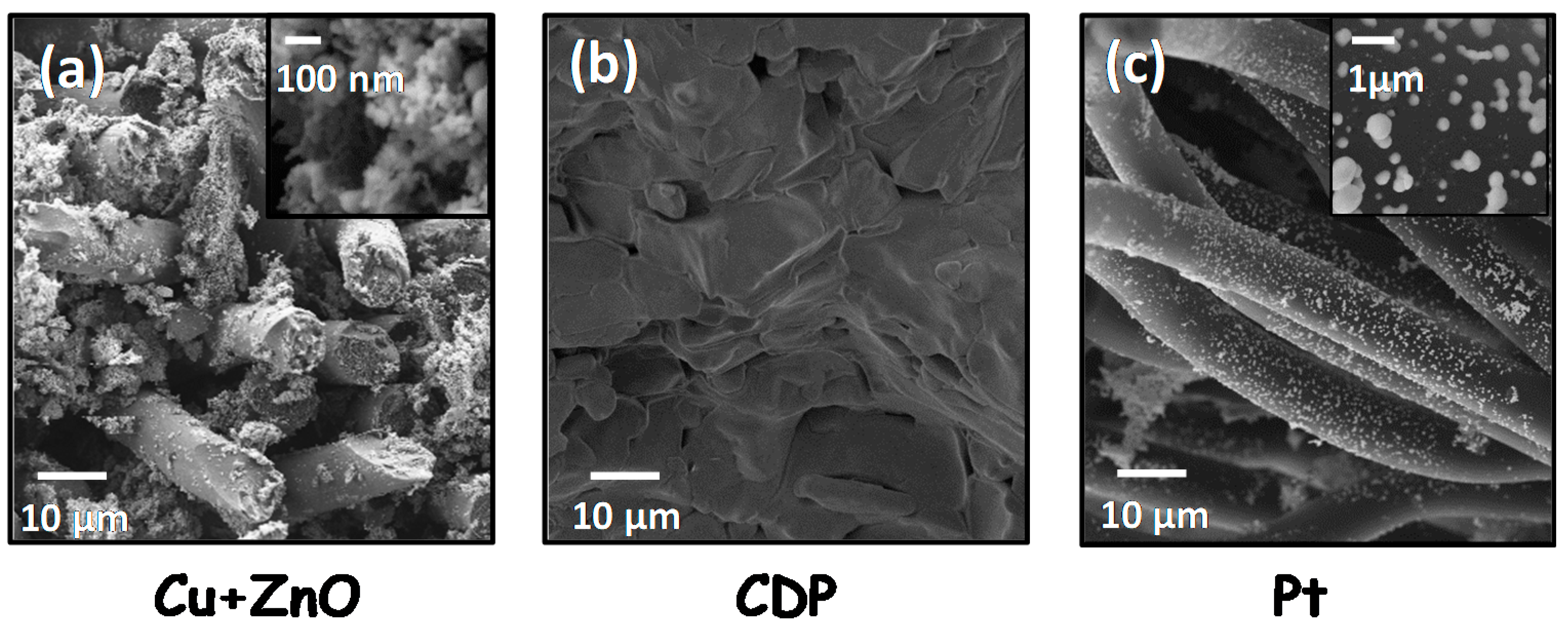
3.1. Structural Characterization
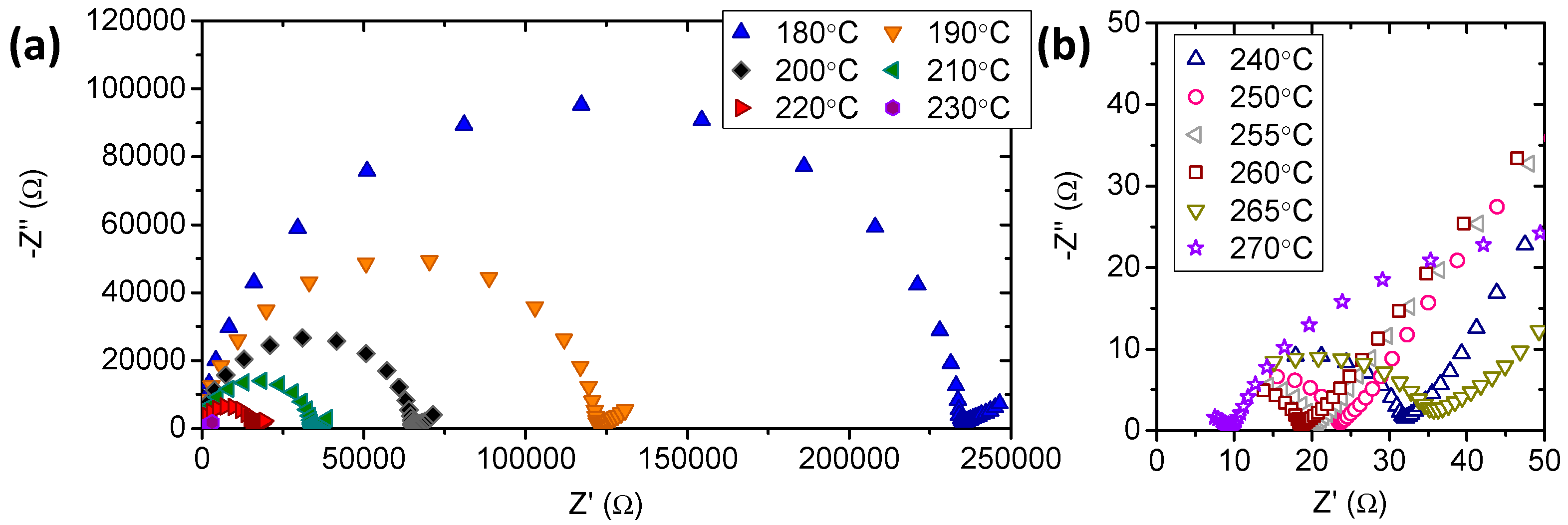
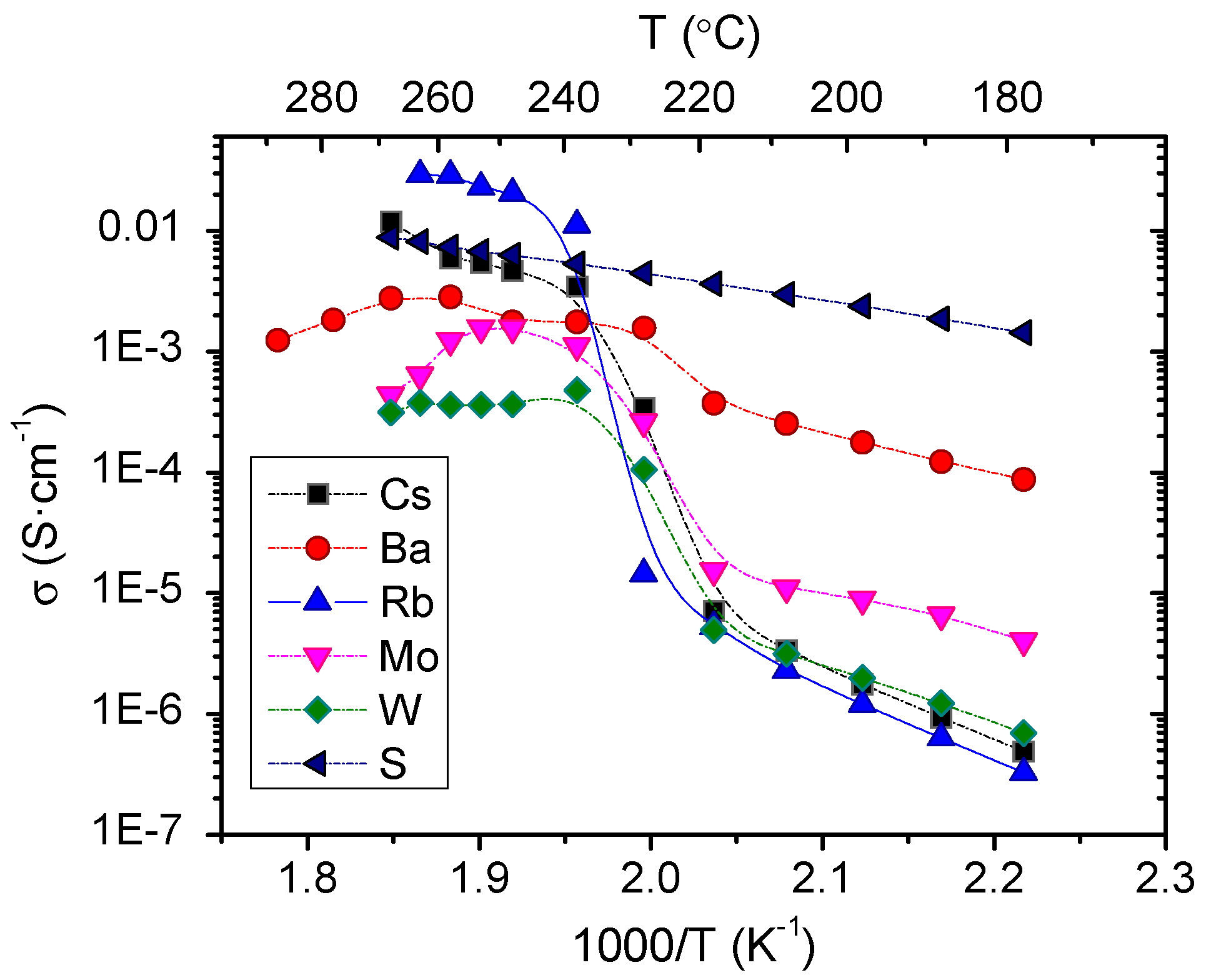
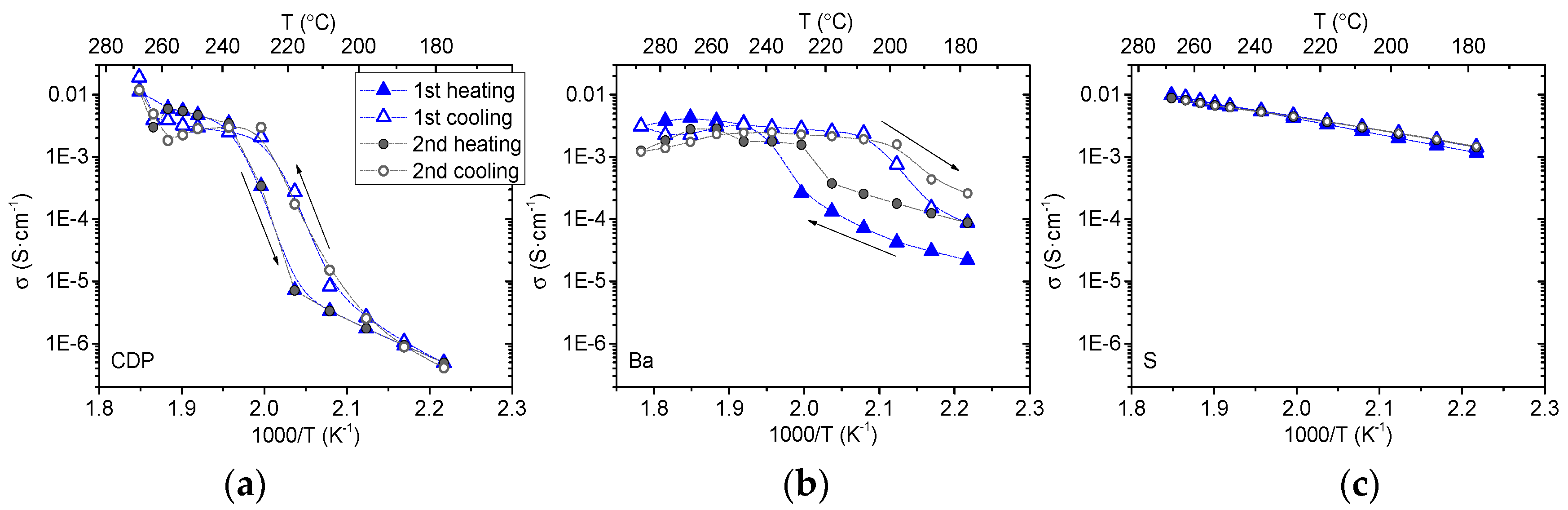
3.2. Electrochemical Characterization
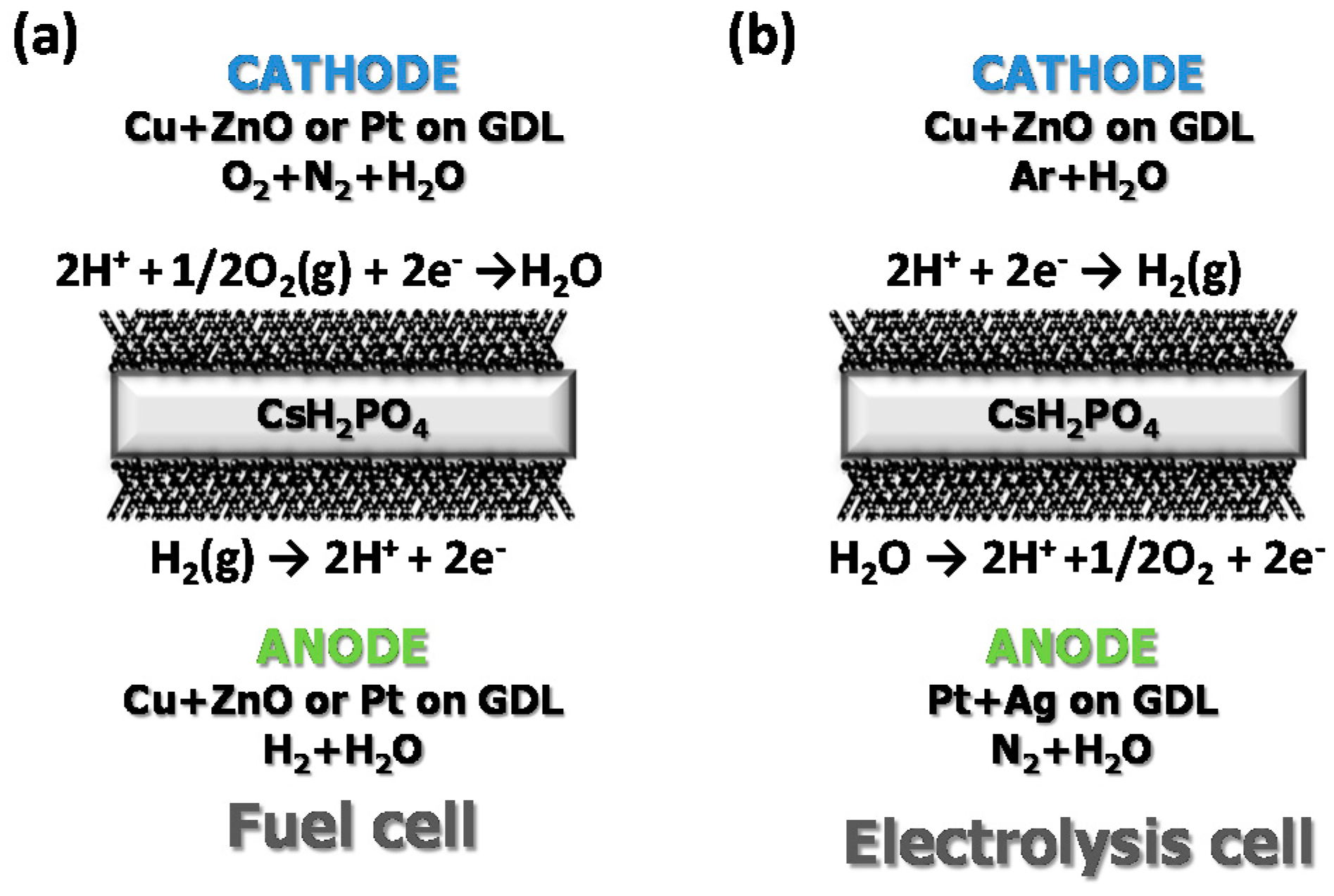
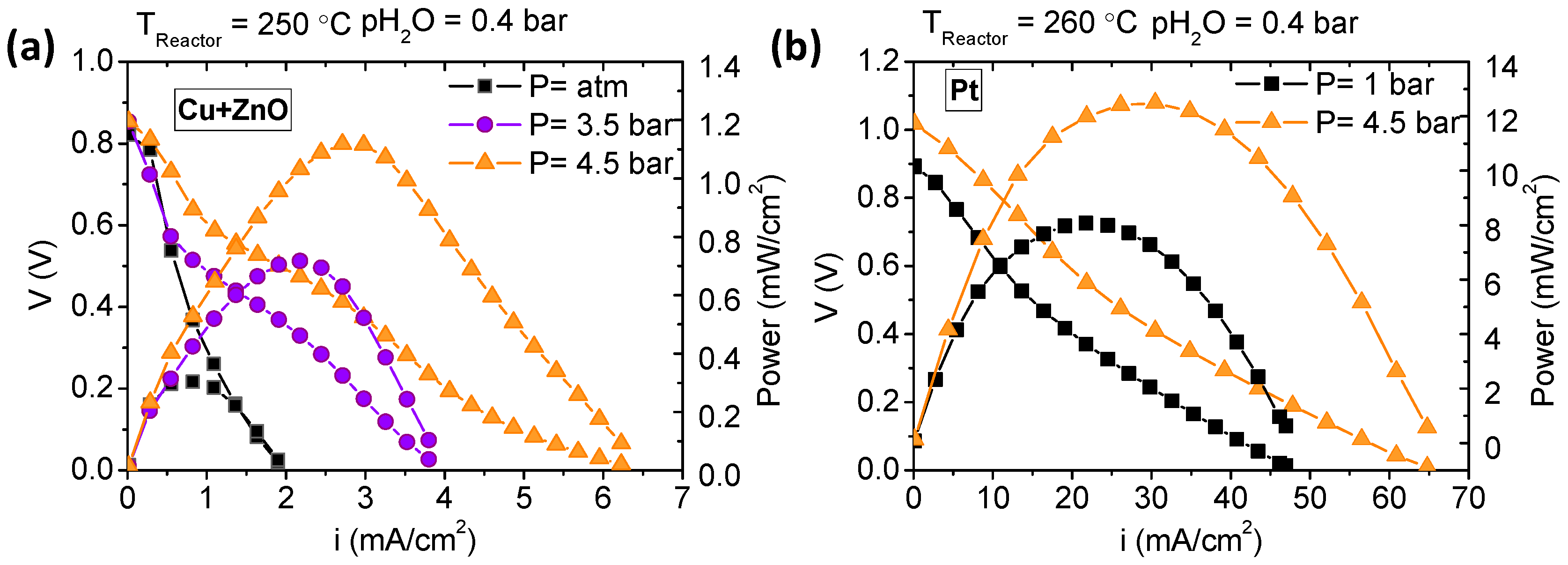
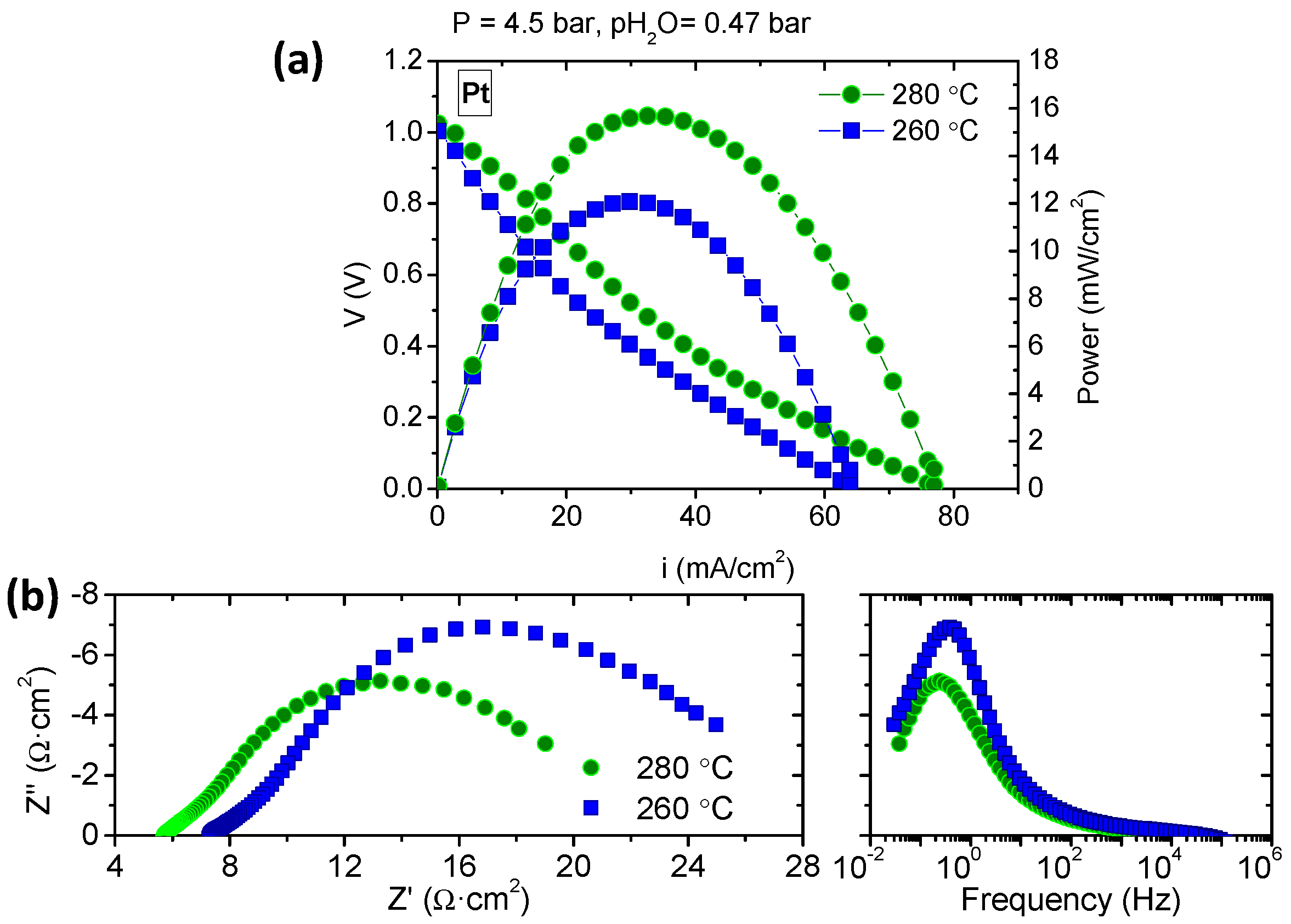
3.3. Fuel Cell Measurements
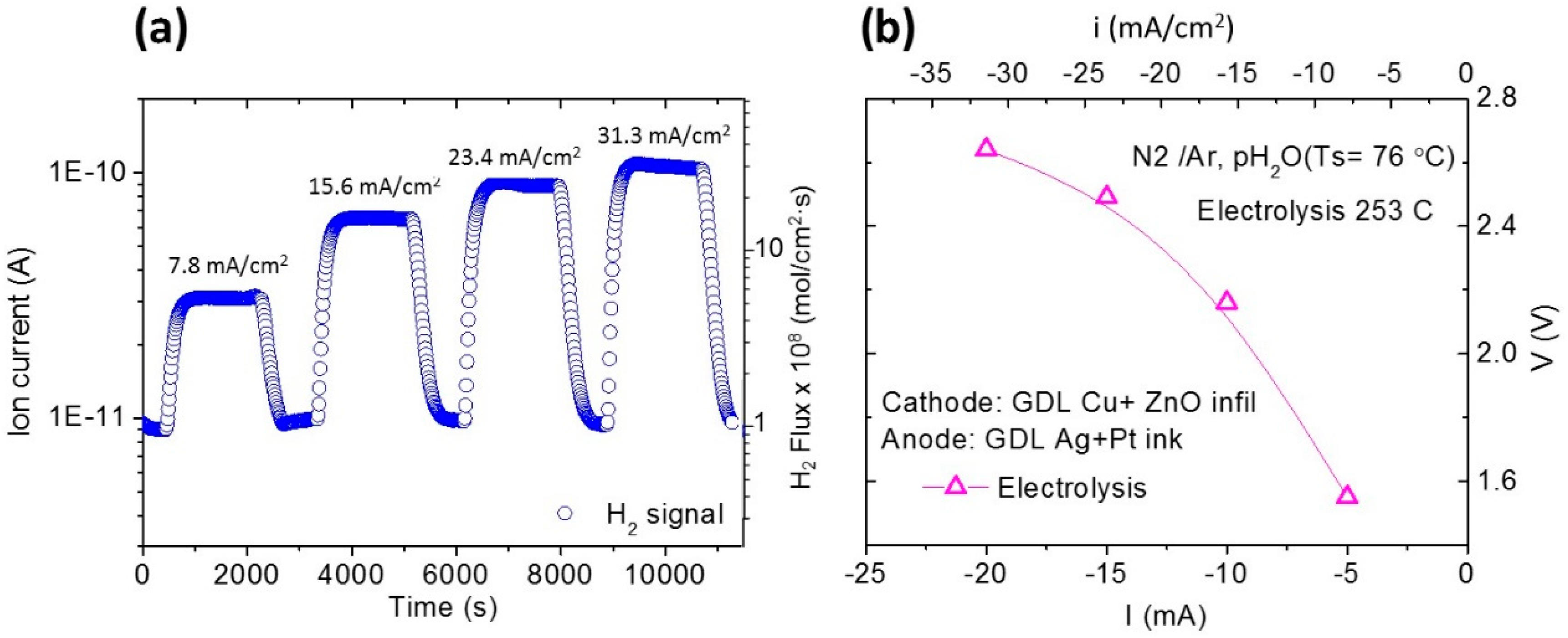
3.4. Electrolysis Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Minh, N.Q. CERAMIC FUEL-CELLS. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Morejudo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martínez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merie, R.B. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef]
- Otomo, J.; Minagawa, N.; Wen, C.-J.; Eguchi, K.; Takahashi, H. Protonic conduction of CsH2PO4 and its composite with silica in dry and humid atmospheres. Solid State Ion. 2003, 156, 357–369. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Louie, M.W.; Kislitsyn, M.; Bhattacharya, K.; Haile, S.M. Phase transformation and hysteresis behavior in Cs1−xRbxH2PO4. Solid State Ion. 2010, 181, 173–179. [Google Scholar] [CrossRef]
- Chisholm, C.R.I.; Boysen, D.A.; Papandrew, A.B.; Zecevic, S.; Cha, S.; Sasaki, K.A.; Varga, A.; Giapis, K.P.; Haile, S.M. From laboratory breakthrough to technological realization: The development path for solid acid fuel cells. Electrochem. Soc. Interface 2009, 18, 53–59. [Google Scholar]
- Taninouchi, Y.K.; Uda, T.; Awakura, Y.; Ikeda, A.; Haile, S.M. Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260 °C. J. Mater. Chem. 2007, 17, 3182–3189. [Google Scholar] [CrossRef]
- Taninouchi, Y.k.; Uda, T.; Awakura, Y. Dehydration of CsH2PO4 at temperatures higher than 260 °C and the ionic conductivity of liquid product. Solid State Ion. 2008, 178, 1648–1653. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Bagryantseva, I.N. Superprotonic CsH2PO4-CsHSO4 solid solutions. Inorg. Mater. 2012, 48, 187–194. [Google Scholar] [CrossRef]
- Uda, T.; Boysen, D.A.; Chisholm, C.R.I.; Haile, S.M. Alcohol fuel cells at optimal temperatures. Electrochem. Solid-State Lett. 2006, 9, A261–A264. [Google Scholar] [CrossRef]
- Bartley, G.J.J.; Burch, R. Support and morphological effects in the synthesis of methanol over Cu/ZnO, Cu/ZrO2 and Cu/SiO2 catalysts. Appl. Catal. 1988, 43, 141–153. [Google Scholar] [CrossRef]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Hallinder, J. Electrolytes and Electrodes for Electrochemical Cells Operating at 200–300 °C; Technical University of Denmark: Lyngby, Denmark, 2013. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chisholm, C.R.I.; Haile, S.M. X-ray structure refinement of CsHSO4 in phase II. Mater. Res. Bull. 2000, 35, 999–1005. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Wang, H.J.; Boukamp, B.A. Conductivity study of dense BaZr0.9Y0.1O(3−δ) obtained by spark plasma sintering. Solid State Ion. 2012, 213, 36–41. [Google Scholar] [CrossRef]
- Ikeda, A.; Kitchaev, D.A.; Haile, S.M. Phase behavior and superprotonic conductivity in the Cs1−xRbxH2PO4 and Cs1−xKxH2PO4 systems. J. Mater. Chem. A 2014, 2, 204–214. [Google Scholar] [CrossRef]
- Hallinder, J.; Holtappels, P.; Mogensen, M. Materials and Manufacturing of Electrochemical Cells for Reduction of CO2 into Liquid Fuels. Meet. Abstr. 2011, MA2011-02, 1507. [Google Scholar]
- Lee, H.-S.; Tuckerman, M.E. The Structure and Proton Transport Mechanisms in the Superprotonic Phase of CsH2PO4: An Ab Initio Molecular Dynamics Study. J. Phys. Chem. C 2008, 112, 9917–9930. [Google Scholar] [CrossRef]
- Bronowska, W. Comment on “Does the structural superionic phase transition at 231 °C in CsH2PO4 really not exist?” [J. Chem. Phys. 110, 4847 (1999)]. J. Chem. Phys. 2001, 114, 611–612. [Google Scholar] [CrossRef][Green Version]
- Romain, F.; Novak, A. Raman study of the high-temperature phase transition in CsH2PO4. J. Mol. Struct. 1991, 263, 69–74. [Google Scholar] [CrossRef]
- Papandrew, A.B.; Chisholm, C.R.I.; Elgammal, R.A.; Özer, M.M.; Zecevic, S.K. Advanced electrodes for solid acid fuel cells by platinum deposition on CsH2PO4. Chem. Mater. 2011, 23, 1659–1667. [Google Scholar] [CrossRef]
- Boysen, D.A.; Uda, T.; Chisholm, C.R.I.; Haile, S.M. High-Performance Solid Acid Fuel Cells Through Humidity Stabilization. Science 2004, 303, 68–70. [Google Scholar] [CrossRef]
- Yoshimi, S.; Matsui, T.; Kikuchi, R.; Eguchi, K. Temperature and humidity dependence of the electrode polarization in intermediate-temperature fuel cells employing CsH2PO4/SiP2O7-based composite electrolytes. J. Power Sources 2008, 179, 497–503. [Google Scholar] [CrossRef]
- Perez, J.; Gonzalez, E.R.; Ticianelli, E.A. Oxygen electrocatalysis on thin porous coating rotating platinum electrodes. Electrochim. Acta 1998, 44, 1329–1339. [Google Scholar] [CrossRef]
- Prag, C.B. Intermediate Temperature Steam Electrolysis with Phosphate-Based Electrolytes; Technical University of Denmark: Lyngby, Denmark, 2014. [Google Scholar]
- Schiffer, Z.J.; Manthiram, K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. [Google Scholar] [CrossRef]
- Serra, J.M. Electrifying chemistry with protonic cells. Nat. Energy 2019, 4, 178–179. [Google Scholar] [CrossRef]
| Compound | Nomenclature |
|---|---|
| CsH2PO4 | Cs |
| (CsH2PO4)0.8(BaHPO4)0.2 | Ba |
| (CsH2PO4)0.8(RbH2PO4)0.2 | Rb |
| (CsH2PO4)0.8(HSO4)0.2 | S |
| (CsH2PO4)0.8(HMoO4)0.2 | Mo |
| (CsH2PO4)0.8(HWO4)0.2 | W |
| Compound | Eact (T < 230 °C) (eV) | Eact (T > 230 °C) (eV) |
|---|---|---|
| Cs | 1.26 | 0.65 |
| Mo | 0.61 | 0.19 |
| S | 0.42 | 0.42 |
| W | 0.93 | 0.25 |
| Rb | 1.44 | 0.92 |
| Ba | 0.69 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete, L.; Andrio, A.; Escolástico, S.; Moya, S.; Compañ, V.; Serra, J.M. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes 2019, 9, 49. https://doi.org/10.3390/membranes9040049
Navarrete L, Andrio A, Escolástico S, Moya S, Compañ V, Serra JM. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes. 2019; 9(4):49. https://doi.org/10.3390/membranes9040049
Chicago/Turabian StyleNavarrete, Laura, Andreu Andrio, Sonia Escolástico, Sergio Moya, Vicente Compañ, and José M. Serra. 2019. "Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices" Membranes 9, no. 4: 49. https://doi.org/10.3390/membranes9040049
APA StyleNavarrete, L., Andrio, A., Escolástico, S., Moya, S., Compañ, V., & Serra, J. M. (2019). Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes, 9(4), 49. https://doi.org/10.3390/membranes9040049







