Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Membranes
2.1.2. Spent Sulfite Liquor
2.2. Methods
2.2.1. Quantification of Lignosulfonate
2.2.2. Zeta Potential
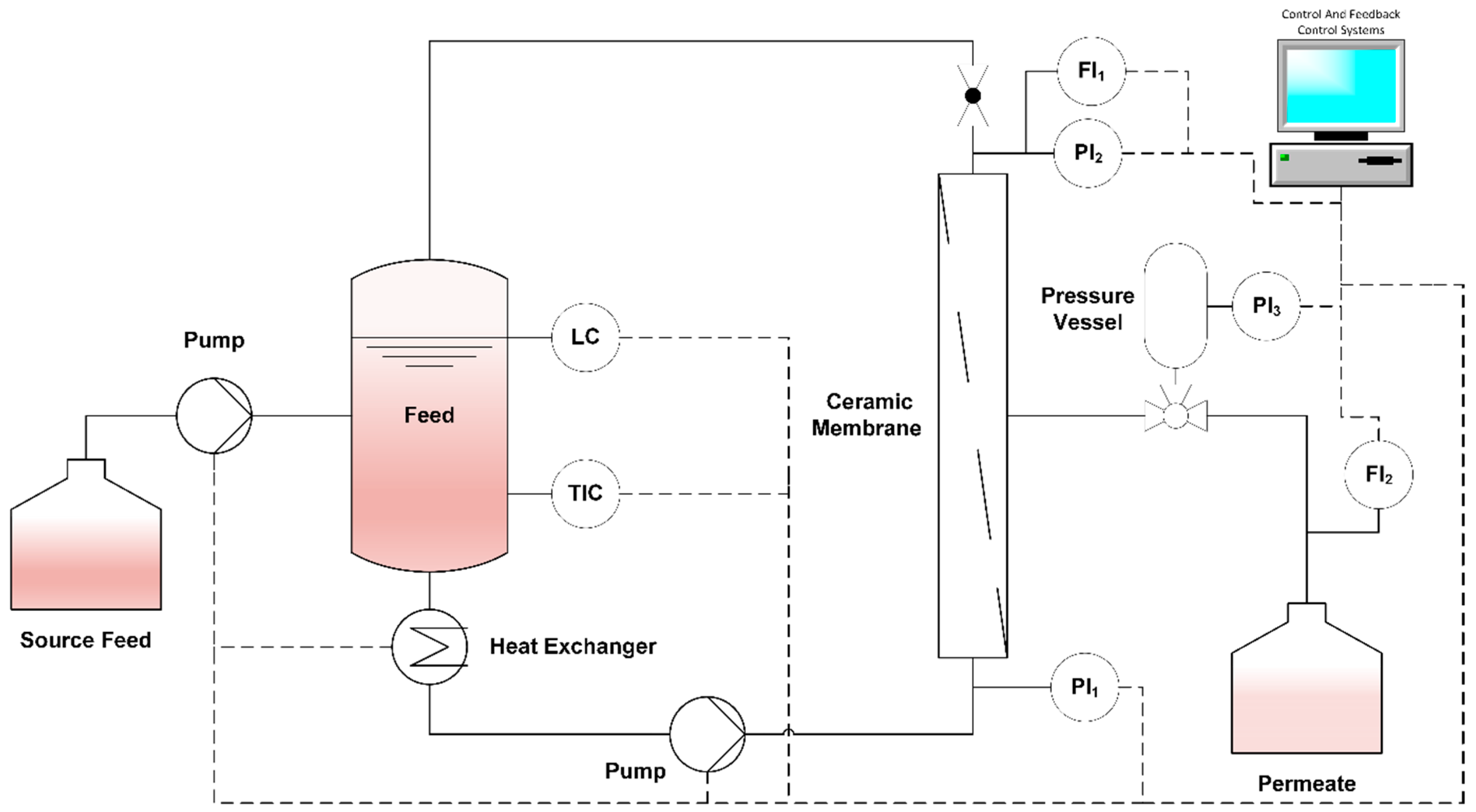
2.2.3. Membrane Filtration
2.2.4. Comparison of Different Membrane Geometries
2.2.5. Membrane Cleaning
3. Results and Discussion
3.1. Zeta Potential
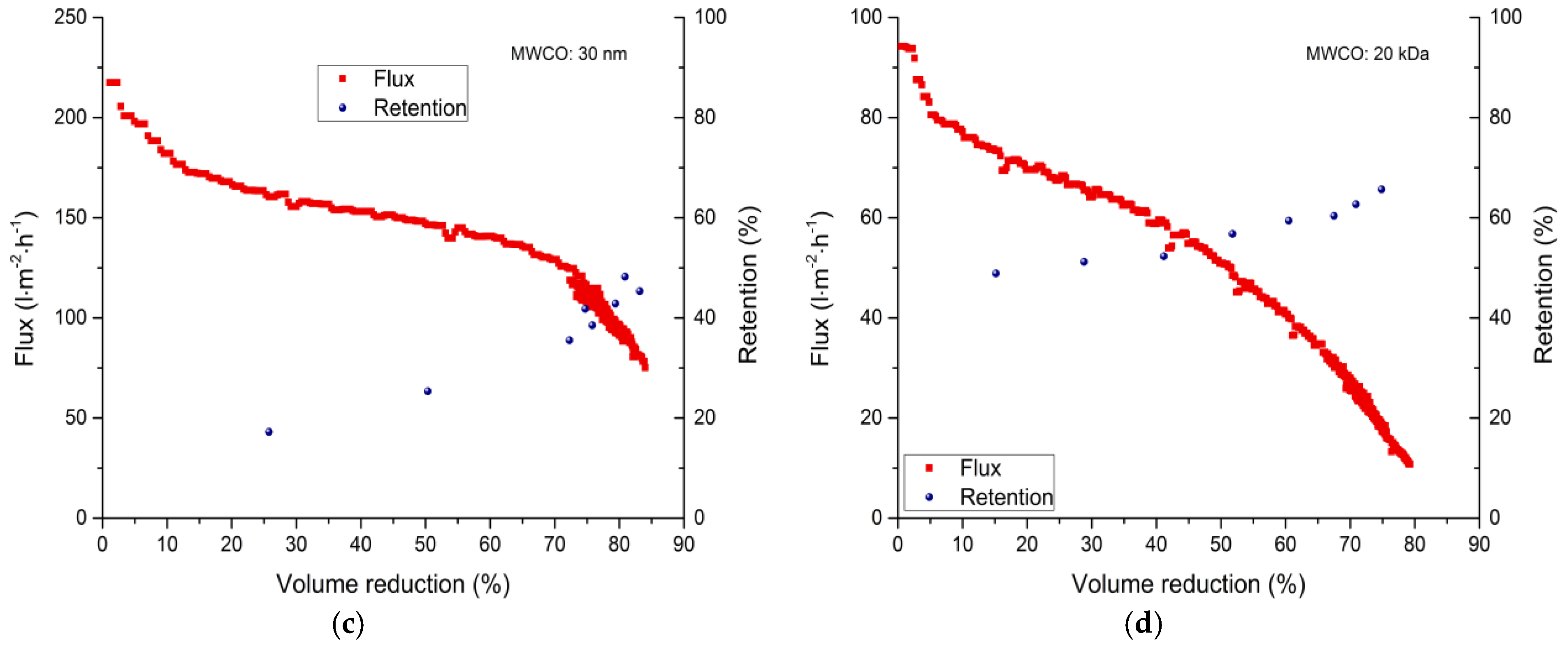
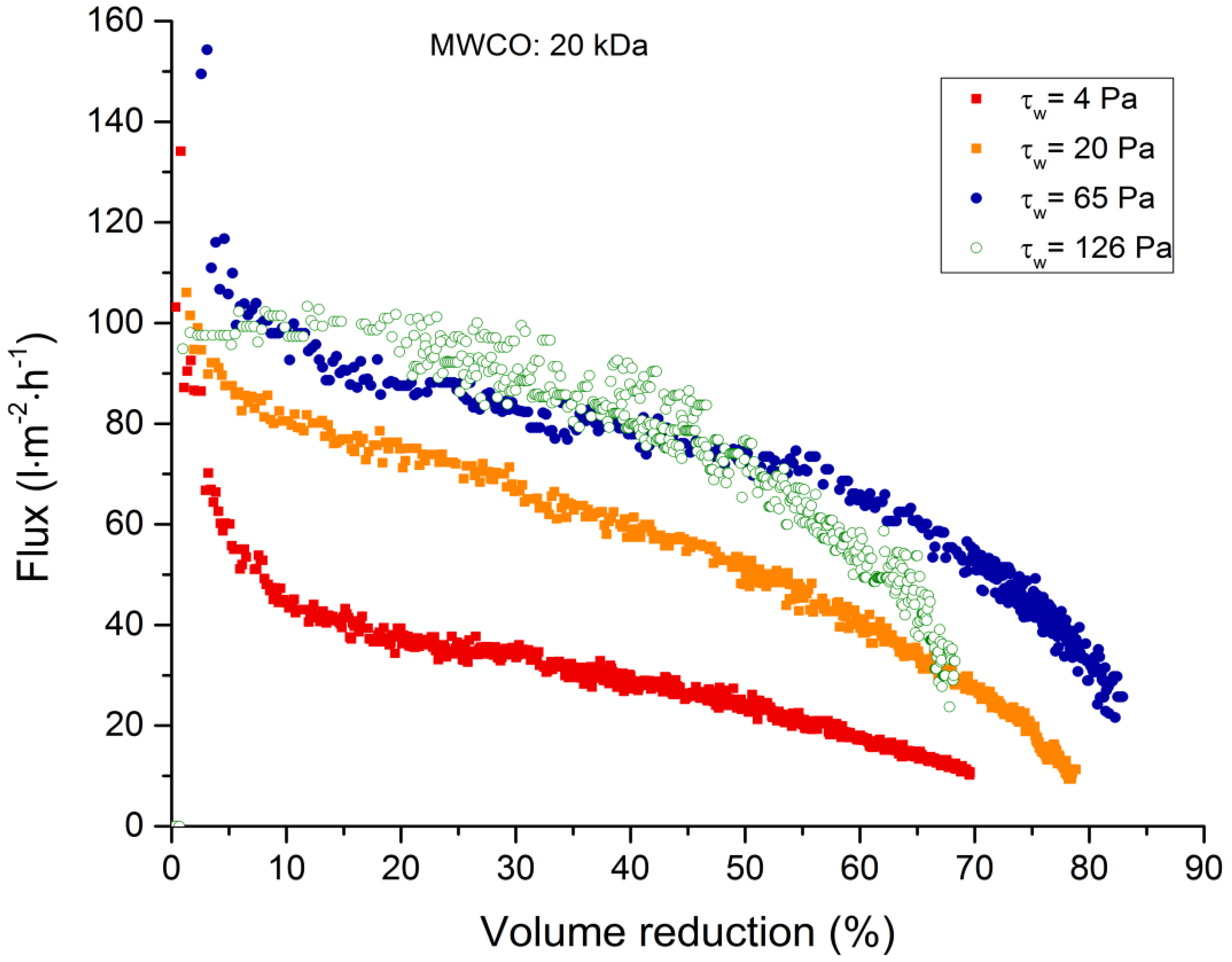
3.2. Membrane Filtration
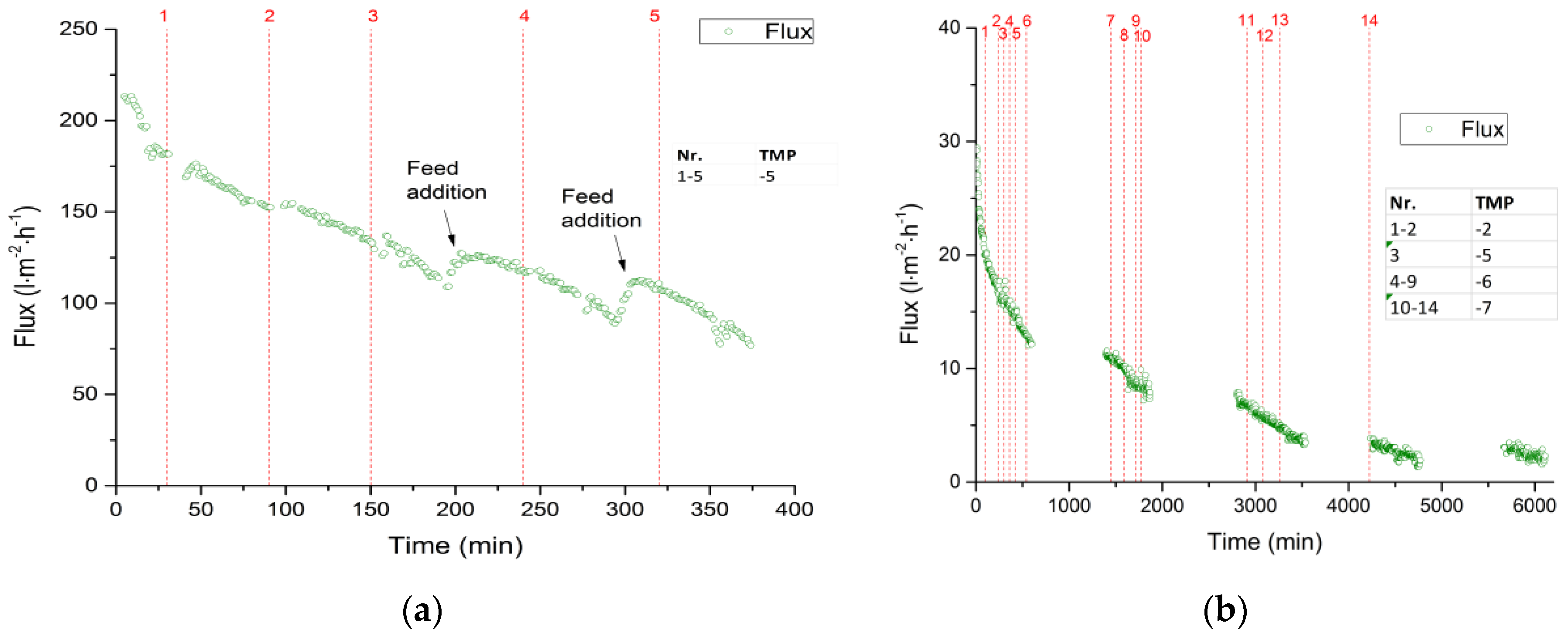
3.2.1. Total Recycle Mode Filtration
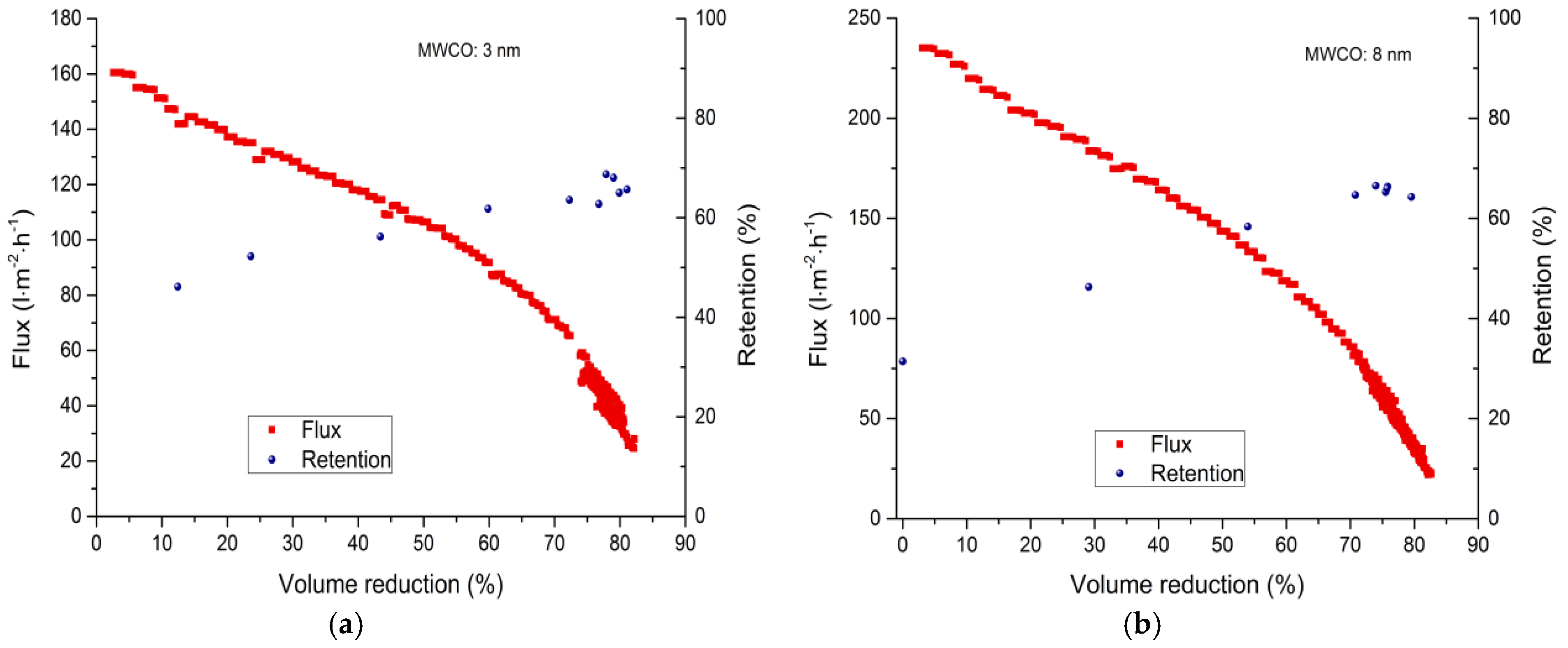
3.2.2. Fed-Batch-Filtration
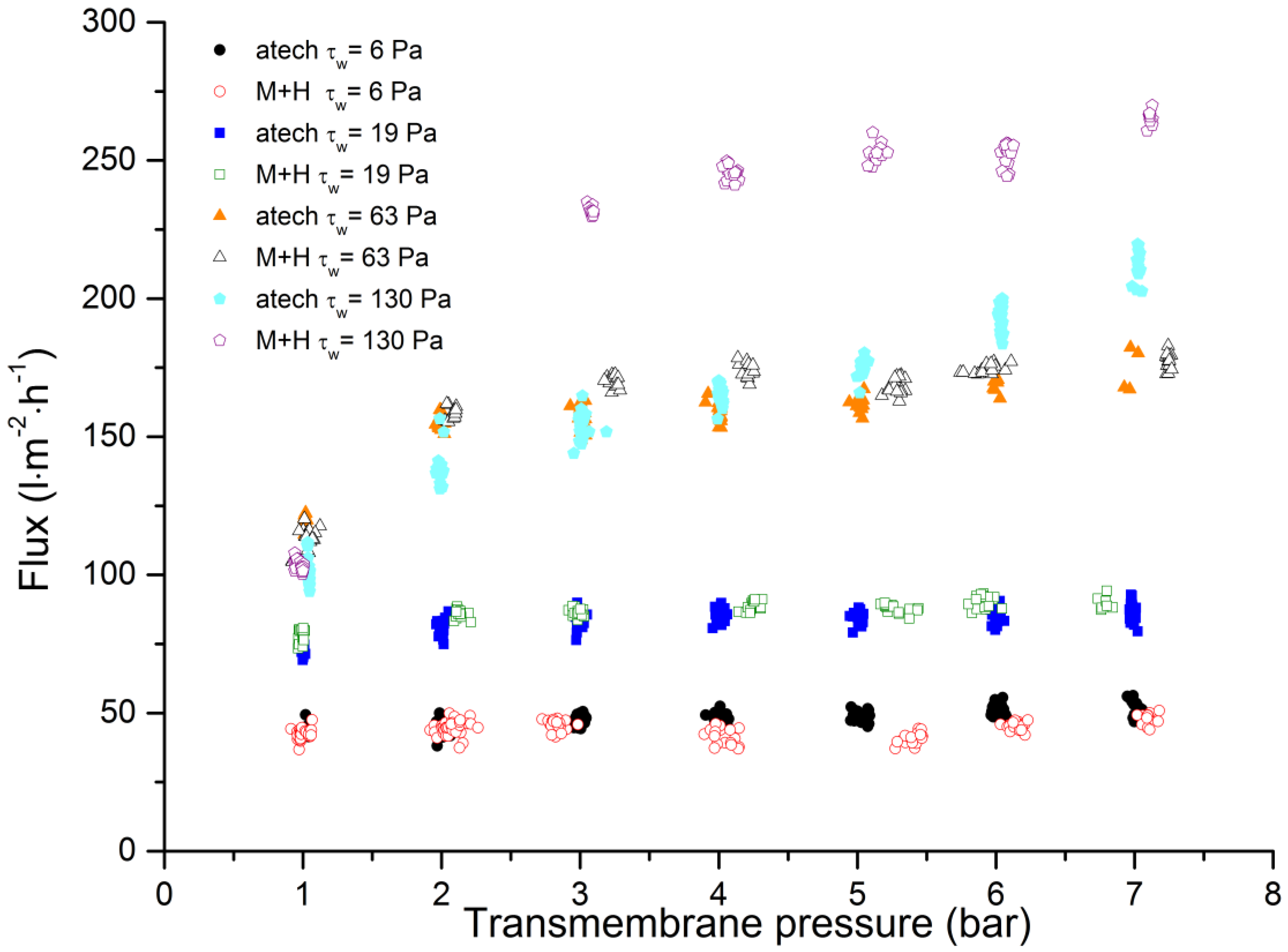
3.3. Comparison of Membrane Geometries
3.4. Backflushing
3.5. Membrane Cleaning
4. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Confederation of European Paper Industries. Key Statistics 2016 European Pulp & Paper Industry. Available online: http://www.cepi.org/system/files/public/documents/publications/statistics/2017/KeyStatistics2016_Final.pdf (accessed on 25 March 2019).
- Busch, R.; Hirth, T.; Liese, A.; Nordhoff, S.; Puls, J.; Pulz, O.; Sell, D.; Syldatk, C.; Ulber, R. Nutzung nachwachsender Rohstoffe in der industriellen Stoffproduktion. Chem. Ing. Tech. 2006, 78, 219–228. [Google Scholar] [CrossRef]
- Stephen Davidson, R.; Dunn, L.A.; Castellan, A.; Nourmamode, A. A study of the photobleaching and photoyellowing of paper containing lignin using fluorescence spectroscopy. J. Photochem. Photobiol. A Chem. 1991, 58, 349–359. [Google Scholar] [CrossRef]
- Pye, E.K. Industrial Lignin Production and Applications. In Biorefineries-Industrial Processes and Products; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; Volume 2, pp. 165–200. [Google Scholar]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. Influence of prefiltration on membrane performance during isolation of lignin-carbohydrate complexes from spent sulfite liquor. Sep. Purif. Technol. 2017, 187, 380–388. [Google Scholar] [CrossRef]
- Žabková, M.; da Silva, E.A.B.; Rodrigues, A.E. Recovery of vanillin from lignin/vanillin mixture by using tubular ceramic ultrafiltration membranes. J. Memb. Sci. 2007, 301, 221–237. [Google Scholar] [CrossRef]
- Axelsson, E.; Olsson, M.R.; Berntsson, T. Increased capacity in kraft pulp mills: Lignin separation and reduced steam demand compared with recovery boiler upgrade. Nord. Pulp Pap. Res. J. 2006, 21, 485–492. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass—Volume II-Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Lab.: Richland, WA, USA, 2007; Volume 2. [Google Scholar]
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Payne, S.A.; Gutschmidt, D. Lignin-based carbon fibers: Carbon nanotube decoration and superior thermal stability. Carbon N. Y. 2014, 80, 91–102. [Google Scholar] [CrossRef]
- Humpert, D.; Ebrahimi, M.; Czermak, P. Membrane Technology for the Recovery of Lignin: A Review. Membranes (Basel) 2016, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, S.; Ebrahimi, M.; Schoenherr, S. Lignin Degradation Processes and the Purification of Valuable Products Lignin Degradation Processes and the Purification of Valuable Products; IntechOpen: London, UK, 2017; pp. 1–36. [Google Scholar]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Lignin fractionation from E. Globulus kraft liquor by ultrafiltration in a three stage membrane sequence. Sep. Purif. Technol. 2018, 192, 140–151. [Google Scholar] [CrossRef]
- Olsson, J. Separation of lignin and hemicelluloses from black liquor and pre-treated black liquor by nanofiltration. Dep. Chem. Eng. 2013, 2, 1–6. [Google Scholar]
- Bhattacharjee, S.; Datta, S.; Bhattacharjee, C. Performance study during ultrafiltration of Kraft black liquor using rotating disk membrane module. J. Clean. Prod. 2006, 14, 497–504. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.-S. Separation of lignin in kraft cooking liquor from a continuous digester by ultrafiltration at temperatures above 100 °C. Desalination 2006, 195, 187–200. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kerker, S.; Daume, S.; Geile, M.; Ehlen, F.; Unger, I.; Schütz, S.; Czermak, P. Innovative ceramic hollow fiber membranes for recycling/reuse of oilfield produced water. Desalin. Water Treat. 2015, 55, 3554–3567. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; KluwerAcademic Publishers: Dordrecht, The Netherlands, 1996; Volume 53. [Google Scholar]
- Bhattacharya, P.K.; Todi, R.K.; Tiwari, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on ultrafiltration of spent sulfite liquor using various membranes for the recovery of lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Restolho, J.A.; Prates, A.; de Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Ringena, O.; Saake, B.; Lehnen, R. Isolation and fractionation of lignosulfonates by amine extraction and ultrafiltration: A comparative study. Holzforschung 2005, 59, 405–412. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Arayici, S.; Barlas, H. Treatment of Pulp and Paper Mill Wastewater Using Utrafiltration Process: Optimization of the Fouling and Rejections. Ind. Eng. Chem. Res. 2012, 51, 6184–6195. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; García, A.; Coz, A.; Labidi, J. Spent sulphite liquor fractionation into lignosulphonates and fermentable sugars by ultrafiltration. Sep. Purif. Technol. 2015, 152, 172–179. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.S.; Wickström, P. Membrane cleaning—A case study in a sulphite pulp mill bleach plant. Desalination 2001, 141, 259–268. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Ni, J.; Shi, H.; Qian, Y. Treatability of kraft spent liquor by microfiltration and ultrafiltration. Desalination 2004, 160, 131–141. [Google Scholar] [CrossRef]
- Jönsson, A.S.; Wallberg, O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Lee, M.; Wu, Z.; Wang, R.; Li, K. Micro-structured alumina hollow fibre membranes—Potential applications in wastewater treatment. J. Memb. Sci. 2014, 461, 39–48. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Inorganic hollow fibre membranes in catalytic processing. Curr. Opin. Chem. Eng. 2011, 1, 69–76. [Google Scholar] [CrossRef]
- Sappi Stockstadt GmbH Stockstadt Mill. Available online: https://www.sappi.com/node/14786 (accessed on 25 March 2019).
- Yan, M.; Yang, D.; Deng, Y.; Chen, P.; Zhou, H.; Qiu, X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 50–58. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Qian, Y.; Ouyang, X.; Yang, D.; Qui, X. Adsorption and desorption behaviors of lignosulfonate during the self-assembly of multilayers. BioResources 2010, 5, 1178–1196. [Google Scholar]
- Strathmann, H. Trennung von Molekularen Mischungen mit Hilfe Synthetischer Membranen; Steinkopff: Heidelberg, Germany, 1979. [Google Scholar]
- Shapiro, A.; Jaffrin, M.; Weinberg, S. Peristaltic pumping with long wavelength at low Reynolds number. J. Fluid Mech. 1969, 37, 799–825. [Google Scholar] [CrossRef]
- Schlichting, H. Grenzschicht-Theorie; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Böswirth, L.; Bschorer, S. Technische Strömungslehre; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2014. [Google Scholar]
- Li, R.; Yang, D.; Guo, W.; Qiu, X. The adsorption and dispersing mechanisms of sodium lignosulfonate on Al2O3 particles in aqueous solution. Holzforschung 2012, 67, 1–8. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, J.; Li, G.; Song, G. Effect of Complexation on the Zeta Potential of Titanium Dioxide Dispersions. J. Dispers. Sci. Technol. 2003, 24, 527–535. [Google Scholar] [CrossRef]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.-S.; Wimmerstedt, R. Fractionation and concentration of kraft black liquor lignin with ultrafiltration. Desalination 2003, 154, 187–199. [Google Scholar] [CrossRef]
- De, S.; Bhattacharya, P.K. Flux prediction of black liquor in cross flow ultrafiltration using low and high rejecting membranes. J. Memb. Sci. 1996, 109, 109–123. [Google Scholar] [CrossRef]
- Dafinov, A.; Font, J.; Garcia-Valls, R. Processing of black liquors by UF/NF ceramic membranes. Desalination 2005, 173, 83–90. [Google Scholar] [CrossRef]
- Irmler, H.W. Dynamische Filtration mit keramischen Membranen; Vulkan Verlag: Essen, Germany, 2001. [Google Scholar]
- Horiba Instruments. Isoelectric Point Determination (Application Note); Horiba Instruments: Kyoto, Japan, 2011. [Google Scholar]
- Duscher, S. Ceramic membranes for the filtration of liquids: An actual overview. Filtr. Sep. Int. Ed. 2014, 13–21. [Google Scholar]
- Huisman, I.H.; Vellenga, E.; Trägårdh, G.; Trägårdh, C. The influence of the membrane zeta potential on the critical flux for crossflow microfiltration of particle suspensions. J. Memb. Sci. 1999, 156, 153–158. [Google Scholar] [CrossRef]
- Satyanarayana, S.V.; Bhattacharya, P.K.; De, S. Flux decline during ultrafiltration of kraft black liquor using different flow modules: A comparative study. Sep. Purif. Technol. 2000, 20, 155–167. [Google Scholar] [CrossRef]
- Accary, G.; Morvan, D.; Me, S. The Human Line-1 Retrotranspsons Creates DNA Double Strand Breaks. Fire Saf. J. 2008, 93, 173–178. [Google Scholar]
- Abdelrasoul, A.; Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; Pesek, K., Ed.; InTech: London, UK, 2013. [Google Scholar]
- Fan, R. Process Monitoring and Control Using Innovative Optical Sensors for Fermentative Lactic Acid Production in Membrane Bioreactor System; Shaker Verlag: Herzogenrath, Germany, 2016. [Google Scholar]
- Persson, T.; Jönsson, A.-S. Isolation of hemicelluloses by ultrafiltration of thermomechanical pulp mill process water—Influence of operating conditions. Chem. Eng. Res. Des. 2010, 88, 1548–1554. [Google Scholar] [CrossRef]
- Kekana, P.; Sithole, B.; Ramjugernath, D. Stirred cell ultrafiltration of lignin from black liquor generated from South African kraft mills. S. Afr. J. Sci. 2016, 112, 1–7. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Nordin, A.-K.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Chan, C.C.V.; Bérubé, P.R.; Hall, E.R. Relationship between types of surface shear stress profiles and membrane fouling. Water Res. 2011, 45, 6403–6416. [Google Scholar] [CrossRef] [PubMed]
- Cabassud, C.; Laborie, S.; Lainé, J.M. How slug flow can improve ultrafiltration flux in organic hollow fibres. J. Memb. Sci. 1997, 128, 93–101. [Google Scholar] [CrossRef]
- Le Berre, O.; Daufin, G. Skimmilk crossflow microfiltration performance versus permeation flux to wall shear stress ratio. J. Memb. Sci. 1996, 117, 261–270. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Lin, S.-J. Filtration flux–shear stress–cake mass relationships in microalgae rotating-disk dynamic microfiltration. Chem. Eng. J. 2014, 244, 429–437. [Google Scholar] [CrossRef]
- Lee, M.; Wu, Z.; Li, K. Advances in ceramic membranes for water treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 43–82. [Google Scholar]
- Bottino, A.; Capannelli, G.; Comite, A.; Di Felice, R. Polymeric and ceramic membranes in three-phase catalytic membrane reactors for the hydrogenation of methylenecyclohexane. Desalination 2002, 144, 411–416. [Google Scholar] [CrossRef]
- Bolduan, P.; Latz, M. Filtration by Means of Ceramic Membranes—Practical Examples from the Chemical and Food Industries. Available online: https://pdfs.semanticscholar.org/cf37/6522f35b0586689ddd212ee7b6521d781881.pdf (accessed on 25 March 2019).
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Ehlen, F.; Schütz, S.; Unger, I.; Kariveti, S.; Wang, C.; Ebrahimi, M.; Kerker, S.; Czermak, P. Development of Ceramic Hollow Fiber Membranes and their Application in Water/Oil Separation Processes. In Proceedings of the 90th DKG Annual Meeting, Byreuth, Germany, 15–19 March 2015. [Google Scholar]
- Schmeda Lopez, D.R. Morphological, Mechanical and Gas Transport Properties of Stainless Steel and Composite Hollow Fibres; The University of Queensland: Brisbane, Australia, 2014. [Google Scholar]
- Liu, S.; Li, K.; Hughes, R. Preparation of porous aluminium oxide (Al2O3) hollow fibre membranes by a combined phase-inversion and sintering method. Ceram. Int. 2003, 29, 875–881. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Busse, N.; Kerker, S.; Schmitz, O.; Hilpert, M.; Czermak, P. Treatment of the Bleaching Effluent from Sulfite Pulp Production by Ceramic Membrane Filtration. Membranes (Basel) 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Redkar, S.; Kuberkar, V.; Davis, R.H. Modeling of concentration polarization and depolarization with high-frequency backpulsing. J. Memb. Sci. 1996, 121, 229–242. [Google Scholar] [CrossRef]
- Mores, W.D.; Davis, R.H. Yeast-Fouling Effects in Cross-Flow Microfiltration with Periodic Reverse Filtration. Ind. Eng. Chem. Res. 2003, 42, 130–139. [Google Scholar] [CrossRef]
- Weis, A.; Michael, B.; Marianne, N. The chemical cleaning of polymeric UF membranes fouled with spent sulphite liquor over multiple operational cycles. J. Memb. Sci. 2003, 216, 67–79. [Google Scholar] [CrossRef]
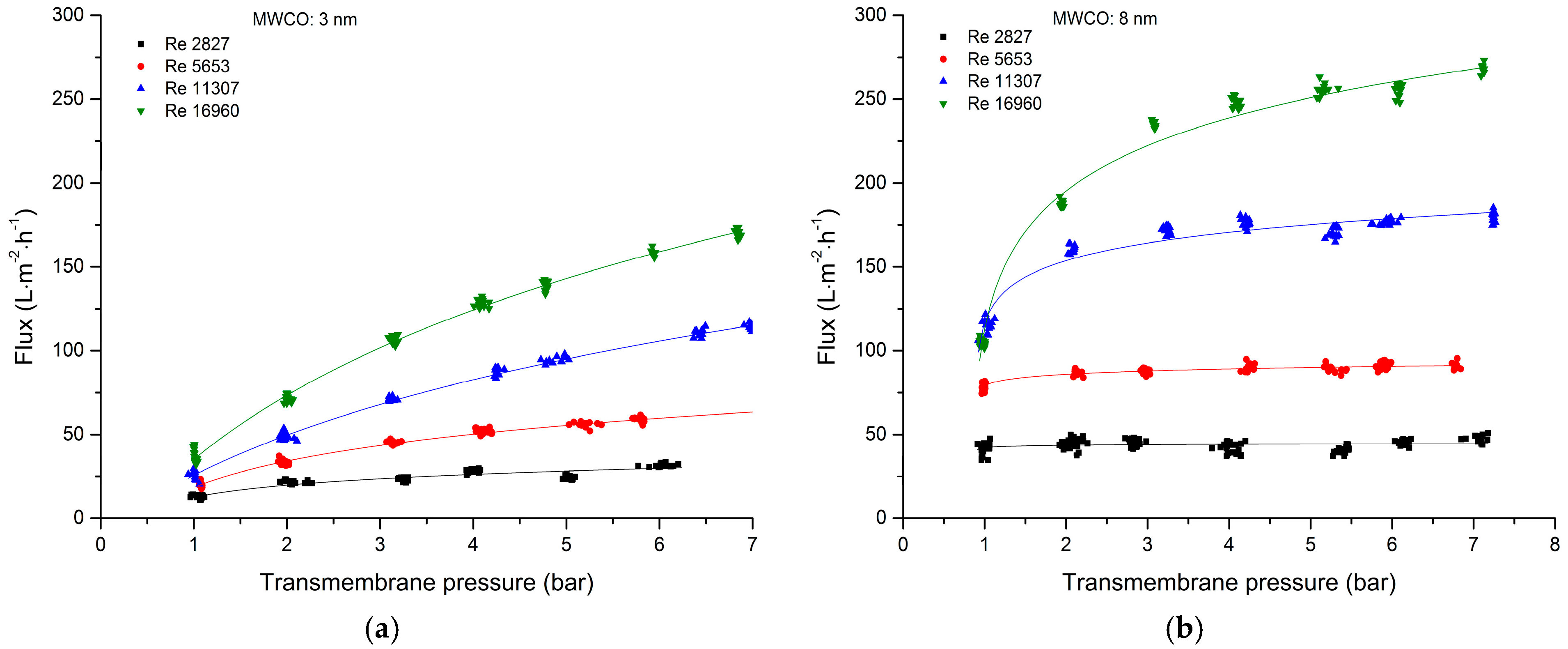
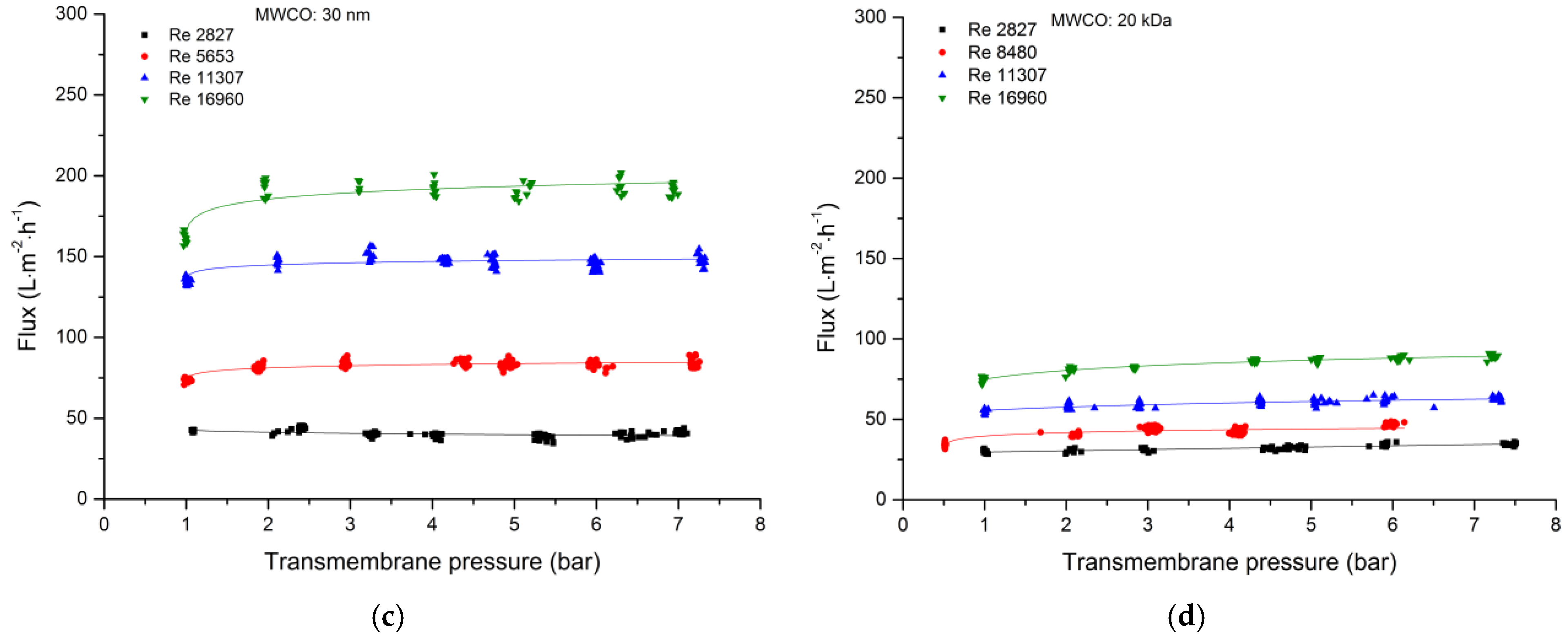

| Cut-Off | Manufacturer | Material Active Layer Inner Surface | Length (m) | Inner Diameter (mm) | Membrane Geometry | Membrane Area (m2) | Pure Water Flux (L·m−2·h−1·bar−1) |
|---|---|---|---|---|---|---|---|
| 20 kDa | atech innovations | Al2O3 | 0.25 | 6 | Tubular | 0.0047 | 262 |
| 3 nm | Mann+Hummel | TiO2 | 0.25 | 2 | Hollow fiber | 0.0038 | 49 |
| 8 nm | Mann+Hummel | TiO2 | 0.25 | 2 | Hollow fiber | 0.0030 | 251 |
| 30 nm | Mann+Hummel | Al2O3 | 0.25 | 2 | Hollow fiber | 0.0038 | 563 |
| Component | Value |
|---|---|
| Density (20 °C) | 1060 kg·m−3 |
| pH (20 °C) | 2.7 |
| Dry residue (after 72 h at 105 °C) | 12.5% |
| Ignition loss (550 °C) | 88.8% |
| Chemical oxygen demand | 138.3 mg·kg−1 |
| Glucose | 0.39 g·kg−1 |
| Xylose | 11.48 g·kg−1 |
| Galactose and rhamnose | 1.02 g·kg−1 |
| Arabinose | 0.20 g·kg−1 |
| Mannose | 0.56 g·kg−1 |
| Methanol | 320 mg·kg−1 |
| Ethanol | 50 mg·kg−1 |
| Hydroxymethylfurfural | 24 mg·kg−1 |
| Acetic acid | 9.0 g·kg−1 |
| Material | Zeta Potential | |
|---|---|---|
| Measured | Literature | |
| Al2O3 | (33.9 ± 7.3) mV | ~38 mV |
| TiO2 | (26.6 ± 5.3) mV | ~30 mV |
| Membrane | 0% VR (L·m−2·h−1) | 30% VR (L·m−2·h−1) | 50% VR (L·m−2·h−1) | 80% VR (L·m−2·h−1) |
|---|---|---|---|---|
| 3 nm | 160 | 128 | 106 | 40–35 |
| 8 nm | 235 | 184 | 144 | 42–34 |
| 30 nm | 218 | 156 | 147 | 97–91 |
| 20 kDa | 94 | 64 | 51 | 12–11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humpert, D.; Ebrahimi, M.; Stroh, A.; Czermak, P. Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes. Membranes 2019, 9, 45. https://doi.org/10.3390/membranes9040045
Humpert D, Ebrahimi M, Stroh A, Czermak P. Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes. Membranes. 2019; 9(4):45. https://doi.org/10.3390/membranes9040045
Chicago/Turabian StyleHumpert, Daniel, Mehrdad Ebrahimi, Annika Stroh, and Peter Czermak. 2019. "Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes" Membranes 9, no. 4: 45. https://doi.org/10.3390/membranes9040045
APA StyleHumpert, D., Ebrahimi, M., Stroh, A., & Czermak, P. (2019). Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes. Membranes, 9(4), 45. https://doi.org/10.3390/membranes9040045






