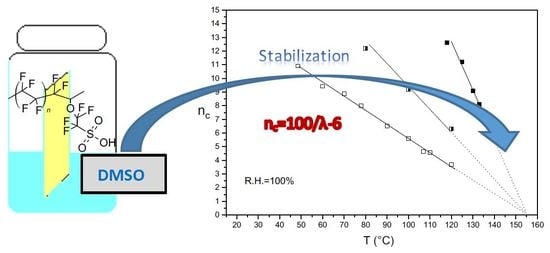
Study of Annealed Aquivion® Ionomers with the INCA Method †
Abstract
1. Introduction
2. Experimental
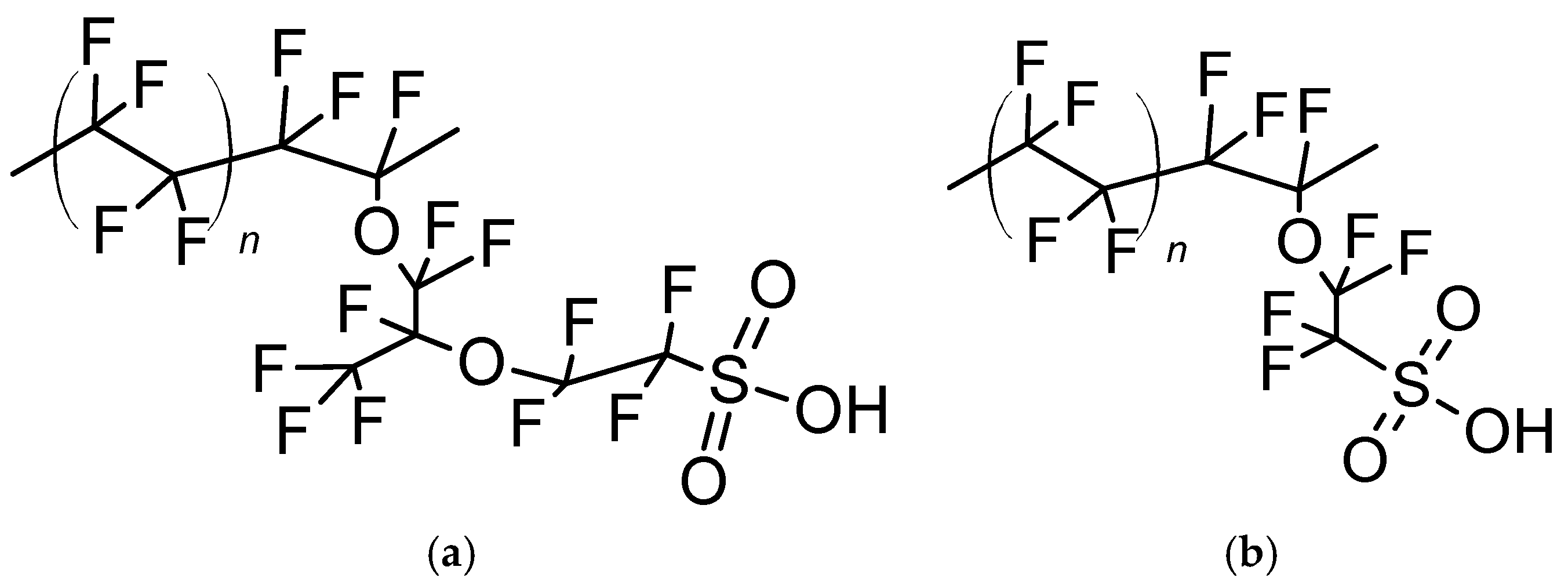
2.1. Chemicals
2.2. Membrane Preparation
2.2.1. Extruded Semi-Crystalline Aquivion® 870 and 980
2.2.2. Un-Crystallized Aquivion® 790 and 830
2.2.3. Annealed Semi-Crystalline Aquivion® 870 and 980
2.2.4. Annealed Aquivion® 830
2.3. Characterization
2.3.1. Water Uptake and nc Measurements
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
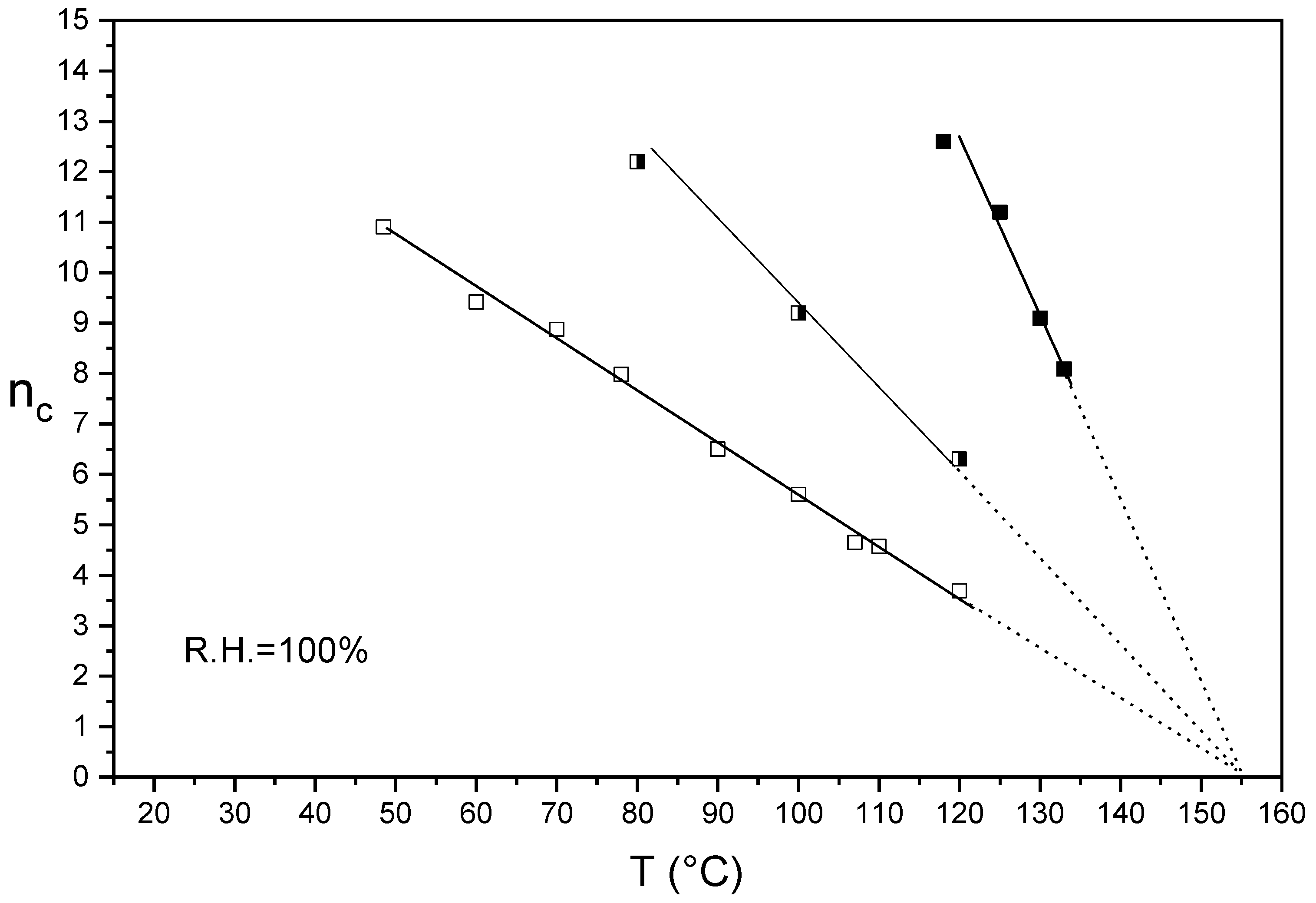
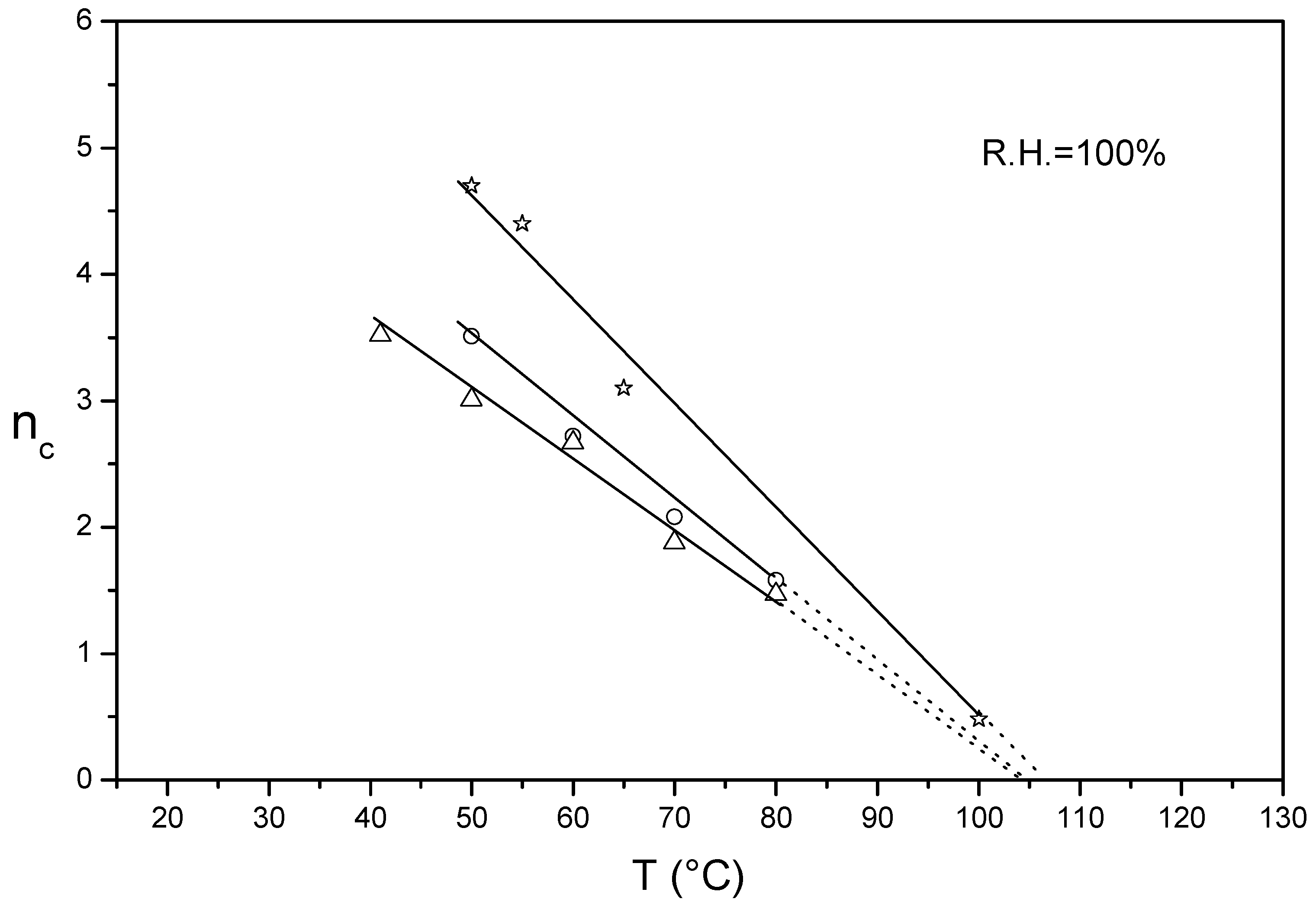
3.1. nc/T Plots of Un-Annealed and Annealed Semi-Crystalline Extruded Aquivion® 870 and 980
3.2. nc/T Plots of Un-Crystallized Aquivion®
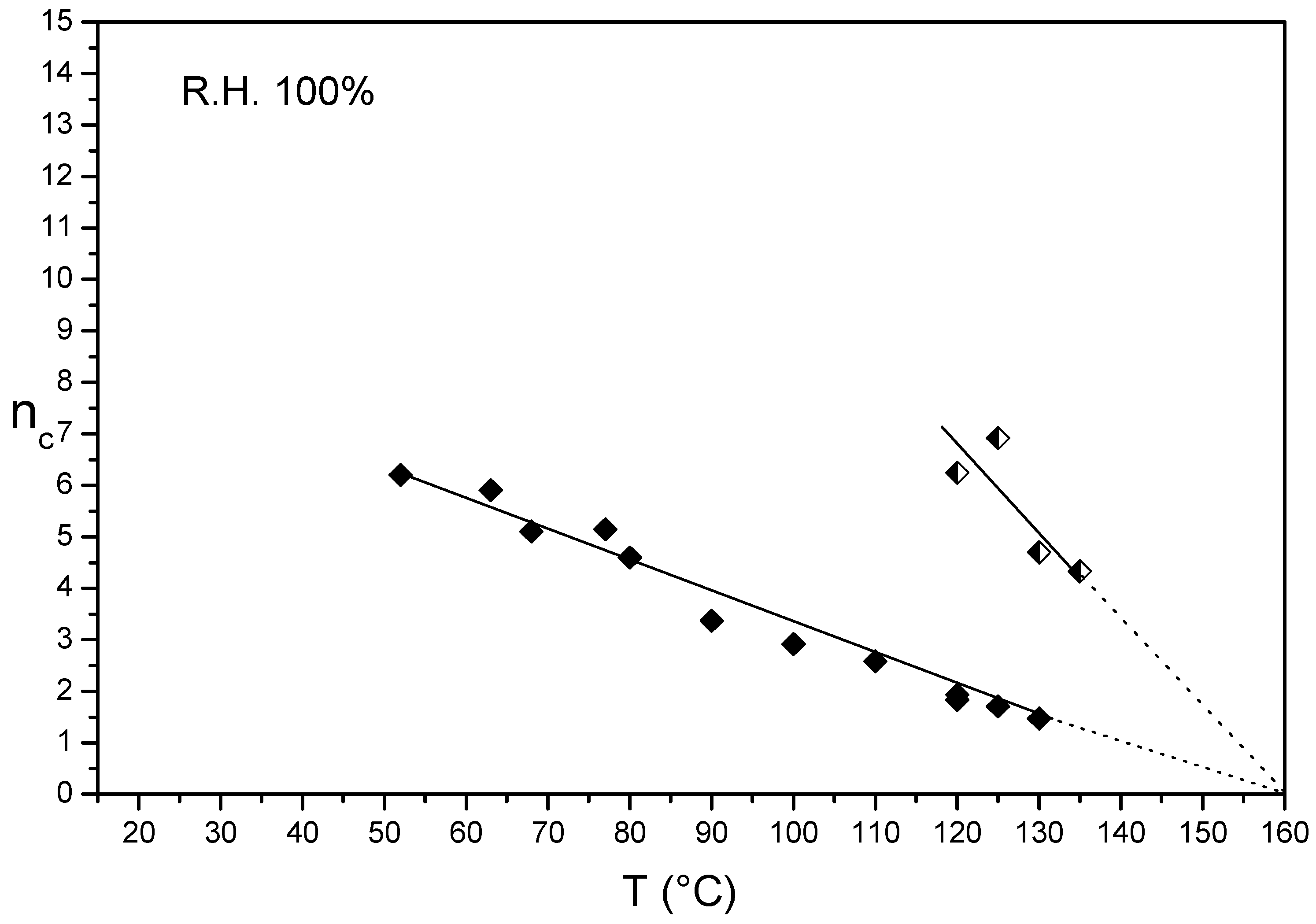
3.3. nc/T Plots of Un-Crystallized and Annealed Aquivion® 830
3.4. Dynamic Mechanical Analysis (DMA) of Pristine and Annealed Aquivion® 870
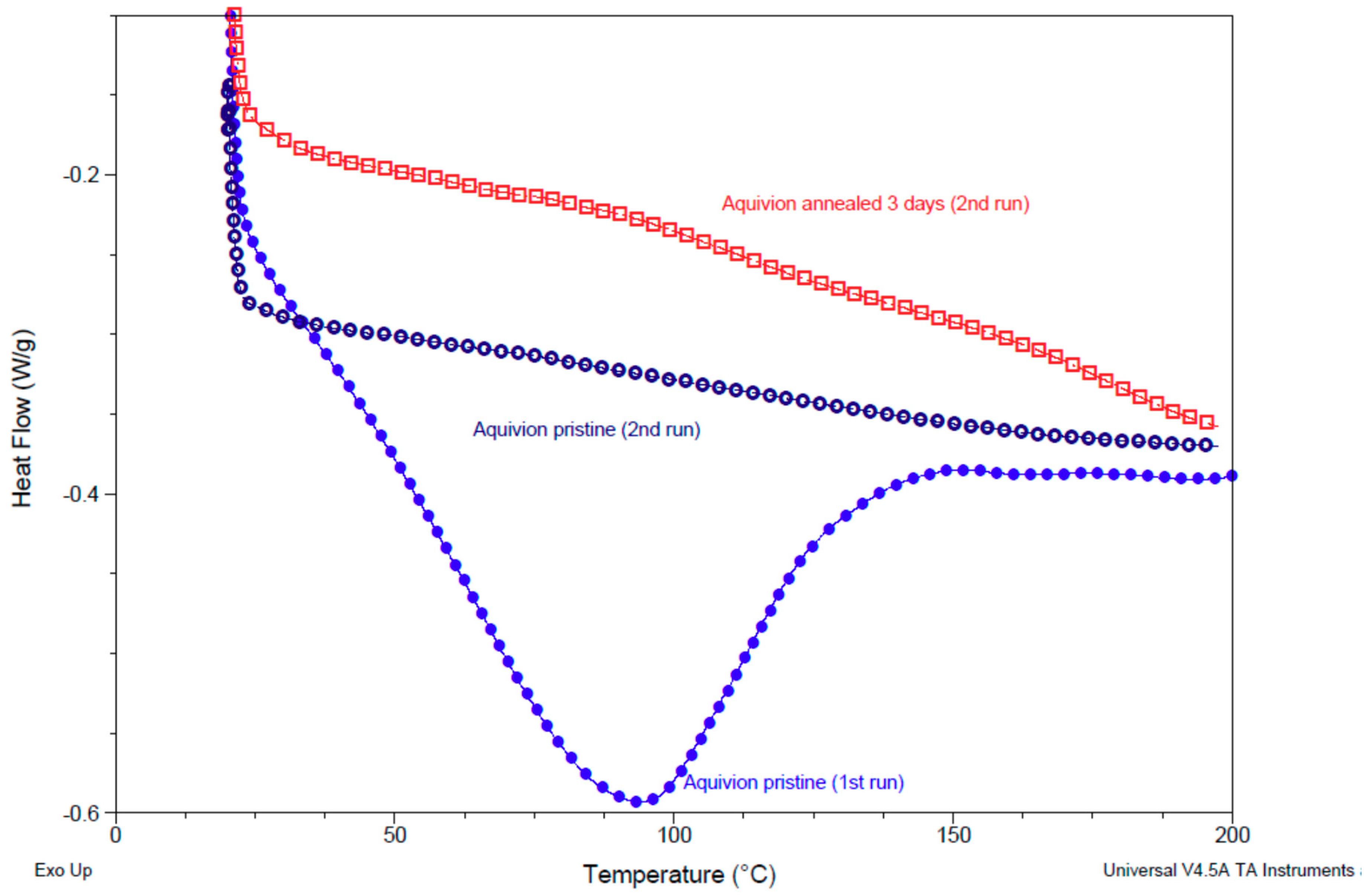
3.5. Differential Scanning Calorimetry (DSC) of Pristine and Annealed Aquivion® 870
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carmo, M.; Fritz, D.L.; Merge, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Gotz, M.; Lefebvre, J.; Mors, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water Uptake by and Transport Through Nafion® 117 Membranes. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric proton conducting membranes for medium temperature fuel cells (110–160°C). J. Membr. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Roberti, E.; Carlotti, G.; Cinelli, S.; Onori, G.; Donnadio, A.; Narducci, R.; Casciola, M.; Sganappa, M. Measurement of the Young’s modulus of Nafion membranes by Brillouin light scattering. J. Power Sources 2010, 195, 7761–7764. [Google Scholar] [CrossRef]
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane Matrices Based on Nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the Effect of Crystal Size, Sulfonation, and Filler Loading on the Mechanical and Conductivity Properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compan, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1–30. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Pabby, A.K.; Rizvi, S.S.H.; Requena, A.M.S. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wang, L.; Husar, A.; Zhou, T.H.; Liu, H.T. A parametric study of PEM fuel cell performances. Int. J. Hydrogen Energy 2003, 28, 1263–1272. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Capitani, D.; Donnadio, A.; Narducci, R.; Pica, M.; Sganappa, M. Novel Nafion–zirconium phosphate nanocomposite membranes with enhanced stability of proton conductivity at medium temperature and high relative humidity. Electrochim. Acta 2007, 52, 8125–8132. [Google Scholar] [CrossRef]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Siracusano, S.; van Dijk, N.; Payne-Johnson, E.; Baglio, V.; Arico, A.S. Nanosized IrOx and IrRuOx electrocatalysts for the O2 evolution reaction in PEM water electrolysers. Appl. Catal. B-Environ. 2018, 164, 488–495. [Google Scholar] [CrossRef]
- Boaretti, C.; Pasquini, L.; Sood, R.; Giancola, S.; Donnadio, A.; Roso, M.; Modesti, M.; Cavaliere, S. Mechanically stable nanofibrous sPEEK/Aquivion® composite membranes for fuel cell applications. J. Membr. Sci. 2018, 545, 66–74. [Google Scholar] [CrossRef]
- Arico, A.S.; di Blasi, A.; Brunaccini, G.; Sergi, F.; Dispenza, G.; Andaloro, L.; Ferraro, M.; Antonucci, V.; Asher, P.; Buche, S.; et al. High Temperature Operation of a Solid Polymer Electrolyte Fuel Cell Stack Based on a New Ionomer Membrane. Fuel Cells 2010, 10, 1013–1023. [Google Scholar] [CrossRef]
- Skulimowska, A.; Dupont, M.; Zaton, M.; Sunde, S.; Merlo, L.; Jones, D.J.; Roziere, J. Proton exchange membrane water electrolysis with short-side-chain Aquivion® membrane and IrO2 anode catalyst. Int. J. Hydrogen Energy 2014, 39, 6307–6316. [Google Scholar] [CrossRef]
- Giancola, S.; Zaton, M.; Reyes-Carmona, A.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Roziere, J.; Jones, D.J. Composite short side chain PFSA membranes for PEM water electrolysis. J. Membr. Sci. 2019, 570, 69–76. [Google Scholar] [CrossRef]
- Rolfi, A.; Oldani, C.; Merlo, L.; Facchi, D.; Ruffo, R. New perfluorinated ionomer with improved oxygen permeability for application in cathode polymeric electrolyte membrane fuel cell. J. Power Sources 2018, 396, 95–101. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Narducci, R.; Sganappa, M. Effects of hydrothermal/thermal treatments on the water-uptake of Nafion membranes and relations with changes of conformation, counter-elastic force and tensile modulus of the matrix. J. Power Sources 2008, 178, 575–583. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R. Evolution of Permanent Deformations (or Memory) in Nafion 117 Membranes with Changes in Temperature, Relative Humidity and Time, and Its Importance in the Development of Medium Temperature PEMFCs. Fuel Cells 2009, 9, 410–420. [Google Scholar] [CrossRef]
- Alberti, G.; di Vona, M.L.; Narducci, R. New results on the visco-elastic behaviour of ionomer membranes and relations between T–RH plots and proton conductivity decay of Nafion® 117 in the range 50–140 °C. Int. J. Hydrogen Energy 2012, 37, 6302–6307. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; di Vona, M.L.; Giancola, S. Annealing of Nafion 1100 in the Presence of an Annealing Agent: A Powerful Method for Increasing Ionomer Working Temperature in PEMFCs. Fuel Cells 2013, 13, 42–47. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; di Vona, M.L.; Giancola, S. More on Nafion Conductivity Decay at Temperatures Higher than 80 °C: Preparation and First Characterization of In-Plane Oriented Layered Morphologies. Ind. Eng. Chem. Res. 2013, 52, 10418–10424. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; di Vona, M.L.; Giancola, S. Preparation and Nc/T plots of un-crystallized Nafion 1100 and semi-crystalline Nafion 1000. Int. J. Hydrogen Energy 2017, 42, 15908–15912. [Google Scholar] [CrossRef]
- Narducci, R.; di Vona, M.L.; Marrocchi, A.; Baldinelli, G. Stabilized SPEEK Membranes with a High Degree of Sulfonation for Enthalpy Heat Exchangers. Coatings 2018, 8, 190. [Google Scholar] [CrossRef]
- Narducci, R.; Knauth, P.; Chailan, J.F.; di Vona, M.L. How to improve Nafion with tailor made annealing. Rsc Adv. 2018, 8, 27268–27274. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; di Vona, M.L. Solid State Proton Conductors: Properties and Applications in Fuel Cells; Knauth, P., di Vona, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2012; Chapter 8; ISBN 978-0-470-66937-2. [Google Scholar]
- Subianto, S.; Pica, M.; Casciola, M.; Cojocaru, P.; Merlo, L.; Hards, G.; Jones, D.J. Physical and chemical modification routes leading to improved mechanical properties of perfluorosulfonic acid membranes for PEM fuel cells. J. Power Sources 2013, 233, 216–230. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R.J. On the decay of Nafion proton conductivity at high temperature and relative humidity. Power Sources 2006, 162, 141–145. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.Y.; Wei, B.; Zhang, Z.; Li, H.; Wang, H.J. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Yin, C.S.; Wang, Z.; Luo, Y.; Li, J.J.; Zhou, Y.W.; Zhang, X.W.; Zhang, H.N.; Fang, P.F.; He, C.Q. Thermal annealing on free volumes, crystallinity and proton conductivity of Nafion membranes. J. Phys. Chem. Solids 2018, 120, 71–78. [Google Scholar] [CrossRef]
- Mugtasimova, K.R.; Melnikov, A.P.; Galitskaya, E.A.; Kashin, A.M.; Dobrovolskiy, Y.A.; Don, G.M.; Likhomanov, V.S.; Sivak, A.V.; Sinitsyn, V.V. Fabrication of Aquivion-type membranes and optimization of their elastic and transport characteristics. Ionics 2018, 24, 3897–3903. [Google Scholar] [CrossRef]
- Lee, K.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K. Effect of Recast Temperature on Diffusion and Dissolution of Oxygen and Morphological Properties in Recast Nafion. J. Electrochem. Soc. 2004, 151, A639–A645. [Google Scholar] [CrossRef]
- Casciola, M.; Cojocaru, P.; Donnadio, A.; Giancola, S.; Merlo, L.; Nedellec, Y.; Pica, M.; Subianto, S. Zirconium phosphate reinforced short side chain perflurosulfonic acid membranes for medium temperature proton exchange membrane fuel cell application. J. Power Sources 2014, 262, 407–413. [Google Scholar] [CrossRef]
- Gebel, G.; Aldebert, P.; Pineri, M. Structure and related properties of solution-cast perfluorosulfonated ionomer film. Macromolecules 1987, 20, 1425–1428. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. Factors affecting the stability of Nafion conductivity at high temperature and relative humidity. Desalination 2006, 200, 639–641. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Moukheiber, E.; de Moor, G.; Flandin, L.; Bas, C. Investigation of ionomer structure through its dependence on ion exchange capacity (IEC). J. Membr. Sci. 2012, 389, 294–304. [Google Scholar] [CrossRef]
- Chailan, J.; Khadhraoui, M.; Knauth, P. Solid State Proton Conductors: Properties and Applications in Fuel Cells; Knauth, P., di Vona, M.L., Eds.; Wiley: Hoboken, NJ, USA, 2012; Chapter 6; ISBN 978-0-470-66937-2. [Google Scholar]

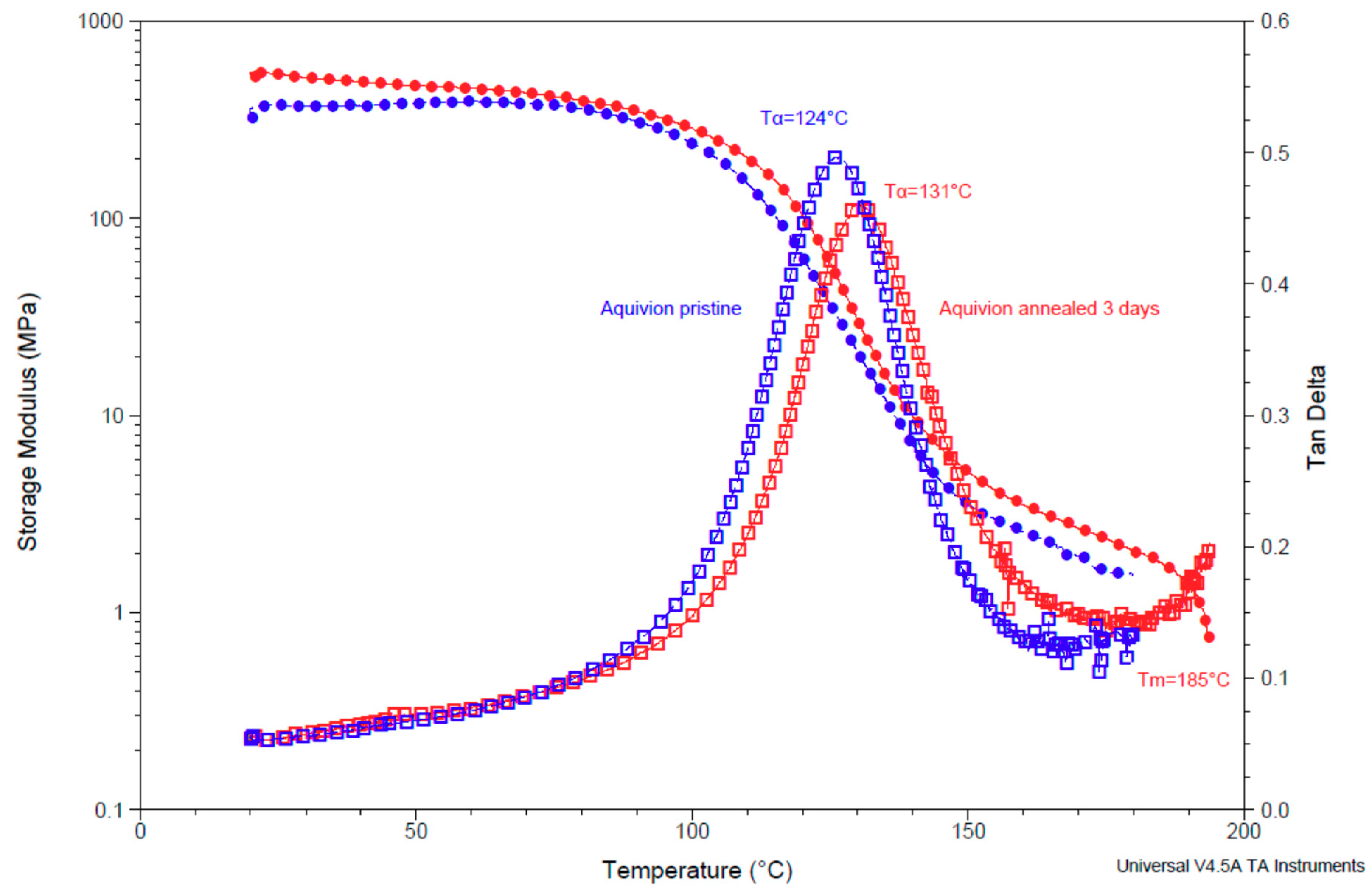
| Sample | Tα/°C | I (tan δ) | E′/MPa (25 °C) | E’/MPa (50 °C) |
|---|---|---|---|---|
| Aquivion 870 pristine | 124 ± 3 | 0.49 | 350 ± 30 | 360 ± 40 |
| Aquivion 870 3 days annealed | 131 ± 1 | 0.46 | 540 ± 10 | 490 ± 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giancola, S.; Arciniegas, R.A.B.; Fahs, A.; Chailan, J.-F.; Di Vona, M.L.; Knauth, P.; Narducci, R.
Study of Annealed Aquivion® Ionomers with the INCA Method
Giancola S, Arciniegas RAB, Fahs A, Chailan J-F, Di Vona ML, Knauth P, Narducci R.
Study of Annealed Aquivion® Ionomers with the INCA Method
Giancola, Stefano, Raul Andres Becerra Arciniegas, Armand Fahs, Jean-Franҫois Chailan, Maria Luisa Di Vona, Philippe Knauth, and Riccardo Narducci.
2019. "Study of Annealed Aquivion® Ionomers with the INCA Method
Giancola, S., Arciniegas, R. A. B., Fahs, A., Chailan, J.-F., Di Vona, M. L., Knauth, P., & Narducci, R.
(2019). Study of Annealed Aquivion® Ionomers with the INCA Method