Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications
Abstract
:1. Introduction
2. Definition of the Gas Separation Membrane
3. Design of Microporous Structure in Membranes
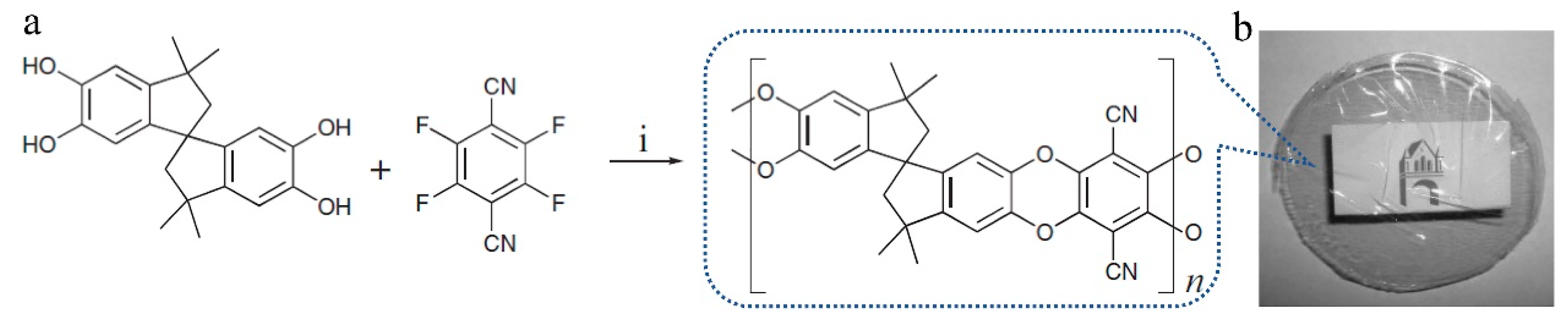
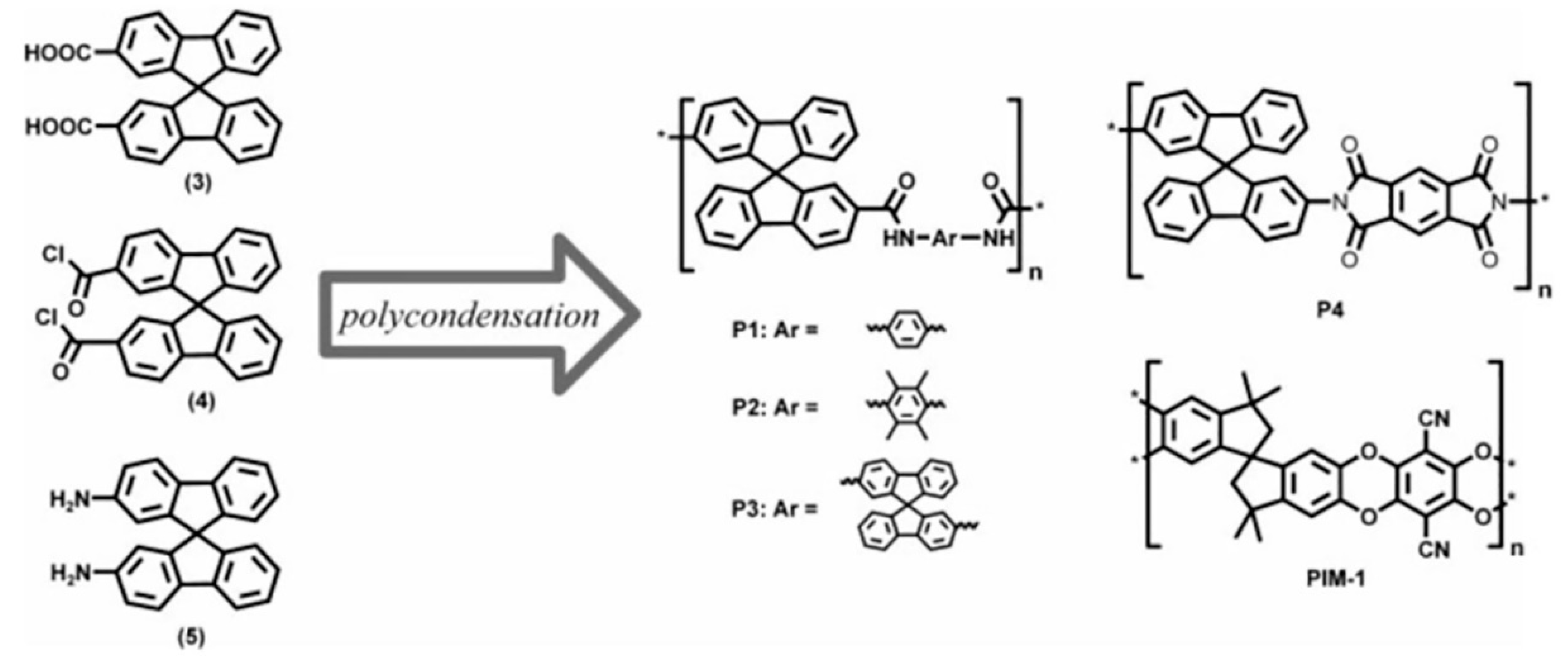
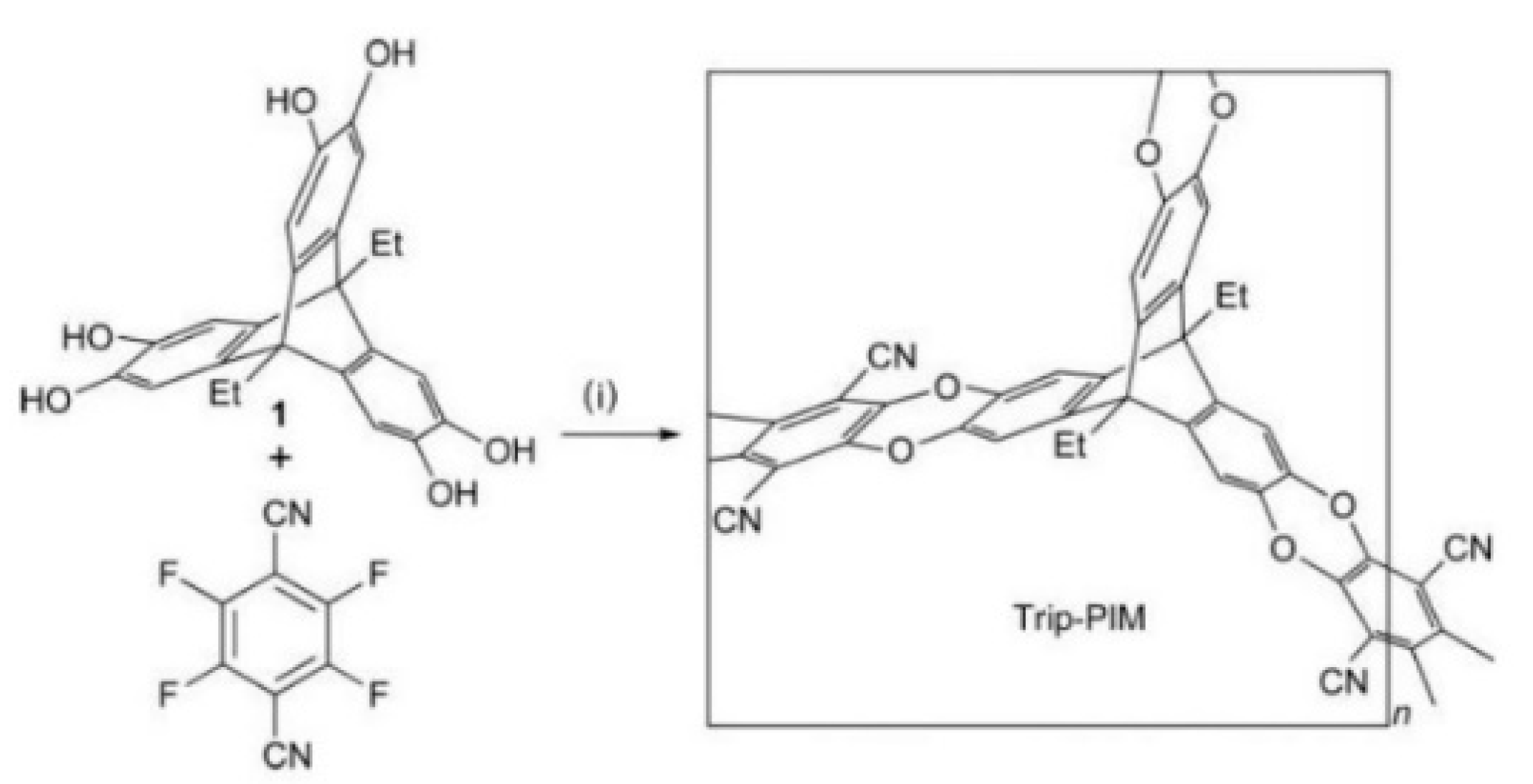
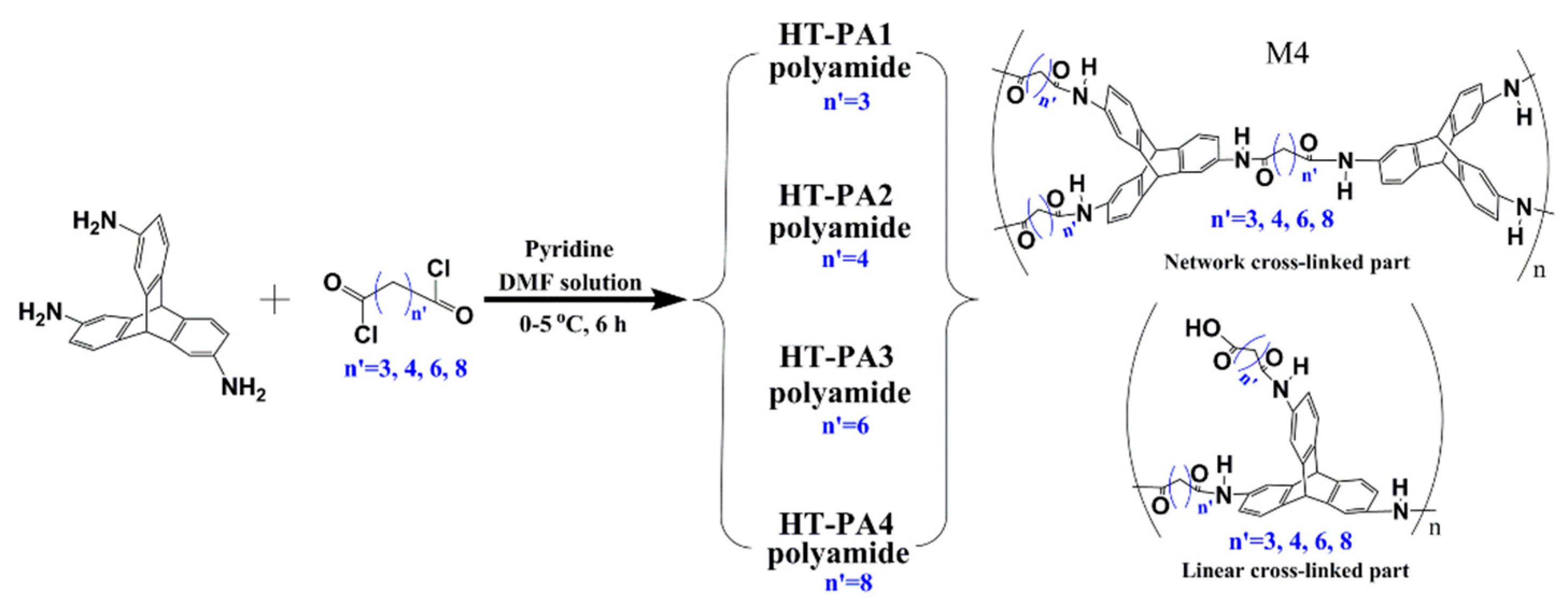
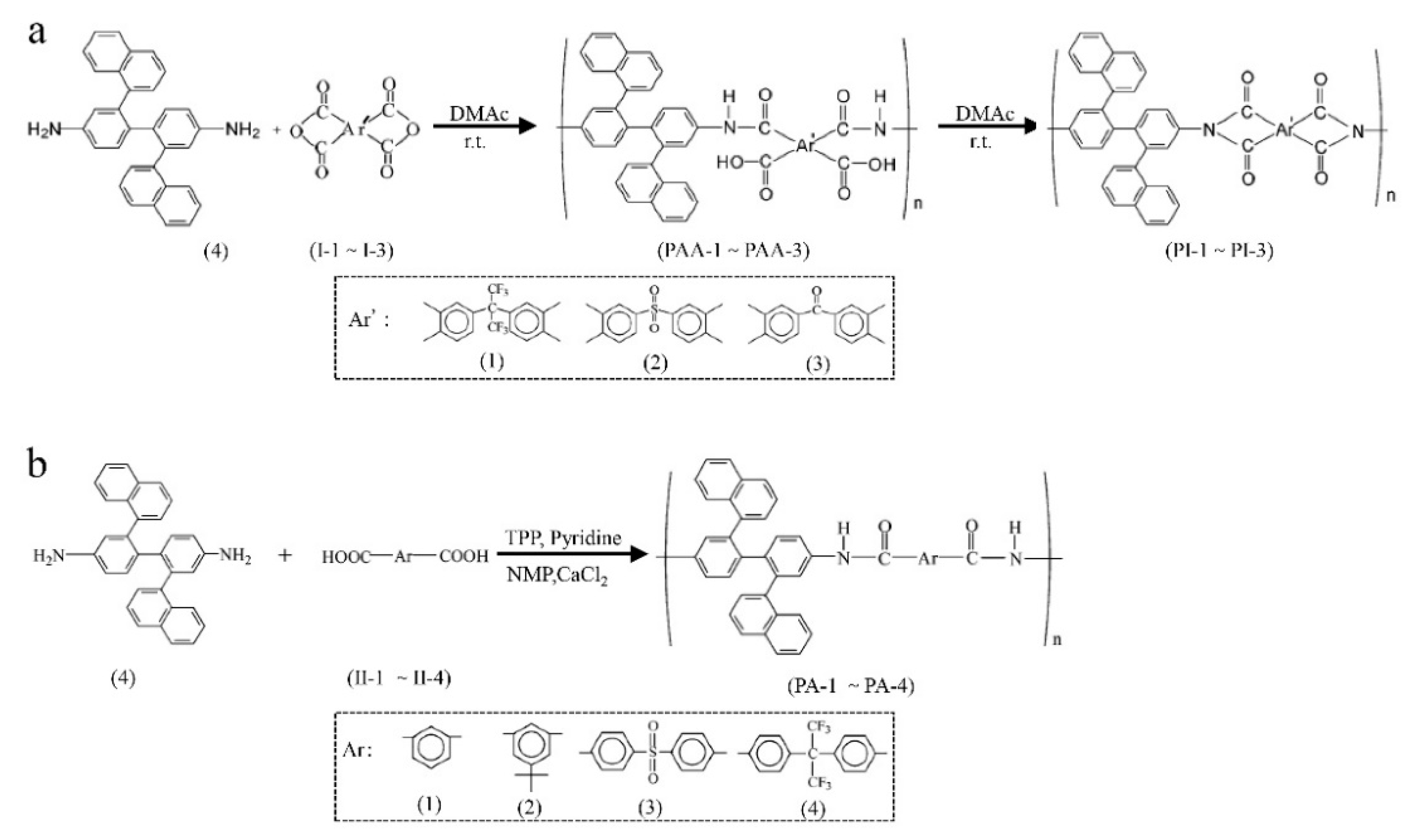
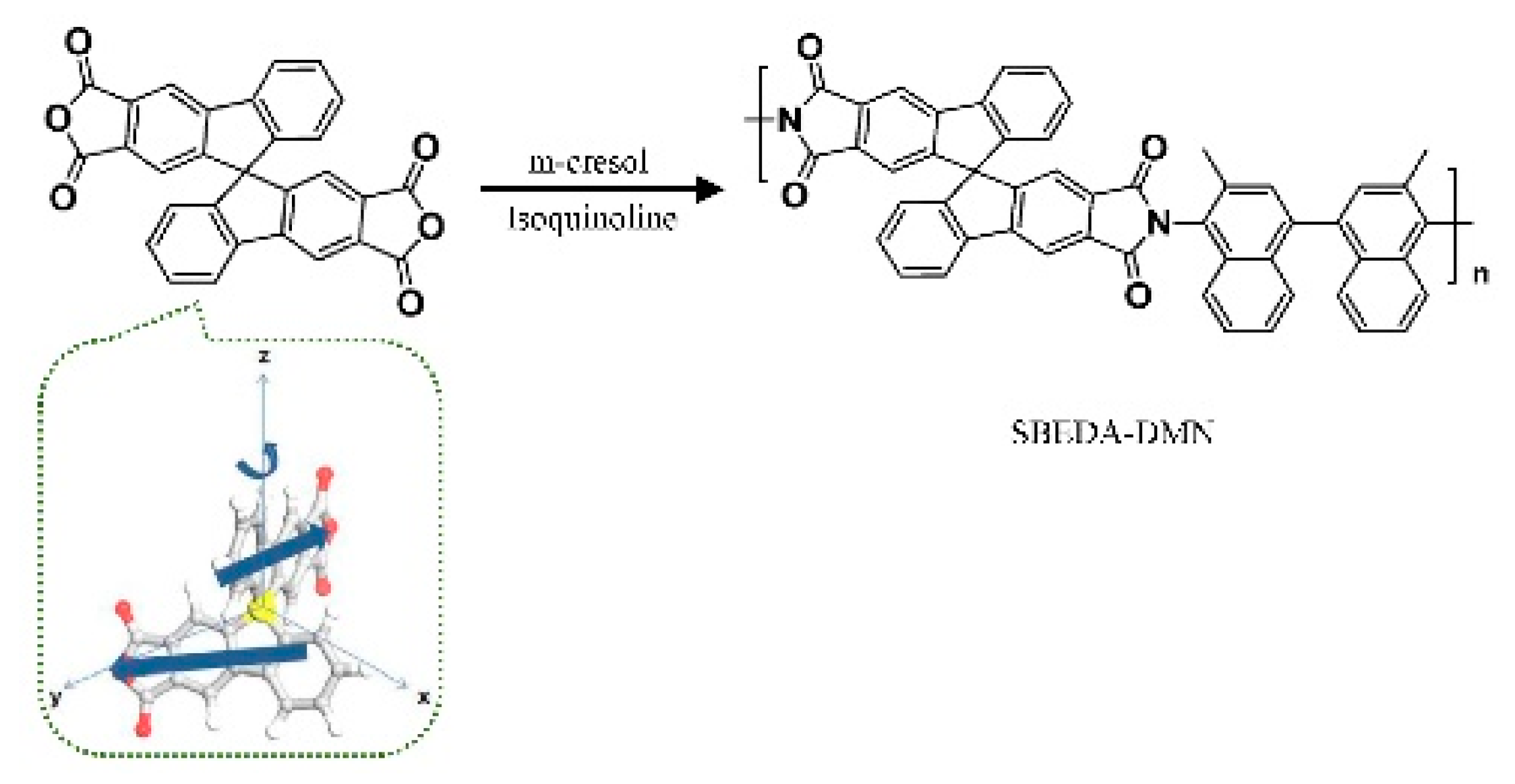
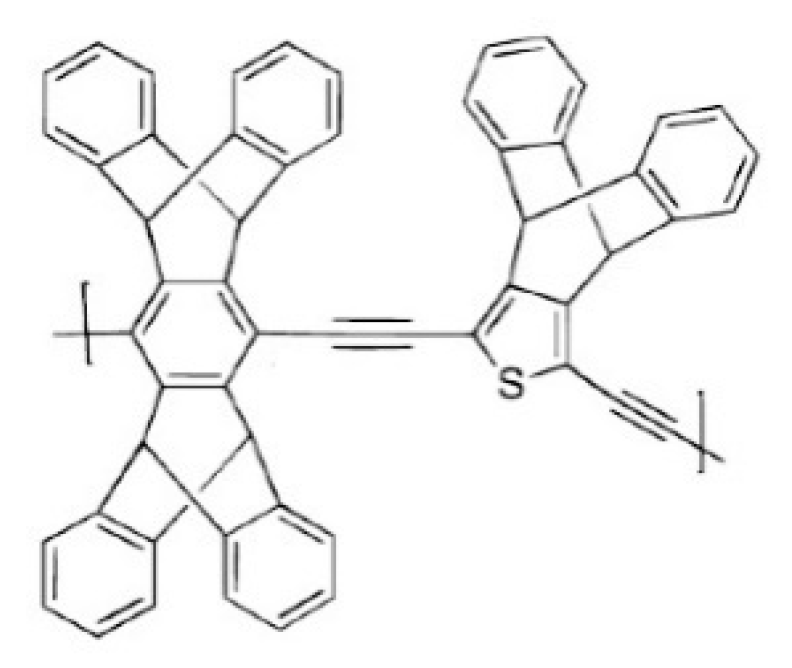
3.1. Non-Network Macromolecular Structure in Membranes
3.2. Network Macromolecular Structure in Membranes
4. BET Surface Area of Microporous Material
5. Solution-Processable Property
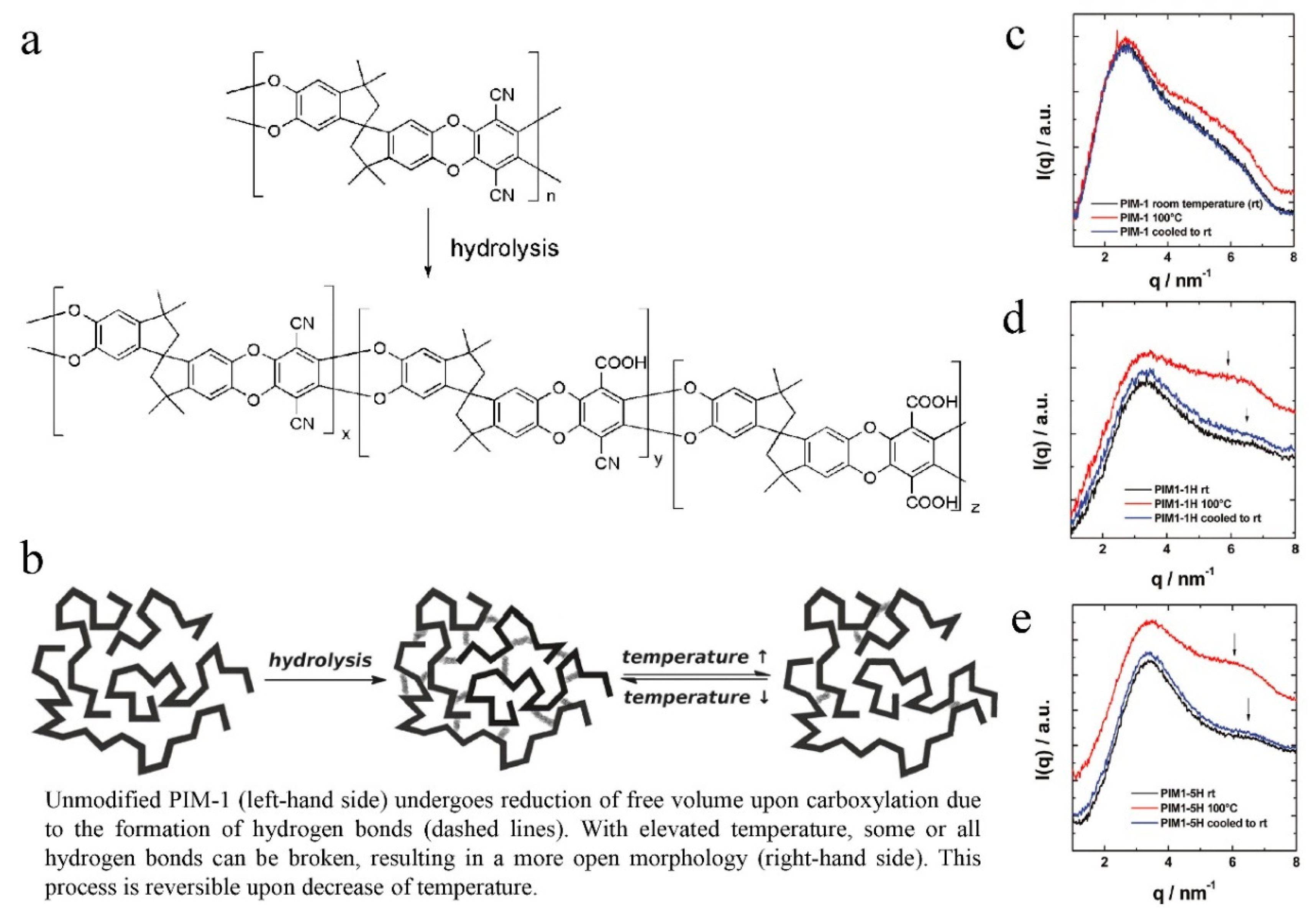
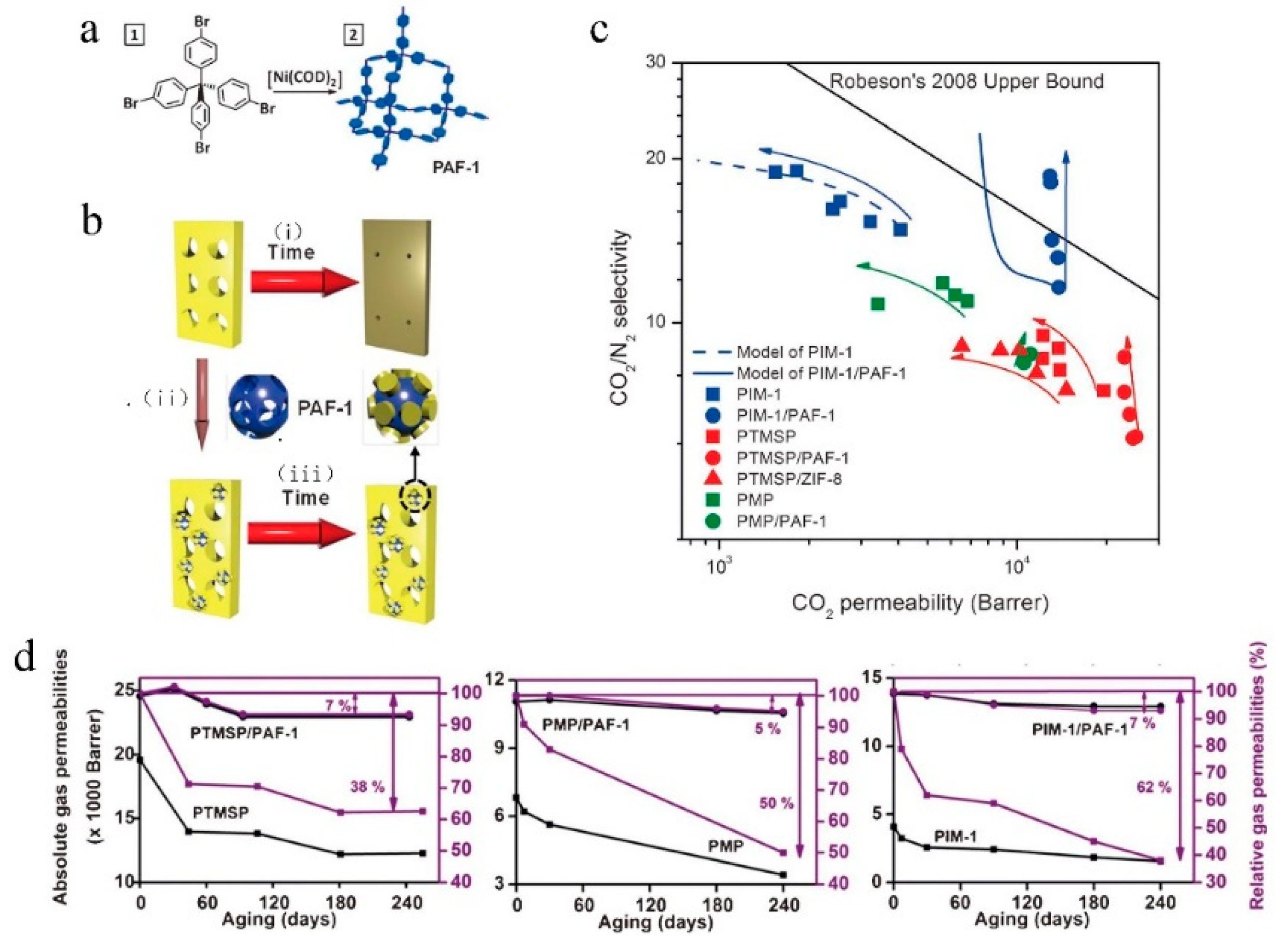
6. Physical Aging Problem
7. Applications
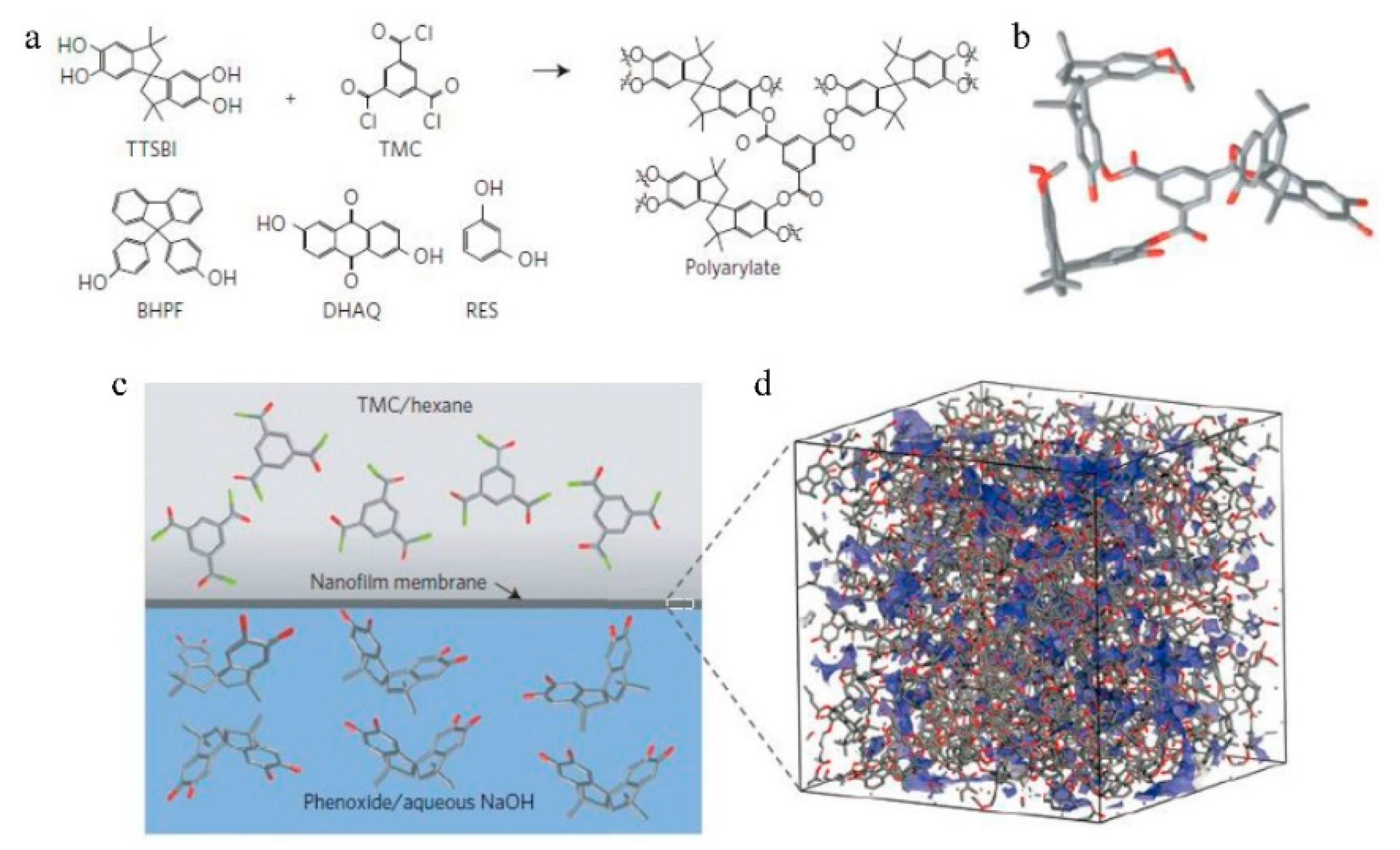
7.1. Nanofiltration
7.2. Pervaporation
7.3. Gas/Vapor Separation
8. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Castel, C.; Favre, E. Membrane separations and energy efficiency. J. Membr. Sci. 2018, 548, 345–357. [Google Scholar] [CrossRef]
- Jin, W.; Liu, G.; Xu, N. Organic-Inorganic Composite Membranes for Molecular Separation; World Scientific Europe Ltd.: London, UK, 2017. [Google Scholar]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, G. Microporous organic materials for membrane-based gas separation. Adv. Mater. 2018, 30, 1700750. [Google Scholar] [CrossRef] [PubMed]
- Meckler, S.M.; Bachman, J.E.; Robertson, B.P.; Zhu, C.; Long, J.R.; Helms, B.A. Thermally Rearranged Polymer Membranes Containing Tröger’s Base Units Have Exceptional Performance for Air Separations. Angew. Chem. Int. Ed. 2018, 57, 4912–4916. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Do-Thanh, C.-L.; Murdock, C.R.; Nelson, K.M.; Tian, C.; Brown, S.; Mahurin, S.M.; Jenkins, D.M.; Hu, J.; Zhao, B.; et al. Efficient CO2 Capture by a 3D Porous Polymer Derived from Tröger’s Base. ACS Macro Lett. 2013, 2, 660–663. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene) Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Cooper, A.I. Conjugated microporous polymers. Adv. Mater. 2009, 21, 1291–1295. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly[1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Nagai, K.; Masuda, T.; Nakagawa, T.; Freeman, B.D.; Pinnau, I. Poly[1-(trimethylsilyl)-1-propyne] and related polymers: Synthesis, properties and functions. Prog. Polym. Sci. 2001, 26, 721–798. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Thomas, S.; Pinnau, I.; Du, N.; Guiver, M.D. Hydrocarbon/hydrogen mixed-gas permeation properties of PIM-1, an amorphous microporous spirobisindane polymer. J. Membr. Sci. 2009, 338, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, B.; Alghunaimi, F.; Ma, X.; Alaslai, N.; Pinnau, I. Synthesis and characterization of novel triptycene dianhydrides and polyimides of intrinsic microporosity based on 3,3′-dimethylnaphthidine. Polymer 2016, 101, 225–232. [Google Scholar] [CrossRef]
- McKeown, N.B. The synthesis of polymers of intrinsic microporosity (PIMs). Sci. Chi. Chem. 2017, 60, 1023–1032. [Google Scholar] [CrossRef]
- Rakow, N.A.; Wendland, M.S.; Trend, J.E.; Poirier, R.J.; Paolucci, D.M.; Maki, S.P.; Lyons, C.S.; Swierczek, M.J. Visual Indicator for Trace Organic Volatiles. Langmuir 2010, 26, 3767–3770. [Google Scholar] [CrossRef]
- Ma, C.J.; Urban, J. Polymers of Intrinsic Microporosity (PIMs) Gas Separation Membranes: A mini Review. Proc. Nat. Res. Soc. 2018, 2, 02002. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.R.; Jin, J.; Freeman, B.D.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371. [Google Scholar] [CrossRef]
- Bernardo, P.; Bazzarelli, F.; Tasselli, F.; Clarizia, G.; Mason, C.R.; Maynard-Atem, L.; Budd, P.M.; Lanč, M.; Pilnáček, K.; Vopička, O.; et al. Effect of physical aging on the gas transport and sorption in PIM-1 membranes. Polymer 2017, 113, 283–294. [Google Scholar] [CrossRef]
- Müller, N.; Handge, U.A.; Abetz, V. Physical ageing and lifetime prediction of polymer membranes for gas separation processes. J. Membr. Sci. 2016, 516, 33–46. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical aging, plasticization and their effects on gas permeation in “rigid” polymers of intrinsic microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Hong, S.J.; Hihara, E.; Dang, C. Analysis of adiabatic heat and mass transfer of microporous hydrophobic hollow fiber membrane-based generator in vapor absorption refrigeration system. J. Membr. Sci. 2018, 564, 415–427. [Google Scholar] [CrossRef]
- Ghadimi, A.; Norouzbahari, S.; Lin, H.; Rabiee, H.; Sadatnia, B. Geometric restriction of microporous supports on gas permeance efficiency of thin film composite membranes. J. Membr. Sci. 2018, 563, 643–654. [Google Scholar] [CrossRef]
- Li, L.; Sirkar, K.K. Influence of microporous membrane properties on the desalination performance in direct contact membrane distillation. J. Membr. Sci. 2016, 513, 280–293. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Uhlhorn, R.J.R.; Keizer, K.; Burggraaf, A.J. Gas transport and separation with ceramic membranes. Part II. Synthesis and separation properties of microporous membranes. J. Membr. Sci. 1992, 66, 271–287. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark Alexander, V.; Olivier James, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Swaidan, R.; Al-Saeedi, M.; Ghanem, B.; Litwiller, E.; Pinnau, I. Rational Design of Intrinsically Ultramicroporous Polyimides Containing Bridgehead-Substituted Triptycene for Highly Selective and Permeable Gas Separation Membranes. Macromolecules 2014, 47, 5104–5114. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Litwiller, E.; Pinnau, I. Ultra-microporous triptycene-based polyimide membranes for high-performance gas separation. Adv. Mater. 2014, 26, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, B.; Wang, Z. Highly Selective Separation of CO2, CH4, and C2–C4 Hydrocarbons in Ultramicroporous Semicycloaliphatic Polyimides. ACS Appl. Mater. Interfaces 2018, 10, 26618–26627. [Google Scholar] [CrossRef] [PubMed]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- McKeown, N.B.; Gahnem, B.; Msayib, K.J.; Budd, P.M.; Tattershall, C.E.; Mahmood, K.; Tan, S.; Book, D.; Langmi, H.W.; Walton, A. Towards Polymer-Based Hydrogen Storage Materials: Engineering Ultramicroporous Cavities within Polymers of Intrinsic Microporosity. Angew. Chem. Int. Ed. 2006, 45, 1804–1807. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P. Nanofiltration extends the range of membrane filtration. Environ. Prog. 1988, 7, 58–62. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, B.S.; Hashem, M.; Harris, K.D.M.; Msayib, K.J.; Xu, M.; Budd, P.M.; Chaukura, N.; Book, D.; Tedds, S.; Walton, A.; et al. Triptycene-Based Polymers of Intrinsic Microporosity: Organic Materials That Can Be Tailored for Gas Adsorption. Macromolecules 2010, 43, 5287–5294. [Google Scholar] [CrossRef]
- Weber, J.; Su, Q.; Antonietti, M.; Thomas, A. Exploring Polymers of Intrinsic Microporosity—Microporous, Soluble Polyamide and Polyimide, Macromol. Macromol. Rapid Commun. 2007, 28, 1871–1876. [Google Scholar] [CrossRef]
- Weber, J.; Du, N.; Guiver, M.D. Influence of Intermolecular Interactions on the Observable Porosity in Intrinsically Microporous Polymers. Macromolecules 2011, 44, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Guiver, M.D. High-Performance Carboxylated Polymers of Intrinsic Microporosity (PIMs) with Tunable Gas Transport Properties. Macromolecules 2009, 42, 6038–6043. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of Intrinsic Microporosity Containing Trifluoromethyl and Phenylsulfone Groups as Materials for Membrane Gas Separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cao, S.; Zavala-Rivera, P.; Ping Lu, L.; Li, W.; Ji, Y.; Al-Muhtaseb, S.A.; Cheetham, A.K.; Sivaniah, E. Photo-oxidative enhancement of polymeric molecular sieve membranes. Nat. Commun. 2013, 4, 1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Heuchel, M.; Fritsch, D.; Budd, P.M.; McKeown, N.B.; Hofmann, D. Atomistic packing model and free volume distribution of a polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2008, 318, 84–99. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Ferrari, M.-C.; Jansen, J.C.; Monteleone, M.; Esposito, E.; Fuoco, A.; Hart, K.; Liyana-Arachchi, T.P.; Colina, C.M.; et al. The synthesis, chain-packing simulation and long-term gas permeability of highly selective spirobifluorene-based polymers of intrinsic microporosity. J. Mater. Chem. A 2018, 6, 10507–10514. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ando, S. Evaluation of free volume and anisotropic chain orientation of Tröger’s base (TB)-based microporous polyimide/copolyimide membranes. Polymer 2017, 123, 39–48. [Google Scholar] [CrossRef]
- Seong, J.G.; Zhuang, Y.; Kim, S.; Do, Y.S.; Lee, W.H.; Guiver, M.D.; Lee, Y.M. Effect of methanol treatment on gas sorption and transport behavior of intrinsically microporous polyimide membranes incorporating Tröger’s base. J. Membr. Sci. 2015, 480, 104–114. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Cui, Z.; Lee, J.; Lee, Y.M.; Guiver, M.D. Intrinsically microporous soluble polyimides incorporating tröger’s base for membrane gas separation. Macromolecules 2014, 47, 3254–3262. [Google Scholar] [CrossRef]
- Williams, R.; Burt, L.A.; Esposito, E.; Jansen, J.C.; Tocci, E.; Rizzuto, C.; Lanč, M.; Carta, M.; McKeown, N.B. A highly rigid and gas selective methanopentacene-based polymer of intrinsic microporosity derived from Tröger’s base polymerization. J. Mater. Chem. A 2018, 6, 5661–5667. [Google Scholar] [CrossRef]
- Alghunaimi, F.; Ghanem, B.; Wang, Y.; Salinas, O.; Alaslai, N.; Pinnau, I. Synthesis and gas permeation properties of a novel thermally-rearranged polybenzoxazole made from an intrinsically microporous hydroxyl-functionalized triptycene-based polyimide precursor. Polymer 2017, 121, 9–16. [Google Scholar] [CrossRef]
- Alghunaimi, F.; Ghanem, B.; Alaslai, N.; Mukaddam, M.; Pinnau, I. Triptycene dimethyl-bridgehead dianhydride-based intrinsically microporous hydroxyl-functionalized polyimide for natural gas upgrading. J. Membr. Sci. 2016, 520, 240–246. [Google Scholar] [CrossRef]
- Alaslai, N.; Ghanem, B.; Alghunaimi, F.; Pinnau, I. High-performance intrinsically microporous dihydroxyl-functionalized triptycene-based polyimide for natural gas separation. Polymer 2016, 91, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Swaidan, R.J.; Ghanem, B.; Swaidan, R.; Litwiller, E.; Pinnau, I. Pure-and mixed-gas propylene/propane permeation properties of spiro-and triptycene-based microporous polyimides. J. Membr. Sci. 2015, 492, 116–122. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Al-Saeedi, M.; Litwiller, E.; Pinnau, I. Role of Intrachain Rigidity in the Plasticization of Intrinsically Microporous Triptycene-Based Polyimide Membranes in Mixed-Gas CO2/CH4 Separations. Macromolecules 2014, 47, 7453–7462. [Google Scholar] [CrossRef]
- Rogan, Y.; Starannikova, L.; Ryzhikh, V.; Yampolskii, Y.; Bernardo, P.; Bazzarelli, F.; Jansen, J.C.; McKeown, N.B. Synthesis and gas permeation properties of novel spirobisindane-based polyimides of intrinsic microporosity. Polym. Chem. 2013, 4, 3813–3820. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Al-Harbi, N.M.; Fritsch, D.; Heinrich, K.; Starannikova, L.; Tokarev, A.; Yampolskii, Y. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 2009, 42, 7881–7888. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, B.; Tan, B.; Chen, C.-F.; Xu, H.-B.; Yang, X.-L. Triptycene-based microporous polymers: Synthesis and their gas storage properties. ACS Macro Lett. 2012, 1, 190–193. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Wang, J.-J.; Tan, L.; Liu, J.-M.; Tan, B.; Yang, X.-L.; Xu, H.-B. Synthesis and properties of triptycene-based microporous polymers. Polymer 2013, 54, 6942–6946. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.-L.; Wang, J.-J.; Wang, Z.; Liu, J.-M.; Tan, B.; Yang, X.-L.; Xu, H.-B. Triptycene-based microporous polyimides: Synthesis and their high selectivity for CO2 capture. Polymer 2014, 55, 3642–3647. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, P.-C.; Tan, L.; Liu, J.-M.; Tan, B.; Yang, X.-L.; Xu, H.-B. Triptycene-Based Hyper-Cross-Linked Polymer Sponge for Gas Storage and Water Treatment. Macromolecules 2015, 48, 8509–8514. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, C.-F. Synthesis and structure of 2,6,14- and 2,7,14-trisubstituted triptycene derivatives. J. Org. Chem. 2006, 71, 6626–6629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, C.-F. Triptycene-Based Expanded Oxacalixarenes: Synthesis, Structure, and Tubular Assemblies in the Solid State. J. Org. Chem. 2007, 72, 3880–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, P.-C.; Tan, L.; Luo, L.-N.; Liu, Y.; Liu, J.-M.; Ding, S.-Y.; Tan, B.; Yang, X.-L.; Xu, H.-B. Synthesis and properties of organic microporous polymers from the monomer of hexaphenylbenzene based triptycene. Polymer 2016, 82, 100–104. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 67–69. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, F.; Liu, Q.; Zong, C.; Yang, W.; Cao, X.; Jin, W.; Xu, N. Microporous polyamide membranes for molecular sieving of nitrogen from volatile organic compounds. Angew. Chem. Int. Ed. 2017, 56, 5755–5759. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Msayib, K.J.; Ghanem, B.S.; Kingston, H.J.; Tattershall, C.E.; Makhseed, S.; Reynolds, K.J.; Fritsch, D. Polymers of Intrinsic Microporosity (PIMs): Bridging the Void between Microporous and Polymeric Materials. Chem. Eur. J. 2005, 11, 2610–2620. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Ritter, N.; Antonietti, M.; Thomas, A.; Senkovska, I.; Kaskel, S.; Weber, J. Binaphthalene-Based, Soluble Polyimides: The Limits of Intrinsic Microporosity. Macromolecules 2009, 42, 8017–8020. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Hauser, B.G.; Bae, Y.-S.; Brown, S.E.; Snurr, R.Q.; Mirkin, C.A.; Hupp, J.T. Synthesis, Properties, and Gas Separation Studies of a Robust Diimide-Based Microporous Organic Polymer. Chem. Mater. 2009, 21, 3033–3035. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, S.; Hasell, T.; Liu, M.; Sun, S.; Cheetham, A.K.; Sivaniah, E.; Cooper, A.I. Porous organic cage thin films and molecular-sieving membranes. Adv. Mater. 2016, 28, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Mundstock, A.; Feldhoff, A.; Knebel, A.; Gu, J.; Meng, H.; Caro, J. Covalent Organic Framework–Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Islamoglu, T.; Shar, G.A.; Ding, R.; El-Kaderi, H.M. Targeted synthesis of a mesoporous triptycene-derived covalent organic framework. CrystEngComm 2013, 15, 1524–1527. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Sydlik, S.A.; Chen, Z.; Swager, T.M. Triptycene Polyimides: Soluble Polymers with High Thermal Stability and Low Refractive Indices. Macromolecules 2011, 44, 976–980. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Chang, F.-C.; Leung, M.-K.; Chou, M.-Y.; Muellen, K. High thermal stability and rigid rod of novel organosoluble polyimides and polyamides based on bulky and noncoplanar naphthalene−biphenyldiamine. Macromolecules 2005, 38, 4024–4029. [Google Scholar] [CrossRef]
- Ma, X.; Ghanem, B.; Salines, O.; Litwiller, E.; Pinnau, I. Synthesis and effect of physical aging on gas transport properties of a microporous polyimide derived from a novel spirobifluorene-based dianhydride. ACS Macro Lett. 2015, 4, 231–235. [Google Scholar] [CrossRef]
- Williams, V.E.; Swager, T.M. Iptycene-Containing Poly(aryleneethynylene)s. Macromolecules 2000, 33, 4069–4073. [Google Scholar] [CrossRef]
- Zheng, Z.; Grünker, R.; Feng, X. Synthetic two-dimensional materials: A new paradigm of membranes for ultimate separation. Adv. Mater. 2016, 28, 6529–6545. [Google Scholar] [CrossRef] [PubMed]
- Alghunaimi, F.; Ghanem, B.; Alaslai, N.; Swaidan, R.; Litwiller, E.; Pinnau, I. Gas permeation and physical aging properties of iptycene diamine-based microporous polyimides. J. Membr. Sci. 2015, 490, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Wiegand, J.R.; Gao, P.; Doherty, C.M.; Hill, A.J.; Guo, R. Molecular origins of fast and selective gas transport in pentiptycene-containing polyimide membranes and their physical aging behavior. J. Membr. Sci. 2016, 518, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Wang, H.; Shi, X.; Shen, B.; He, X.; Ghazi, Z.A.; Khan, N.A.; Sin, H.; Khattak, A.M.; Li, L.; et al. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration. Nat. Chem. 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.Y.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. Int. Ed. 2015, 54, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Schott, J.A.; Mahurin, S.M.; Dai, S. Porous Structure Design of Polymeric Membranes for Gas Separation. Small Methods 2017, 1, 1600051. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Rigid and microporous polymers for gas separation membranes. Prog. Polym. Sci. 2015, 43, 1–32. [Google Scholar] [CrossRef]
- Lau, C.H.; Li, P.; Li, F.; Chung, T.-S.; Paul, D.R. Reverse-selective polymeric membranes for gas separations. Prog. Polym. Sci. 2013, 38, 740–766. [Google Scholar] [CrossRef]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMs). J. Membr. Sci. 2012, 401–402, 222–231. [Google Scholar] [CrossRef]
- Badalov, S.; Arnusch, C.J. Ink-jet printing assisted fabrication of thin film composite membranes. J. Membr. Sci. 2016, 515, 79–85. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Gorgojo, P.; Karan, S.; Wong, H.C.; Jimenez-Solomon, M.F.; Cabral, J.T.; Livingston, A.G. Ultrathin Polymer Films with Intrinsic Microporosity: Anomalous Solvent Permeation and High Flux Membranes. Adv. Funct. Mater. 2014, 24, 4729–4737. [Google Scholar] [CrossRef]
- Murphy, T.M.; Langhe, D.S.; Ponting, M.; Baer, E.; Freeman, B.D.; Paul, D.R. Physical aging of layered glassy polymer films via gas permeability tracking. Polymer 2011, 52, 6117–6125. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Claes, S.; Vandezande, P.; Mullens, S.; De Sitter, K.; Peeters, R.; Van Bael, M.K. Preparation and benchmarking of thin film supported PTMSP-silica pervaporation membranes. J. Membr. Sci. 2012, 389, 265–271. [Google Scholar] [CrossRef]
- Claes, S.; Vandezande, P.; Mullens, S.; Leysen, R.; De Sitter, K.; Andersson, A.; Maurer, F.H.J.; Van den Rul, H.; Peeters, R.; Van Bael, M.K. High flux composite PTMSP-silica nanohybrid membranes for the pervaporation of ethanol/water mixtures. J. Membr. Sci. 2010, 351, 160–167. [Google Scholar] [CrossRef]
- Adymkanov, S.V.; Yampol’skii, Y.P.; Polyakov, A.M.; Budd, P.M.; Reynolds, K.J.; McKeown, N.B.; Msayib, K.J. Pervaporation of alcohols through highly permeable PIM-1 polymer films. Polym. Sci. Ser. A 2008, 50, 444–450. [Google Scholar] [CrossRef]
- Liu, G.; Hung, W.-S.; Shen, J.; Li, Q.; Huang, Y.-H.; Jin, W.; Lee, K.-R.; Lai, J.-Y. Mixed matrix membranes with molecular-interaction-driven tunable free volumes for efficient bio-fuel recovery. J. Mater. Chem. A 2015, 3, 4510–4521. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.S.; Paul, D.R. Gas sorption and permeation in PIM-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Wang, N.; Fan, H.; Zhang, R.; Zhang, G.; Ji, S. Enhanced flux of polydimethylsiloxane membrane for ethanol permselective pervaporation via incorporation of MIL-53 particles. J. Membr. Sci. 2015, 492, 322–330. [Google Scholar] [CrossRef]
- Lewandowska, M.; Kujawski, W. Ethanol production from lactose in a fermentation/pervaporation system. J. Food Eng. 2007, 79, 430–437. [Google Scholar] [CrossRef]
- Jitesh, K.D.; Pangarkar, V.G.; Niranjan, K. Pervaporative stripping of acetone, butanol and ethanol to improve ABE fermentation. Bioseparation 2000, 9, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Samanta, H.S.; Ray, S.K. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Sep. Purif. Technol. 2015, 146, 176–186. [Google Scholar] [CrossRef]
- Chang, C.-L.; Chang, M.-S. Preparation of composite membranes of functionalised silicone polymers and PVDF for pervaporation of ethanol-water mixture. Desalination 2002, 148, 39–42. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. 30 years of membrane technology for gas separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar]
- Van der Bruggen, B.; Luis, P. Pervaporation as a tool in chemical engineering: A new era? Curr. Opin. Chem. Eng. 2014, 4, 47–53. [Google Scholar] [CrossRef]
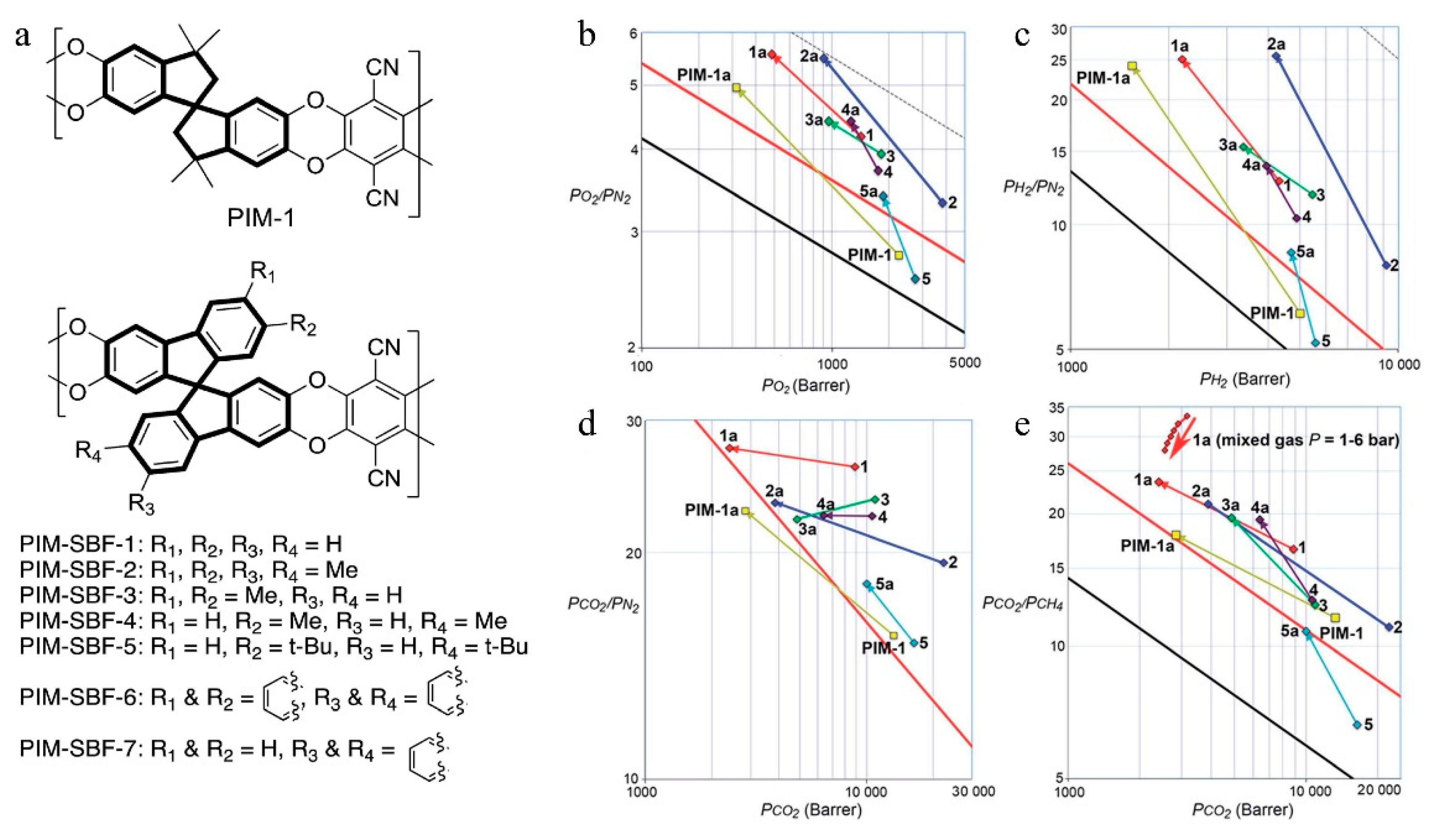
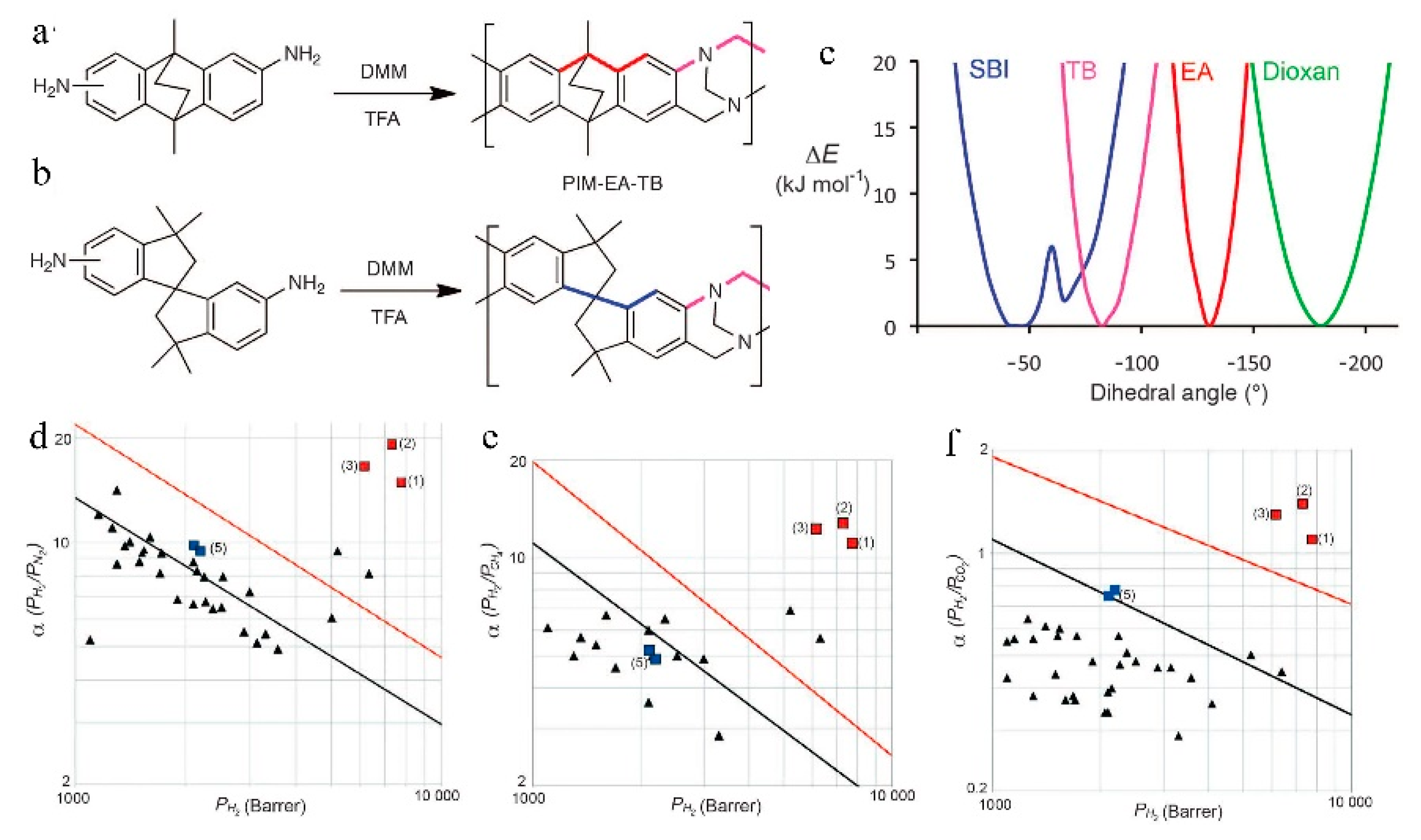
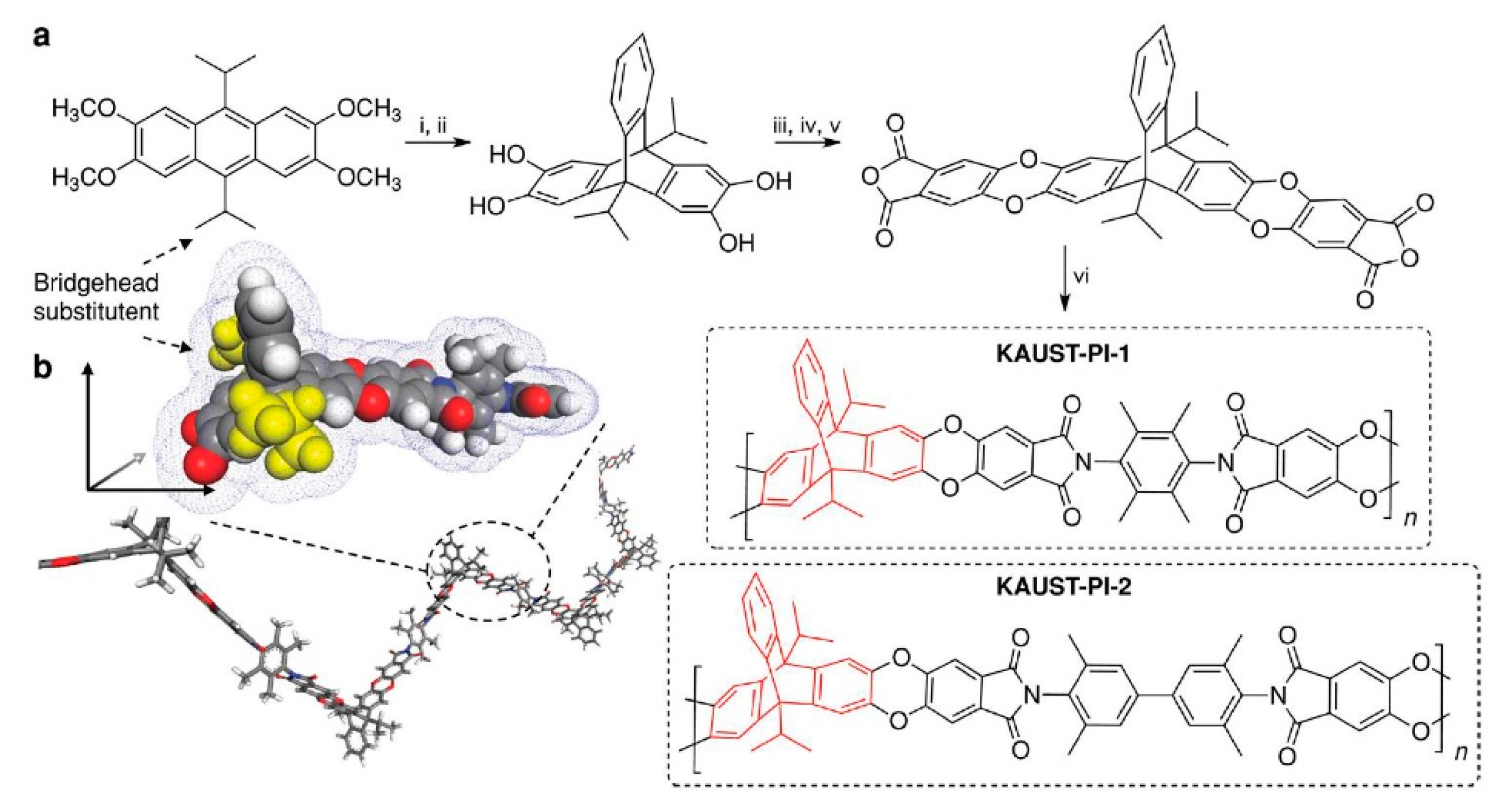
 = Ladder PIMs,
= Ladder PIMs,  = Extended and
= Extended and  = Non-extended PIM-PIs (Extended PIM-PIs means the reactive anhydride was grafted on the dibenzodioxin units by the introduction of 4,5-dichlorophthalonitrile instead of on the 1,1-spirobisindane unit, while, in the non-extended PIM-PIs, anhydride groups are fused directly to the 1,1-spirobisindane unit [61,62]). Solid lines represent state-of-the-art 2008 permeability/selectivity upper bounds. Reproduced from Reference [34].
= Non-extended PIM-PIs (Extended PIM-PIs means the reactive anhydride was grafted on the dibenzodioxin units by the introduction of 4,5-dichlorophthalonitrile instead of on the 1,1-spirobisindane unit, while, in the non-extended PIM-PIs, anhydride groups are fused directly to the 1,1-spirobisindane unit [61,62]). Solid lines represent state-of-the-art 2008 permeability/selectivity upper bounds. Reproduced from Reference [34].
 = Ladder PIMs,
= Ladder PIMs,  = Extended and
= Extended and  = Non-extended PIM-PIs (Extended PIM-PIs means the reactive anhydride was grafted on the dibenzodioxin units by the introduction of 4,5-dichlorophthalonitrile instead of on the 1,1-spirobisindane unit, while, in the non-extended PIM-PIs, anhydride groups are fused directly to the 1,1-spirobisindane unit [61,62]). Solid lines represent state-of-the-art 2008 permeability/selectivity upper bounds. Reproduced from Reference [34].
= Non-extended PIM-PIs (Extended PIM-PIs means the reactive anhydride was grafted on the dibenzodioxin units by the introduction of 4,5-dichlorophthalonitrile instead of on the 1,1-spirobisindane unit, while, in the non-extended PIM-PIs, anhydride groups are fused directly to the 1,1-spirobisindane unit [61,62]). Solid lines represent state-of-the-art 2008 permeability/selectivity upper bounds. Reproduced from Reference [34].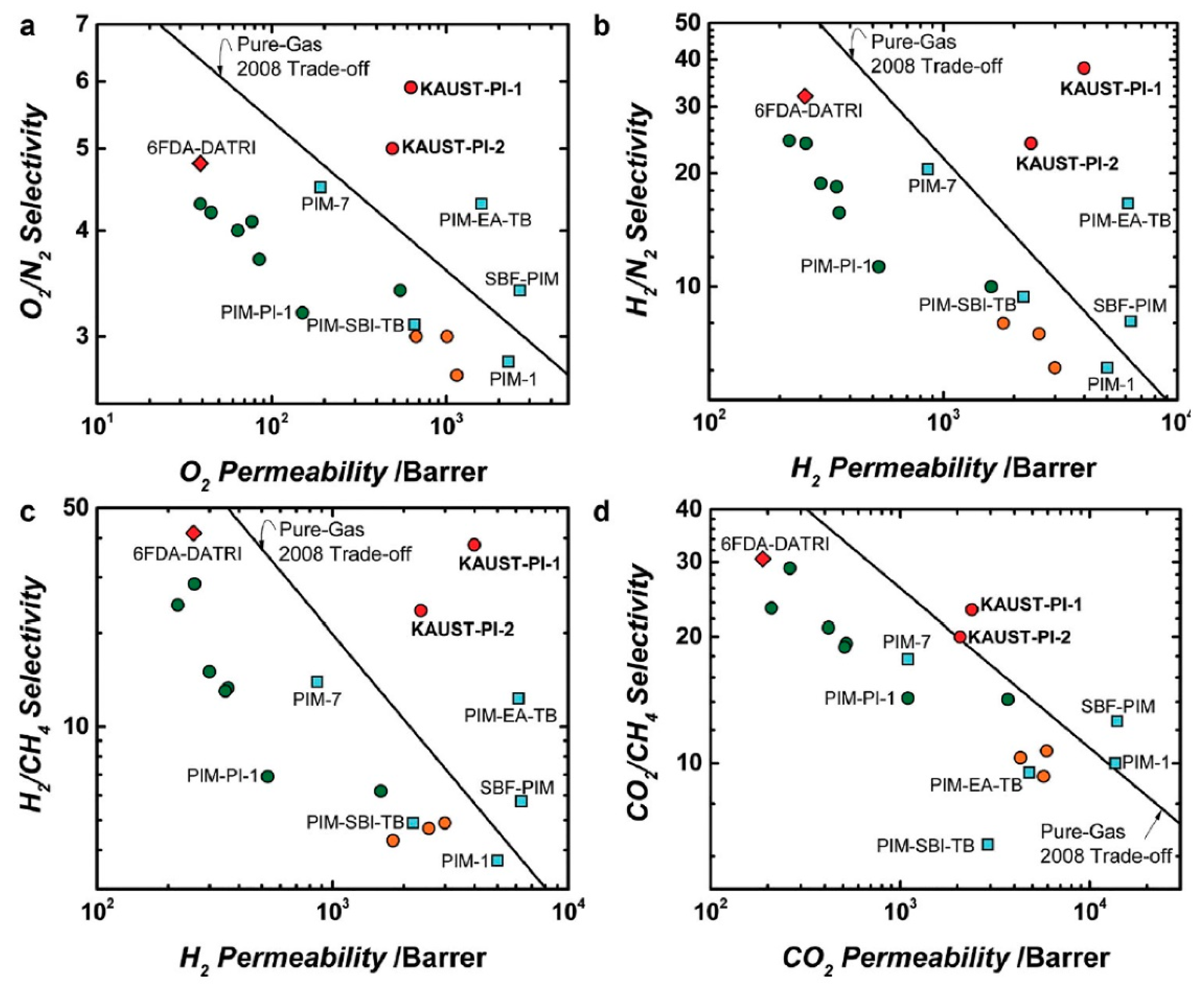
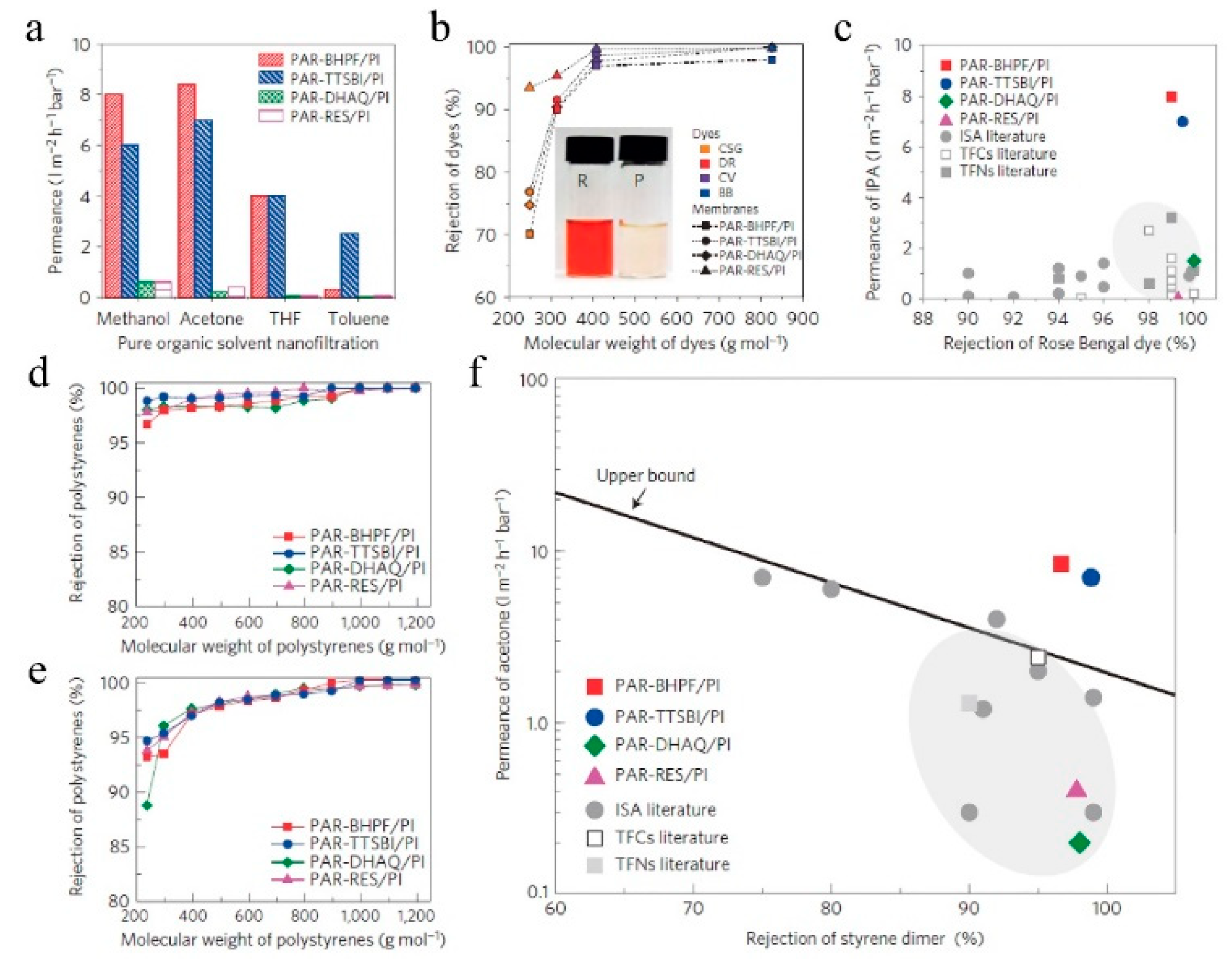
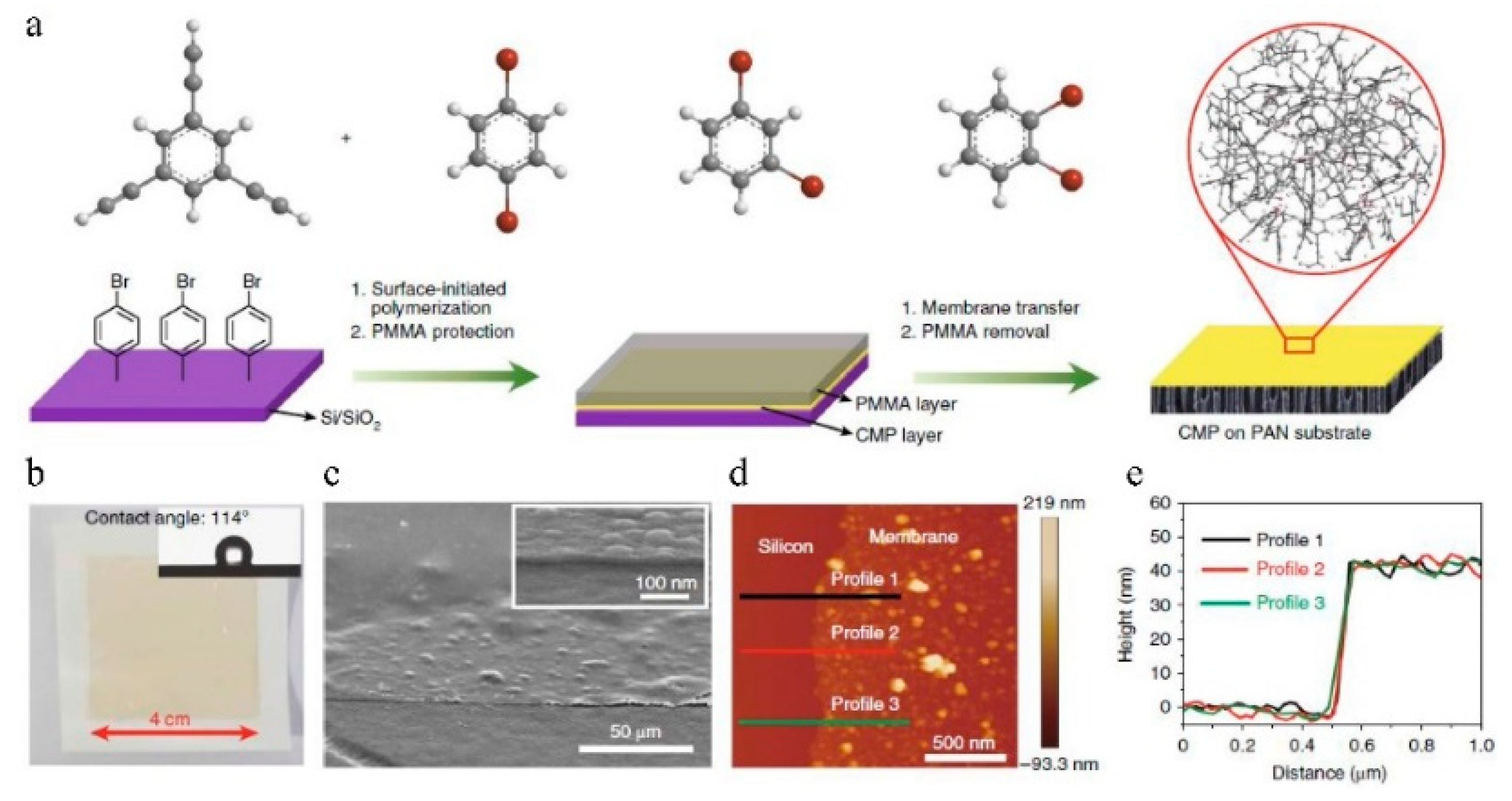
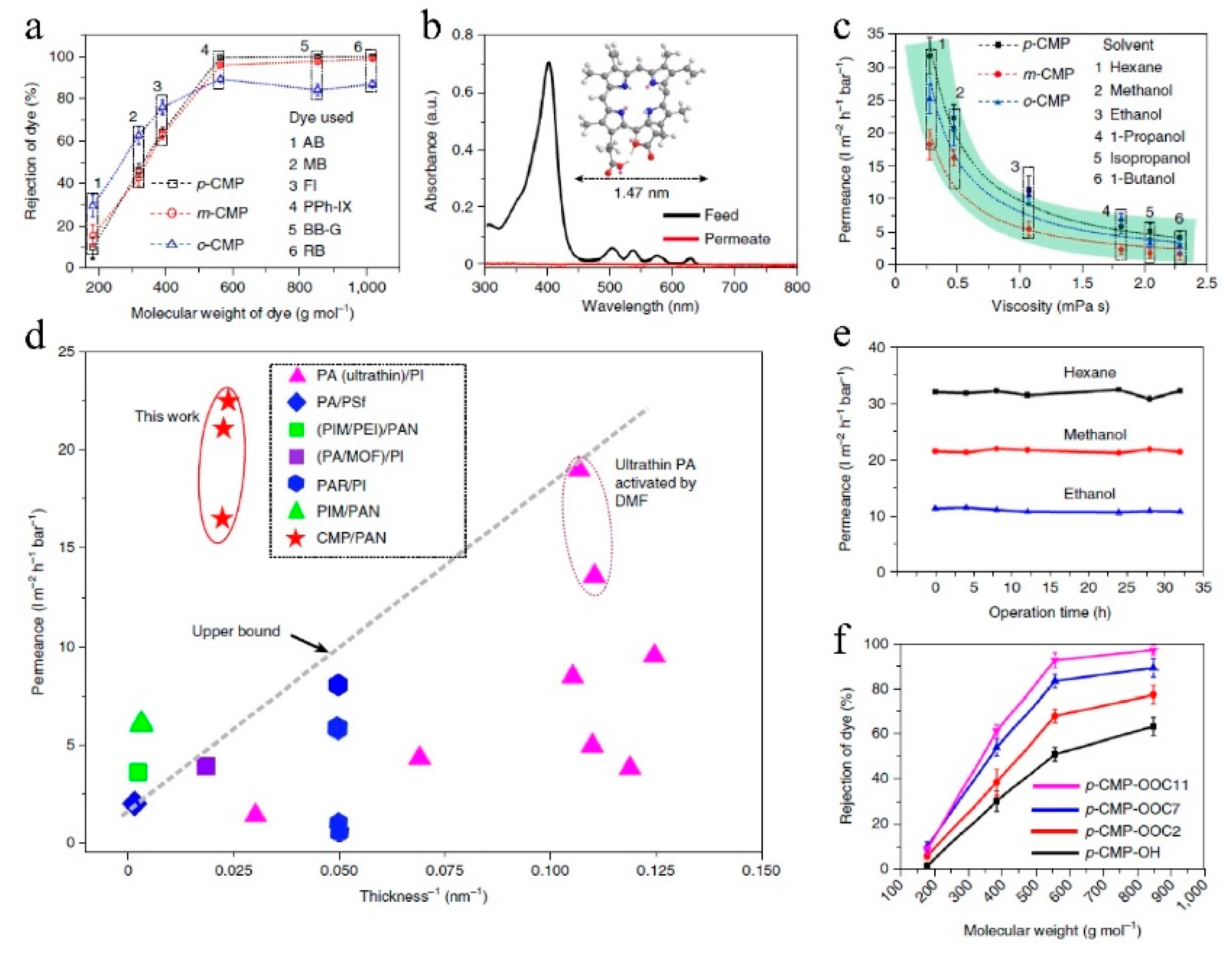
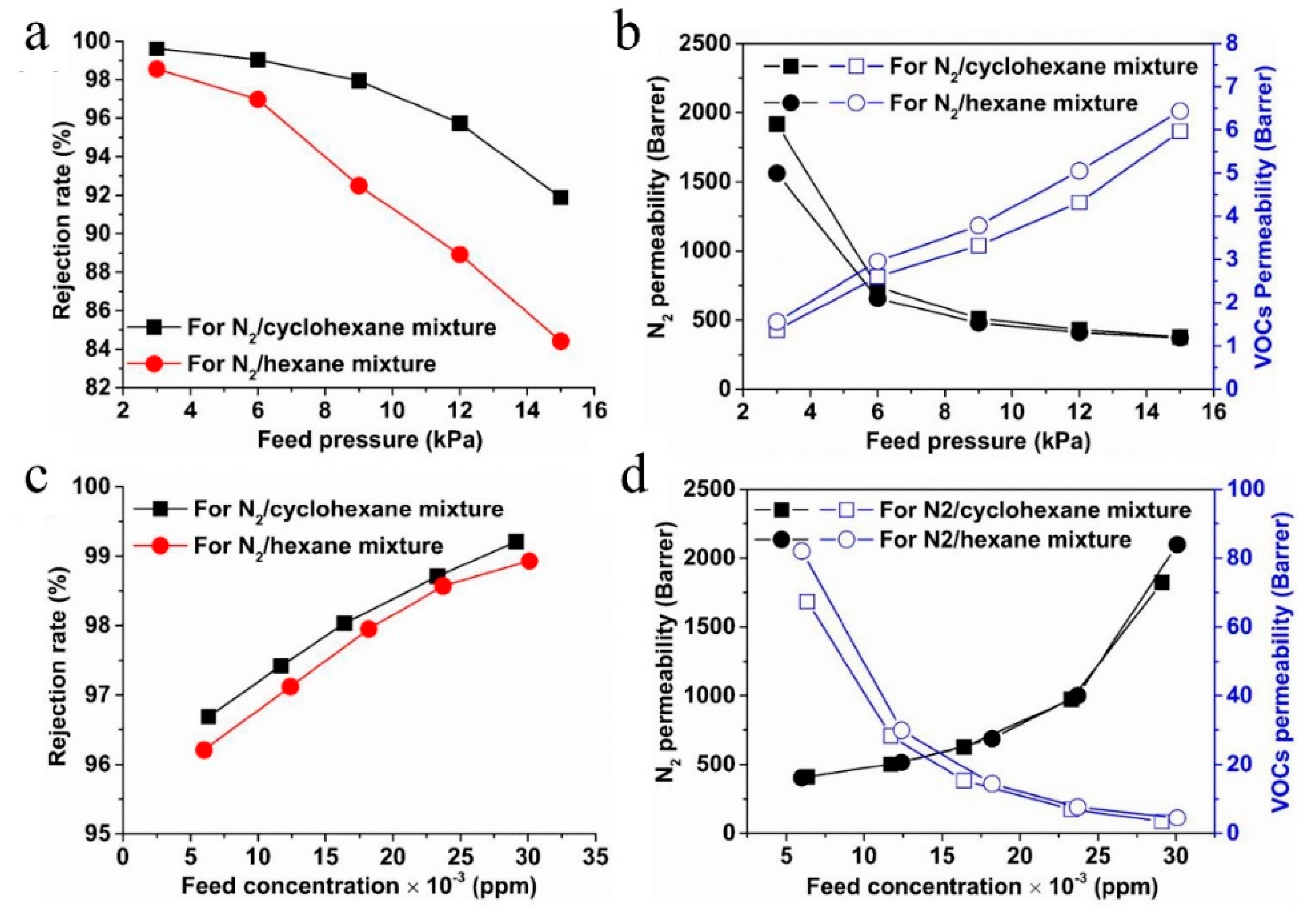
| No. | Polymer | Solvent | Pressure (bar) | 90% Retention (g/mol) | 99% Retention (g/mol) | Permeance (l/(m2hbar)) | Permeance (kg/(m2hbar)) | Time (h) |
|---|---|---|---|---|---|---|---|---|
| 1 | StarmemTM240 | n-Heptane | 30 | ~400 | ~1400 | 0.1 | 0.1 | 50 |
| 2 | PIM-1 | n-Heptane | 4 | 560 | - | 7.3 | 5.0 | 3 |
| 3 | PIM-1 | n-Heptane | 10 | 290 | 420 | 4.2 | 2.9 | 230 |
| 4 | PIM-1 | n-Heptane | 20 | 240 | 400 | 4.1 | 2.8 | 250 |
| 5 | PIM-1 | n-Heptane | 30 | <200 | 410 | 4.0 | 2.8 | 280 |
| 6 | StarmemTM240 | Toluene | 30 | 380 | 1200 | 0.7 | 0.6 | 140 |
| 7 | PIM-1/20%PEI | Toluene | 10 | 480 | 890 | 3.2 | 2.8 | >200 |
| 8 | PIM-1/20%PEI | Toluene | 20 | 430 | 790 | 3.1 | 2.7 | >200 |
| 9 | PIM-1/20%PEI | Toluene | 30 | 460 | 860 | 3.1 | 2.7 | >200 |
| 10 | PIM-1-CO1-50/20%PEI | Toluene | 10 | 350 | 680 | 0.9 | 0.8 | >200 |
| 11 | PIM-1-CO1-50/20%PEI | Toluene | 20 | 330 | 680 | 0.9 | 0.8 | >200 |
| 12 | PIM-1-CO1-50/20%PEI | Toluene | 30 | 340 | 680 | 0.9 | 0.8 | >200 |
| Membrane | Feed Ethanol Concentration, wt% | T, ℃ | Separation Factor | Flux, Kg/m2h | Reference |
|---|---|---|---|---|---|
| PIM | 10 | 30 | 10.7 | 0.47 | [102] |
| 10 | 50 | 10.2 | 1.10 | ||
| PDMS | 5 | 70 | 7.6 | 1.667 | [105] |
| PDMS-PAN membrane | 6 | 30 | 8 | 3.5 | [106] |
| PTMSP | 10 | 50 | 12 | 3.5 | [101] |
| Pervap4060 | 10 | 50 | 7 | 1.9 | [101] |
| Pevatech PDMS | 10 | 50 | 6 | 3.3 | [101] |
| SBR | 10 | 30 | 3.8 | 0.015 | [107] |
| poly (styrene-co-utylacrylate) copolymer | 5 | 30 | 16 | 0.3 | [108] |
| TMVS-g-PVDF | 10 | 30 | ~3.1 | 2.85 | [109] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Jin, W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes 2019, 9, 3. https://doi.org/10.3390/membranes9010003
Zhou H, Jin W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes. 2019; 9(1):3. https://doi.org/10.3390/membranes9010003
Chicago/Turabian StyleZhou, Haoli, and Wanqin Jin. 2019. "Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications" Membranes 9, no. 1: 3. https://doi.org/10.3390/membranes9010003
APA StyleZhou, H., & Jin, W. (2019). Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes, 9(1), 3. https://doi.org/10.3390/membranes9010003




