Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR)
Abstract
1. Introduction
2. Materials and Methods
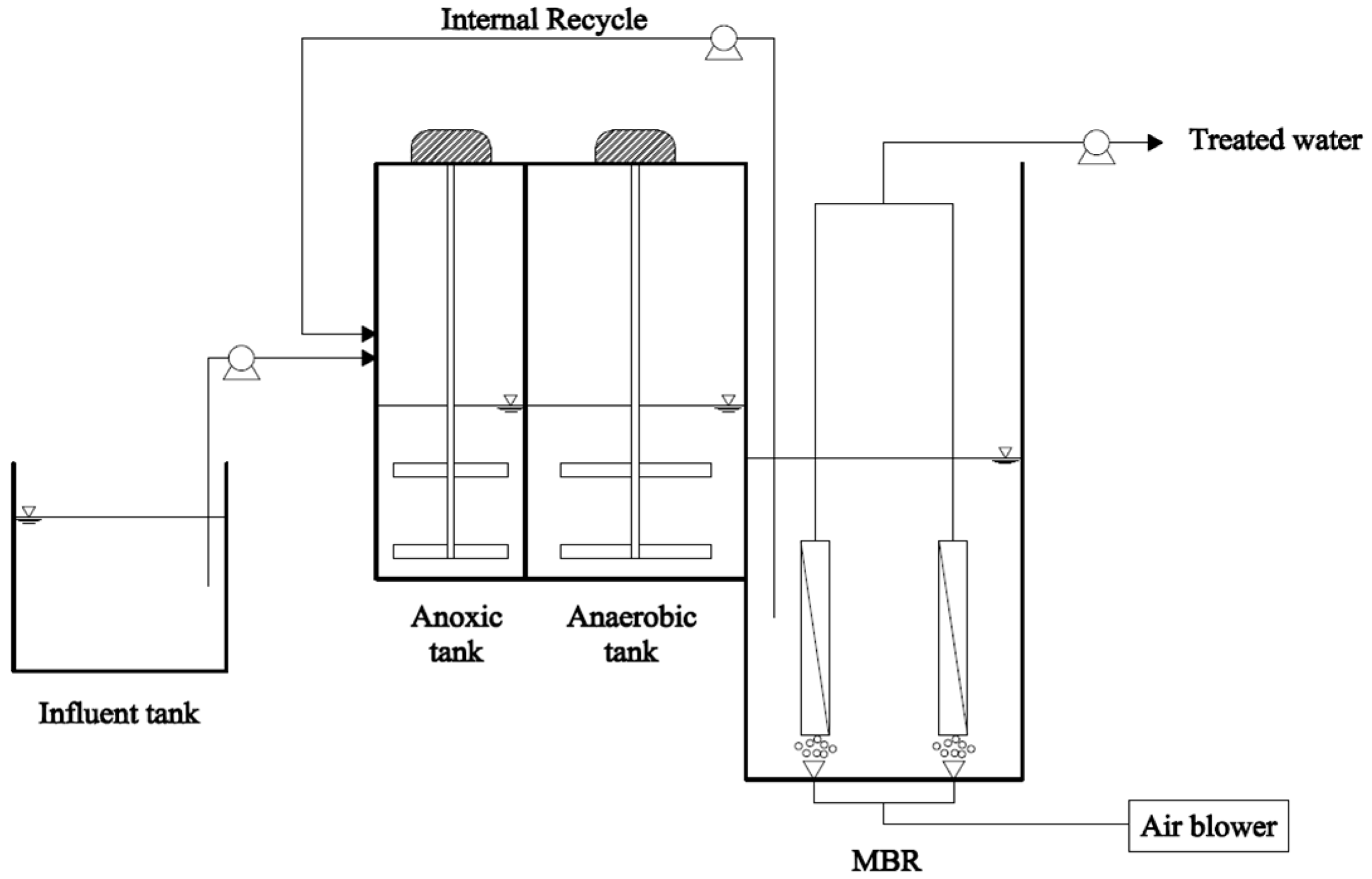
2.1. Experimental Set-Up
2.2. Synthetic Wastewater, Seed Sludge and Sulfur-Based Carriers
2.3. Analytical Methods
3. Results and Discussion
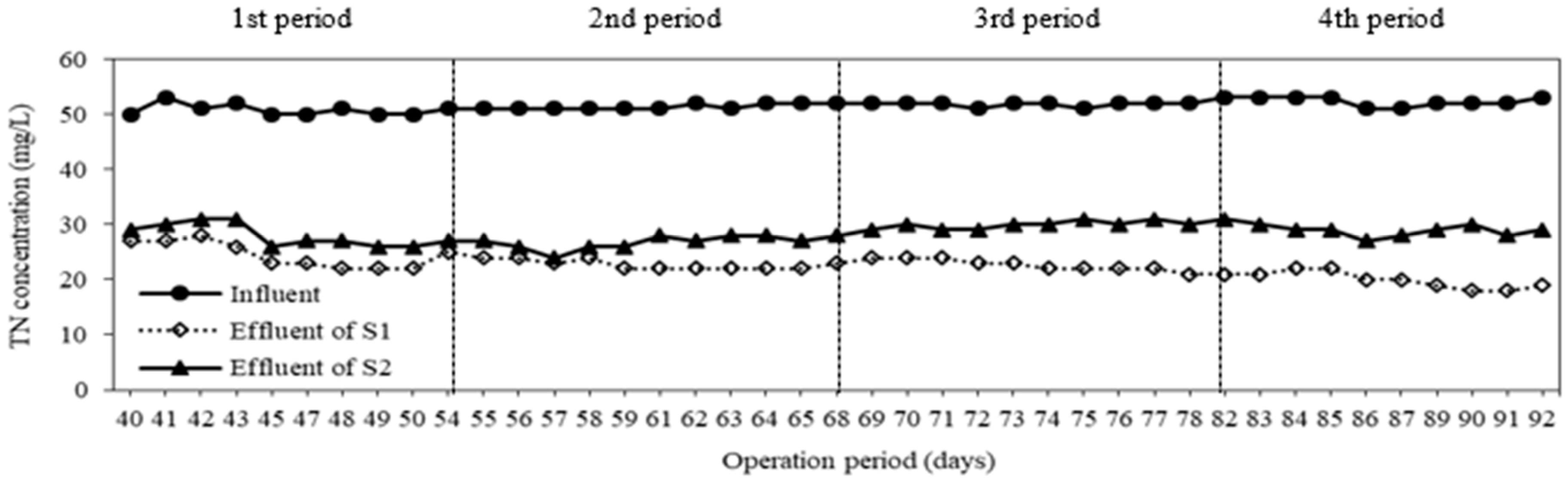
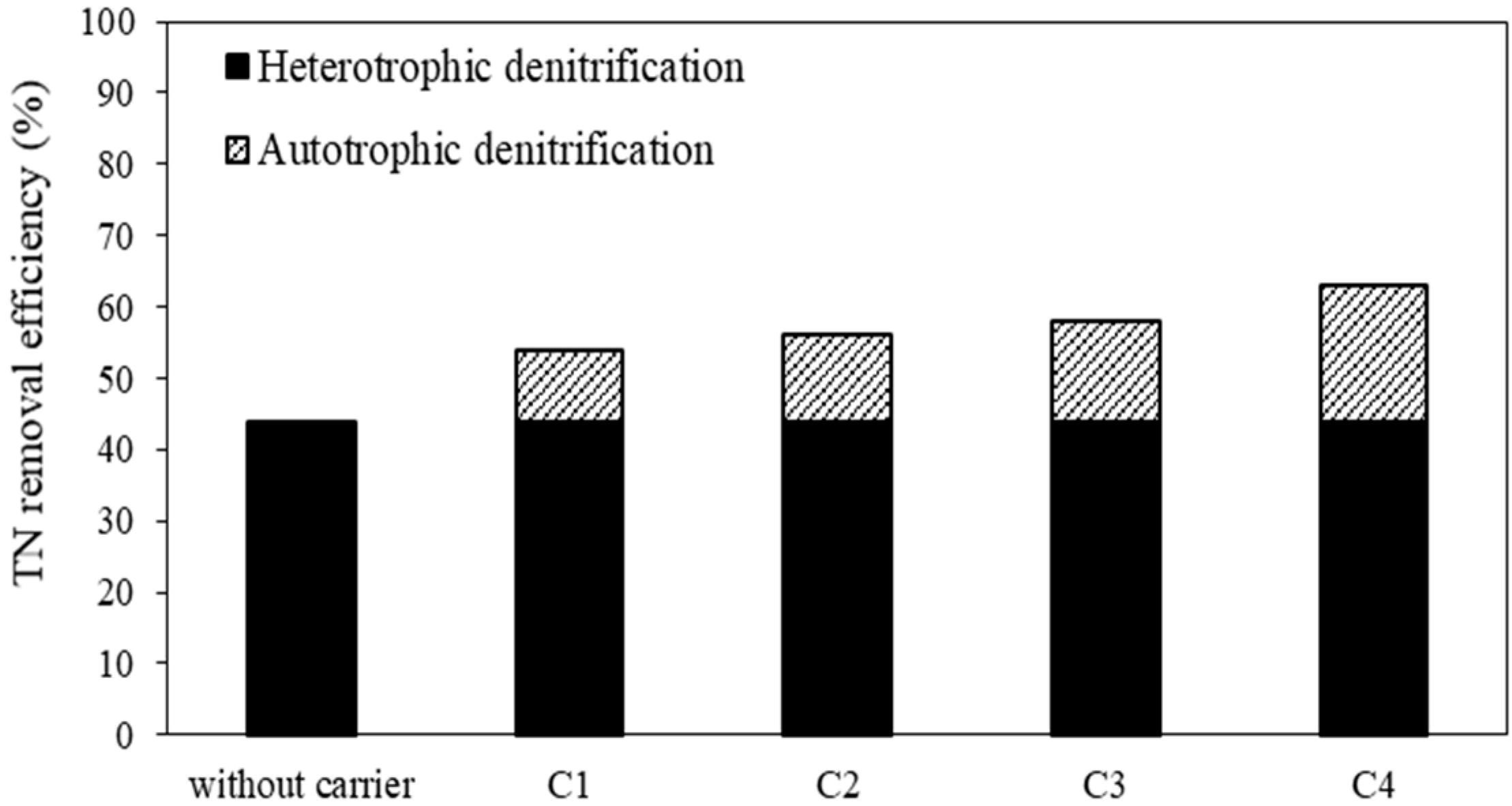
3.1. Removal of Nitrogen
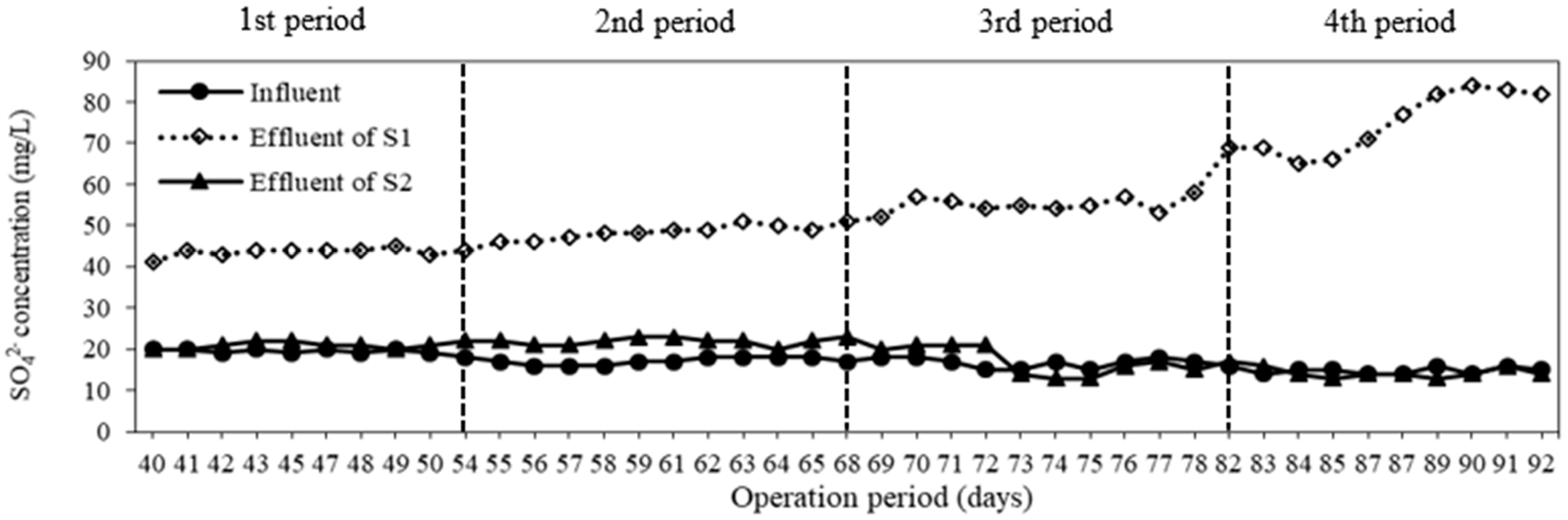
3.2. Sulfate Generation
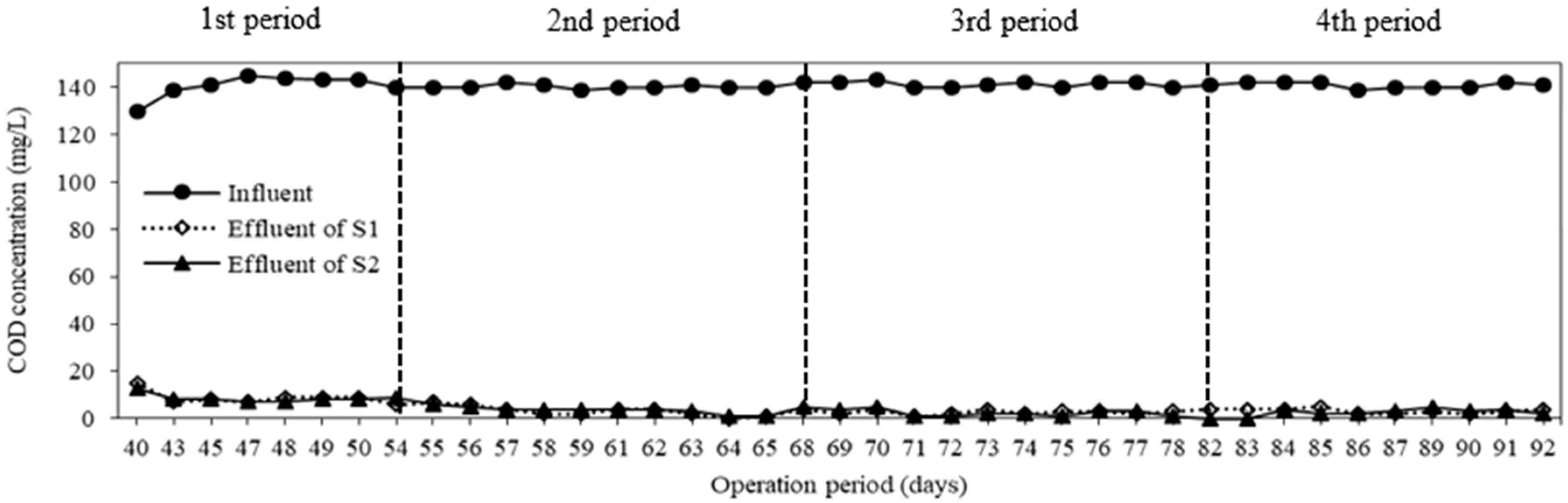
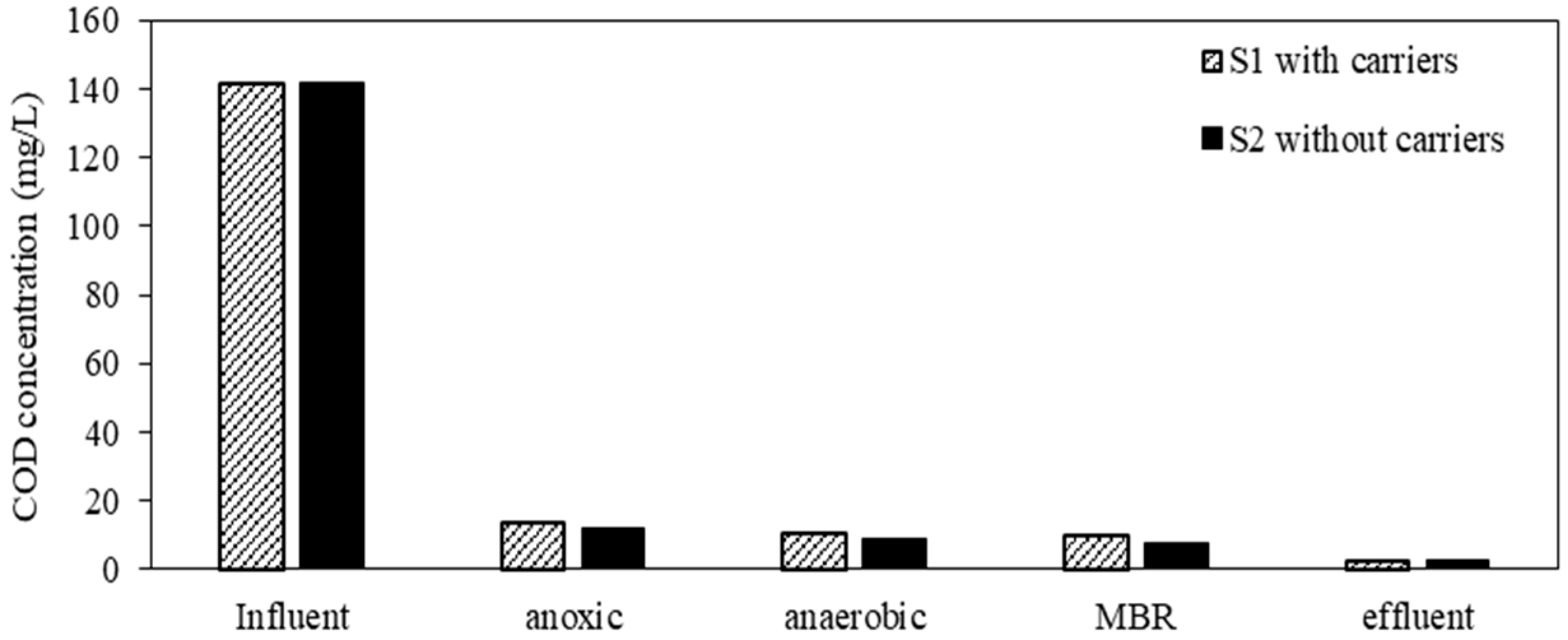
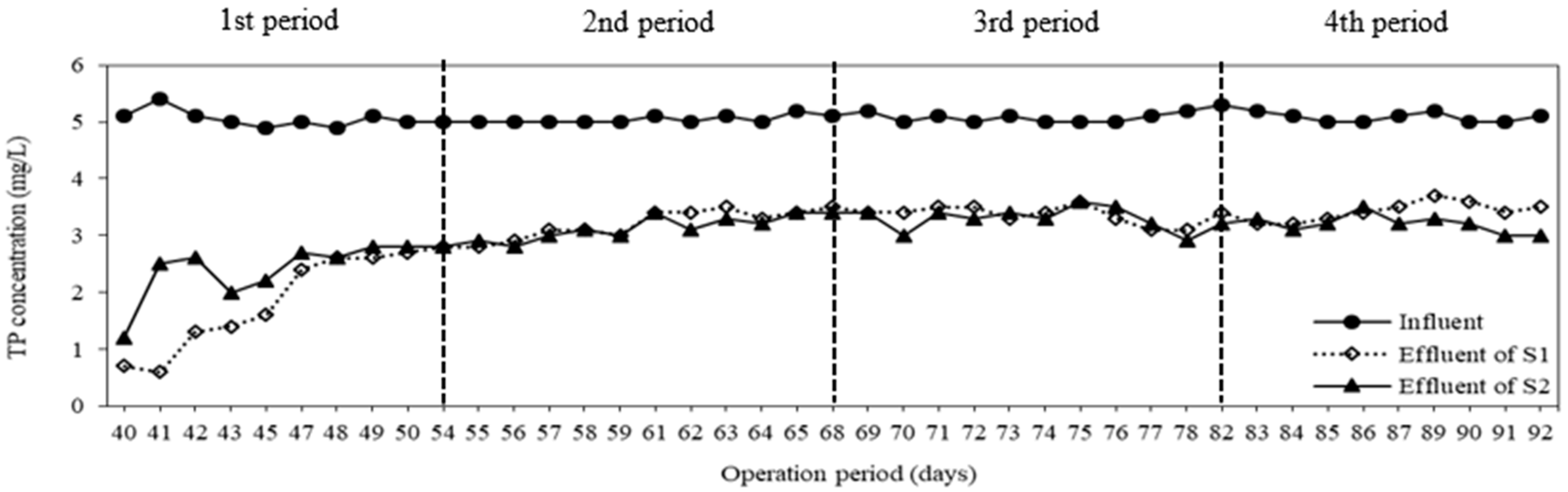
3.3. COD and TP Removal
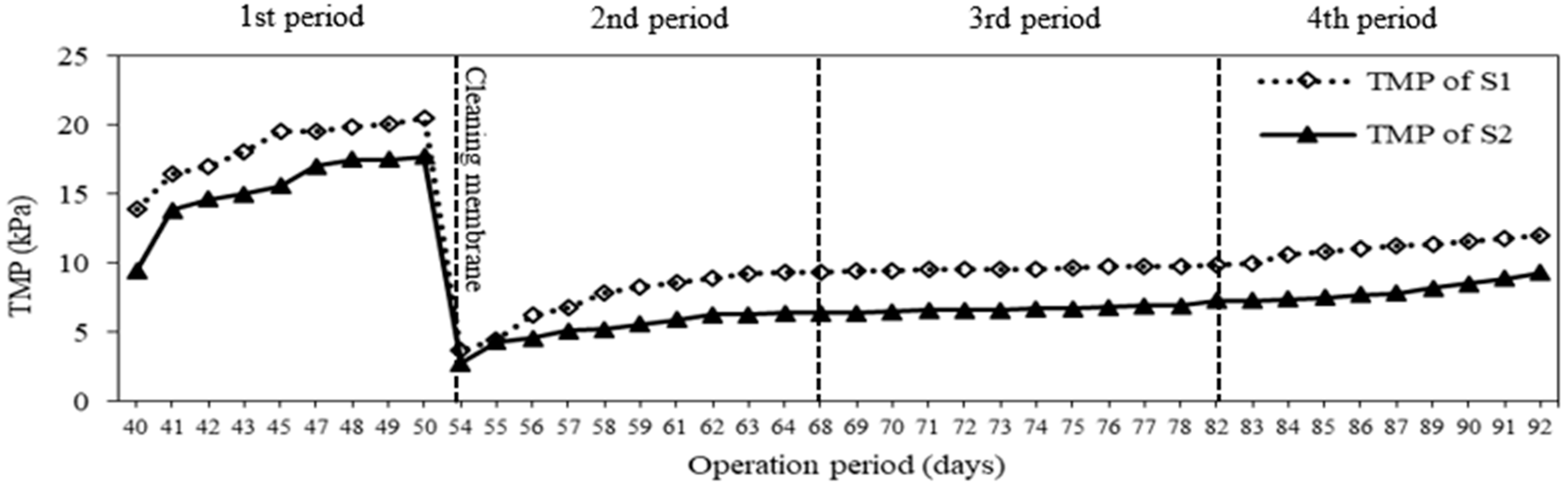
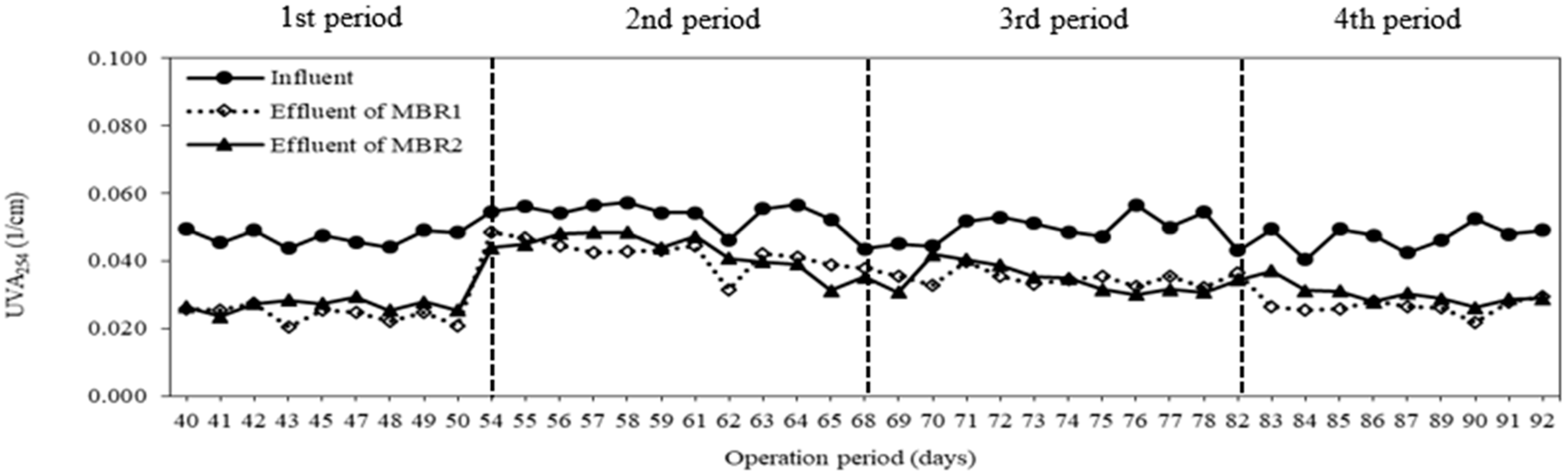
3.4. Membrane Fouling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morita, M.; Uemoto, H.; Watanabe, A. Nitrogen-removal bioreactor capable of simultaneous nitrification and denitrification for application to industrial wastewater treatment. Biochem. Eng. J. 2008, 41, 59–66. [Google Scholar] [CrossRef]
- Fernández-Nava, Y.; Marañón, E.; Soons, J.; Castrillón, L. Denitrification of high nitrate concentration wastewater using alternative carbon source. J. Hazard. Mater. 2010, 173, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Crespi, M.; Ramazzotti, V. Evidence that N-nitroso compounds contribute to the causation of certain human cancers. In Nitrate Contamination; Bogardi, I., Kuzelka, R.D., Eds.; Springer: Berlin, Germany, 1991; Volume 30, pp. 233–252. ISBN 978-3-642-76040-2. [Google Scholar]
- Weisenburger, D.D. Potential health consequences of groundwater contamination by nitrate in Nebraska. In Nitrate Contamination; Bogardi, I., Kuzelka, R.D., Eds.; Springer: Berlin, Germany, 1991; Volume 30, pp. 309–315. ISBN 978-3-642-76040-2. [Google Scholar]
- Shuval, H.I. Infant methemoglobinemia and other health effects of nitrates in drinking water. Prog. Water Technol. 1980, 12, 1731–1736. [Google Scholar] [CrossRef]
- Mirvish, S.S. N-nitroso compounds, nitrate, and nitrite: Possible implications for the causation of human cancer. Prog. Water Technol. 1977, 8, 195–204. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, Y.J. Biological nitrate removal in industrial wastewater treatment: Which electron donor we can choose. Appl. Microbiol. Biotechnol. 2009, 82, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Metcalf and Eddy. Wastewater Engineering: Treatment, Disposal and Reuse, 4th ed.; McGraw-Hill Inc.: Singapore, 2003; ISBN 978-0-07-340118-8. [Google Scholar]
- Prosnansky, M.; Sakakibara, Y.; Kuroda, M. High-rate denitrification and SS rejection by biofilm-electrode reactor (BER) combined with microfiltration. Water Res. 2002, 36, 4801–4810. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Dursun, N.; Kilic, A.; Demirel, S.; Uyanik, S.; Cinar, O. Simultaneous heterotrophic and sulfur-oxidizing autotrophic denitrification process for drinking water treatment: Control of sulfate production. Water Res. 2011, 45, 6661–6667. [Google Scholar] [CrossRef] [PubMed]
- McAdam, E.J.; Judd, S.J. Denitrification from drinking water using a membrane bioreactor: Chemical and biochemical feasibility. Water Res. 2007, 41, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Wan, D.; Qu, J. Study of a combined heterotrophic and sulfur autotrophic denitrification technology for removal of nitrate in water. J. Hazard. Mater. 2009, 169, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nakano, K.; Lee, T.J.; Kanchanatawee, S.; Matsumura, M. On-site nitrate removal of groundwater by an immobilized psychrophilic denitrifier using soluble starch as a carbon source. J. Biosci. Bioeng. 2002, 93, 303–308. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Chung, M.S. Determination of optimal COD/nitrate ratio for biological denitrification. Int. Biodeterior. Biodegrad. 2003, 51, 43–49. [Google Scholar] [CrossRef]
- Manconi, I.; Carucci, A.; Lens, P. Combined Removal of Sulfur Compounds and Nitrate by Autotrophic Denitrification in Bioaugmented Activated Sludge System. Biotechnol. Bioeng. 2007, 98, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Yurtsever, A.; Aktas, Ö.; Ucar, D.; Wang, Z. Sulfur-based autotrophic denitrification of drinking water using a membrane bioreactor. Chem. Eng. J. 2015, 268, 180–186. [Google Scholar] [CrossRef]
- Shrimali, M.; Singh, K.P. New methods of nitrate removal from water. Environ. Pollut. 2001, 112, 351–359. [Google Scholar] [CrossRef]
- Kimura, K.; Nakamura, M.; Watanabe, Y. Nitrate removal by a combination of elemental sulphur-based denitrification and membrane filtration. Water Res. 2002, 36, 1758–1766. [Google Scholar] [CrossRef]
- Kleerebezem, R.; Mendez, R. Autotrophic denitrification for combined hydrogen sulfide removal from biogas and post-denitrification. Water Sci. Technol. 2002, 45, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.I.M. Denitrification of groundwater with elemental sulfur. Water Res. 2002, 36, 1392–1395. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.S.; Lee, I.S.; Bae, J.H. Denitrification of High NO3−-N Containing Wastewater Using Elemental Sulfur; Nitrogen Loading Rate and N2O Production. Environ. Technol. 2002, 23, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Amin, K.; Kim, S.J.; Yoon, S.J.; Kwon, K.W.; Bae, W.K. Autotrophic denitrification of nitrate and nitrite using thiosulfate as an electron donor. Water Res. 2014, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.E.; Yoo, Y.B.; Young, J.C.; Kim, I.S. Effect of organics on sulfur-utilizing autotrophic denitrification under mixotrophic conditions. J. Biotechnol. 2001, 92, 1–8. [Google Scholar] [CrossRef]
- McAdam, E.J.; Judd, S.J. A review of membrane bioreactor potential for nitrate removal from drinking water. Desalination 2006, 196, 135–148. [Google Scholar] [CrossRef]
- Visvanathan, C.; Aim, R.B.; Parameshwaran, K. Membrane separation bioreactors for wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2000, 30, 1–48. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water, Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992; ISBN 9780875532875. [Google Scholar]
- Fu, Z.; Yang, F.; Zhou, F.; Xue, Y. Control of COD/N ratio for nutrient removal in a modified membrane bioreactor (MBR) treating high strength wastewater. Bioresource Technology 2009, 100, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Bui, X.T.; Vo, T.D.H.; Nguyen, D.D.; Nguyen, P.D.; Do, H.L.C.; Ngo, H.H.; Guo, W. Performance and membrane fouling of two lab-scale sponge membrane bioreactors for hospital wastewater treatment at low flux condition. Sep. Purif. Technol. 2016, 165, 123–129. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jung, H.J.; Kim, K.S.; Kim, I.S. Treatment of high nitrate-containing wastewaters by sequential heterotrophic and autotrophic denitrification. J. Environ. Eng. 2004, 130, 1475–1480. [Google Scholar] [CrossRef]
- Wen, X.; Ding, H.; Huang, X.; Liu, R. Treatment of hospital wastewater using a submerged membrane bioreactor. Process Biochem. 2004, 39, 1427–1431. [Google Scholar] [CrossRef]
| Nitrogen Species | S1 with Carriers | S2 without Carriers | |||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | ||
| NH4+-N (mg/L) | 0 | 0 | 0 | 0 | 0 |
| NO2−-N (mg/L) | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.14 ± 0.02 | 0.06 ± 0.02 |
| NO3−-N (mg/L) | 23.5 ± 2.3 | 22.3 ± 1.1 | 22.2 ± 1.0 | 19.5 ± 1.5 | 27.3 ± 1.7 |
| TN (Total Nitrogen) (mg/L) | 24 ± 2 | 23 ± 1 | 22 ± 1 | 19 ± 1 | 28 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, T.-K.-Q.; Lee, J.-J.; Kang, J.-S.; Park, S.; Kim, H.-S. Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR). Membranes 2018, 8, 115. https://doi.org/10.3390/membranes8040115
Vo T-K-Q, Lee J-J, Kang J-S, Park S, Kim H-S. Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR). Membranes. 2018; 8(4):115. https://doi.org/10.3390/membranes8040115
Chicago/Turabian StyleVo, Thi-Kim-Quyen, Jeong-Jun Lee, Joon-Seok Kang, Seogyeong Park, and Han-Seung Kim. 2018. "Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR)" Membranes 8, no. 4: 115. https://doi.org/10.3390/membranes8040115
APA StyleVo, T.-K.-Q., Lee, J.-J., Kang, J.-S., Park, S., & Kim, H.-S. (2018). Nitrogen Removal by Sulfur-Based Carriers in a Membrane Bioreactor (MBR). Membranes, 8(4), 115. https://doi.org/10.3390/membranes8040115





