Modeling Ionic Strength Effects on Hollow-Fiber Nanofiltration Membrane Mass Transfer
Abstract
:1. Introduction
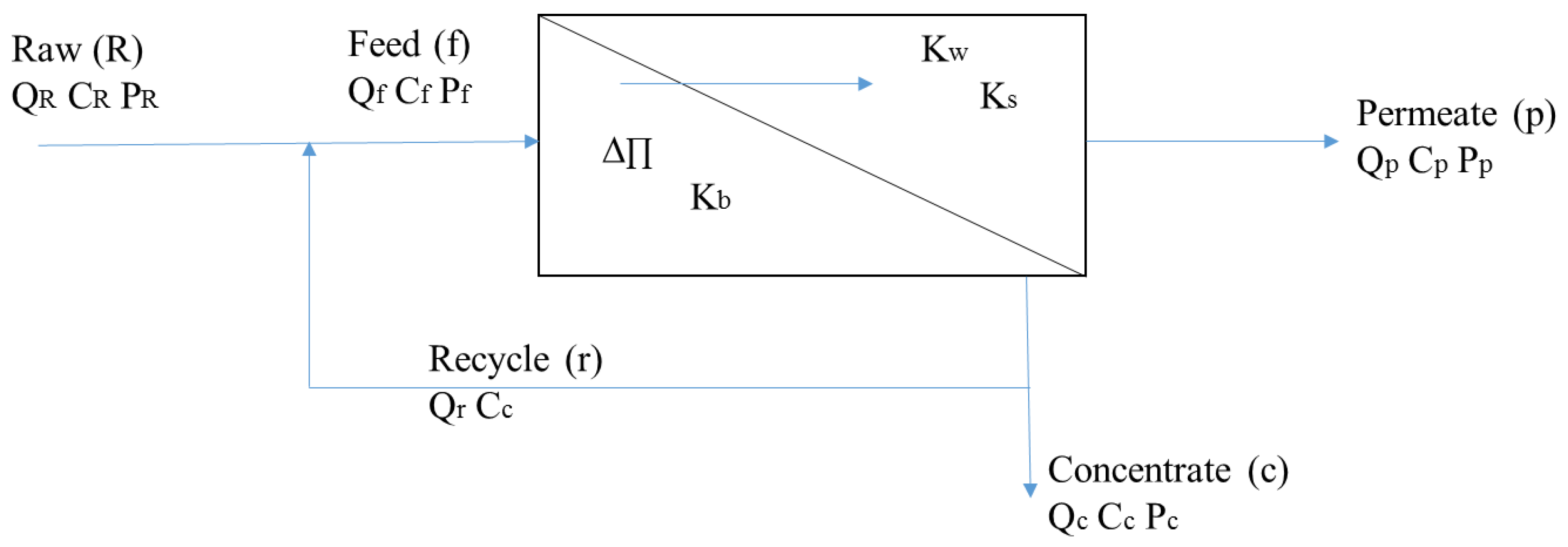
- Cf = solute concentration in the feed stream (mg/L)
- Cp = solute concentration in the permeate stream (mg/L)
- Kw = mass transfer coefficient of water (gal/ft2-day-psi)
- Ks = solute mass transfer coefficient (ft/day)
- ΔP = transmembrane pressure (psi)
- ΔП = transmembrane osmotic pressure (psi)
- R = recovery.
2. Materials and Methods
2.1. Membrane Materials
2.2. Inorganic Solution Chemistry
3. Results
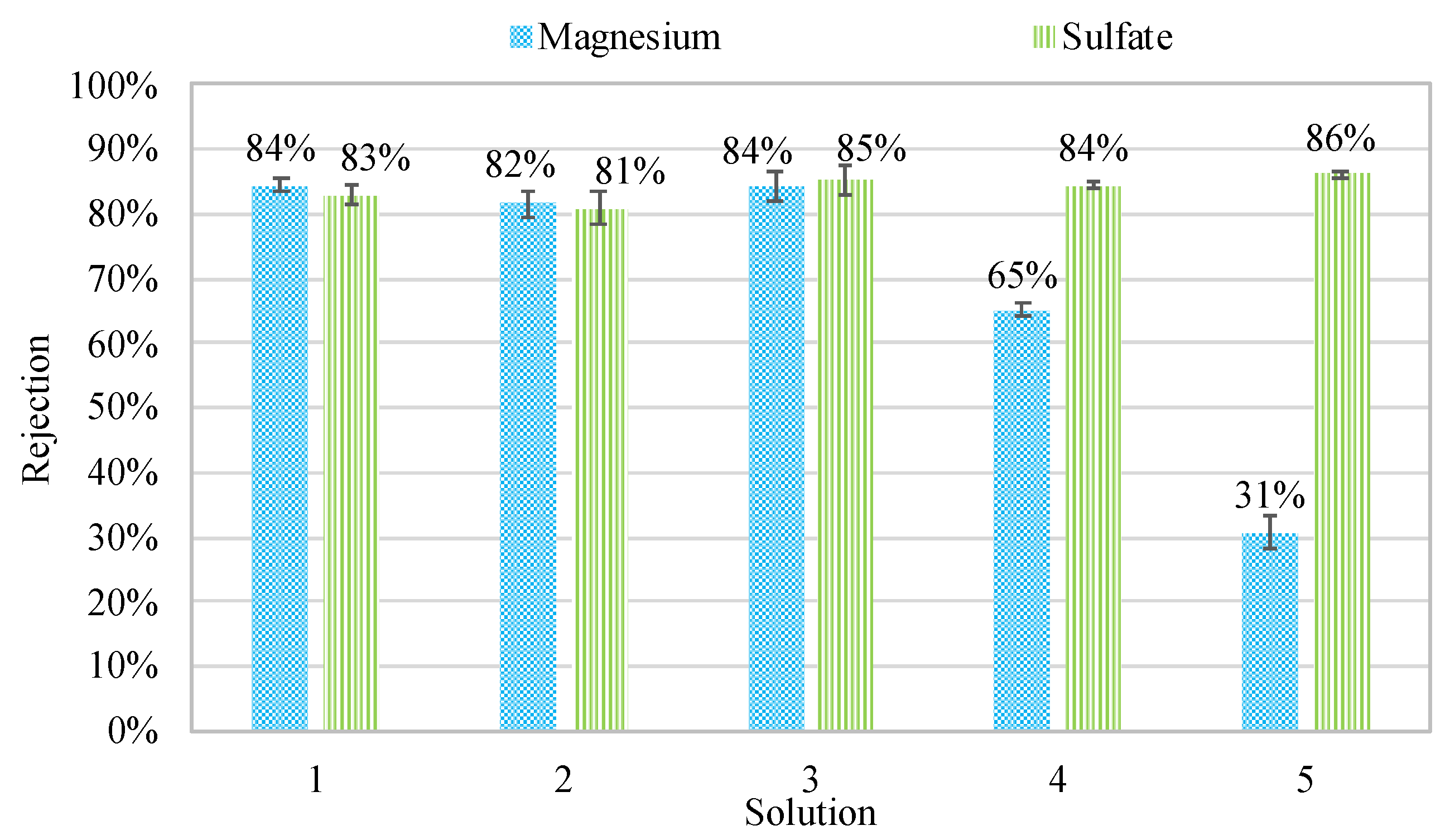
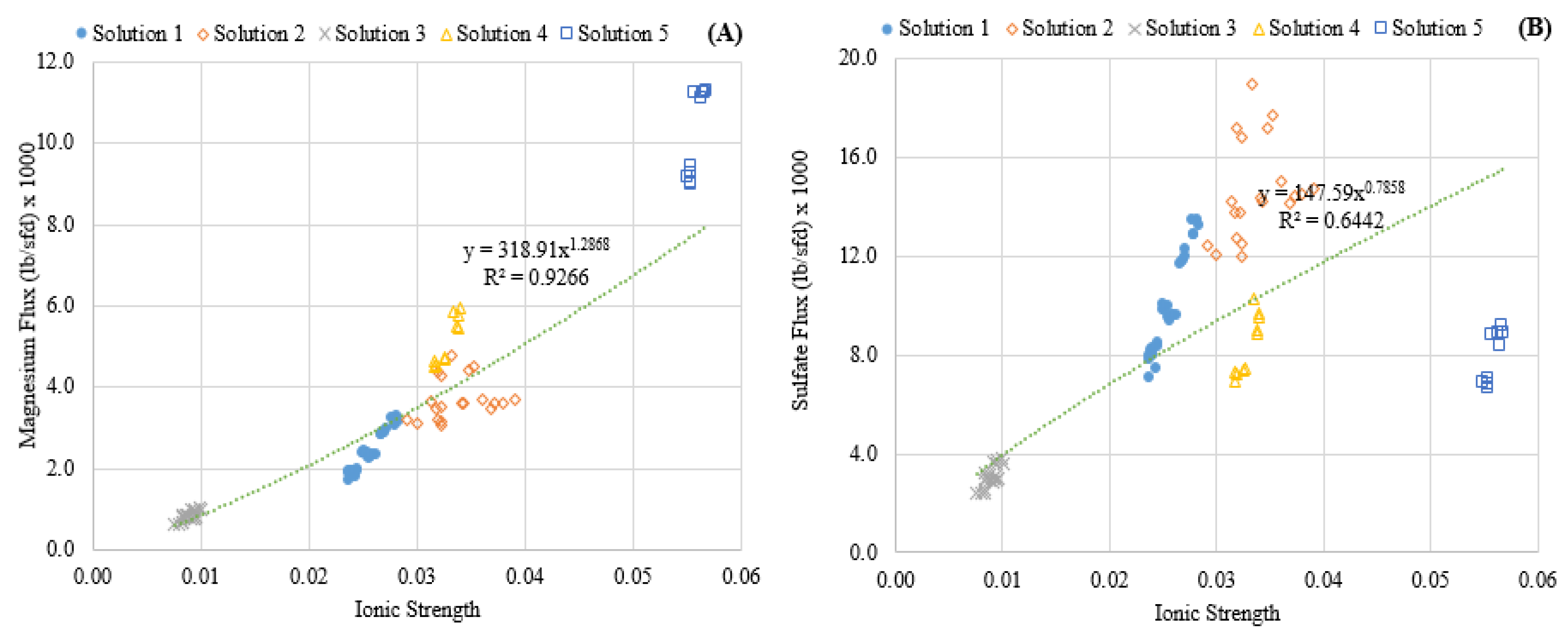
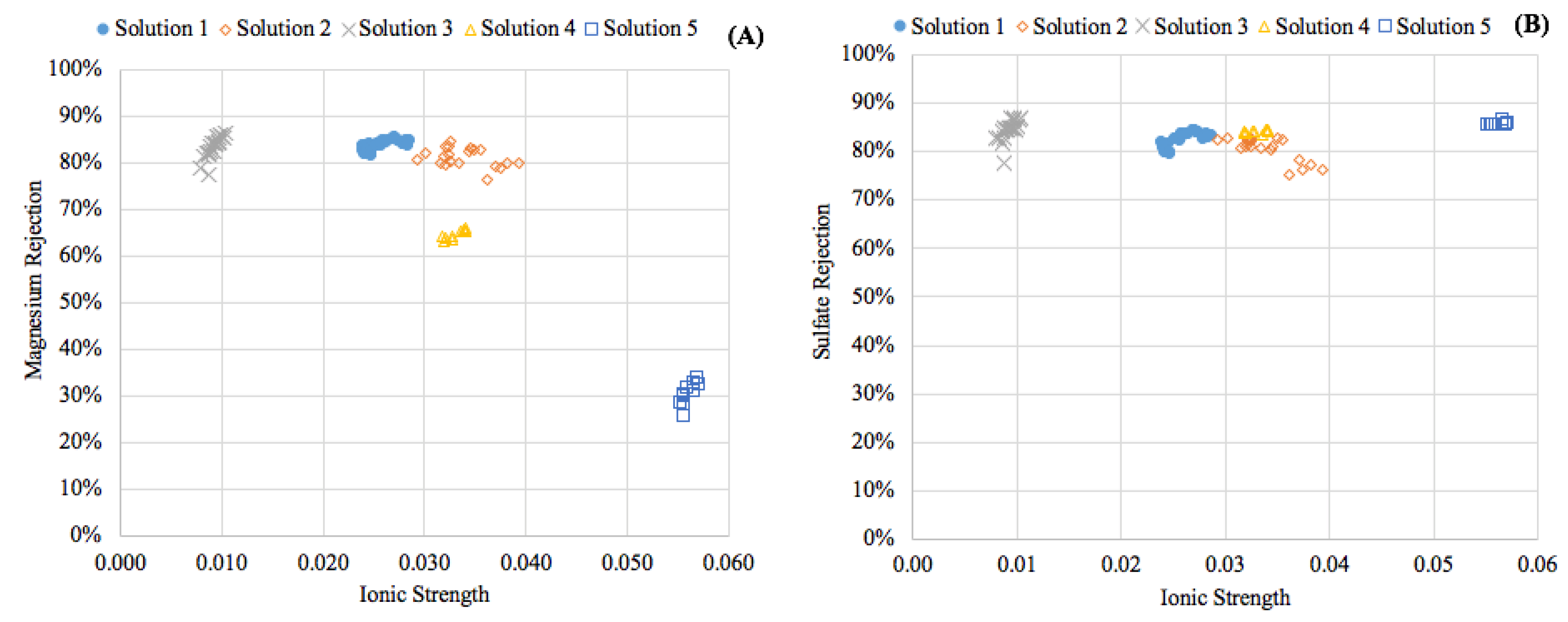
3.1. Experimental Results
3.2. Model Development
- Q = flow (ft3/s)
- C = concentration (mg/L)
- Subscripts f, c, p = feed, concentrate, permeate.
- Fw = flux of water through the membrane (gal/ft2-day)
- Kw = mass transfer coefficient of water (gal/ft2-day-psi)
- ΔP = transmembrane pressure (psi)
- ΔП = transmembrane osmotic pressure (psi)
- A = membrane area (ft2).
- ΔC = [(Cf + Cc)/2]—Cp
- Fs = mass flux of solute (lb/ft2 · day)
- Ks = solute mass transfer coefficient (ft/day)
- μ = ionic strength
- β1 and β2 = constants.
- 1
- Rearranging Equations (6) and (7) and equating yields:
- 2
- Rearranging for ΔC produces:
- 3
- Solving for Cc:
- 4
- Rearranging Equation (3) and substituting into Equation (4) yields:Solving for Cf:
- 5
- Substituting Equation (5) into Equation (9):
- 6
- Substituting Cc in Equation (10) with Equation (8) and simplifying:
- 7
- Rearranging to group common factors Cp and Cf yields:
- 8
- Solving for Cp:
- 9
- Factoring out and rearranging:
- 10
- Multiplying both sides by yields:
- 11
- Multiply both sides by yields:
- 12
- Substituting Equation (6) into Equation (11) produced the HSD-IS model provided in Equation (12):
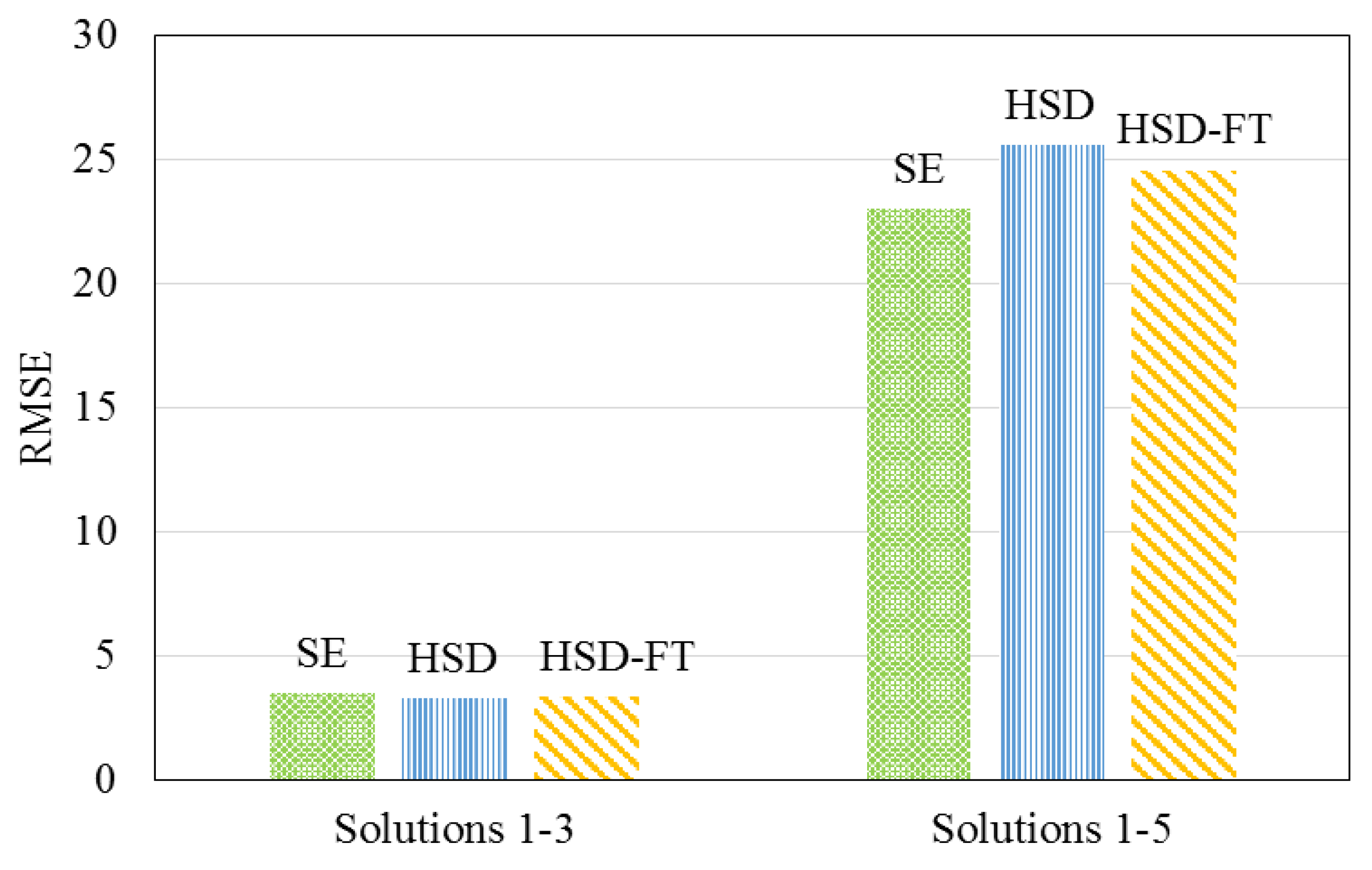
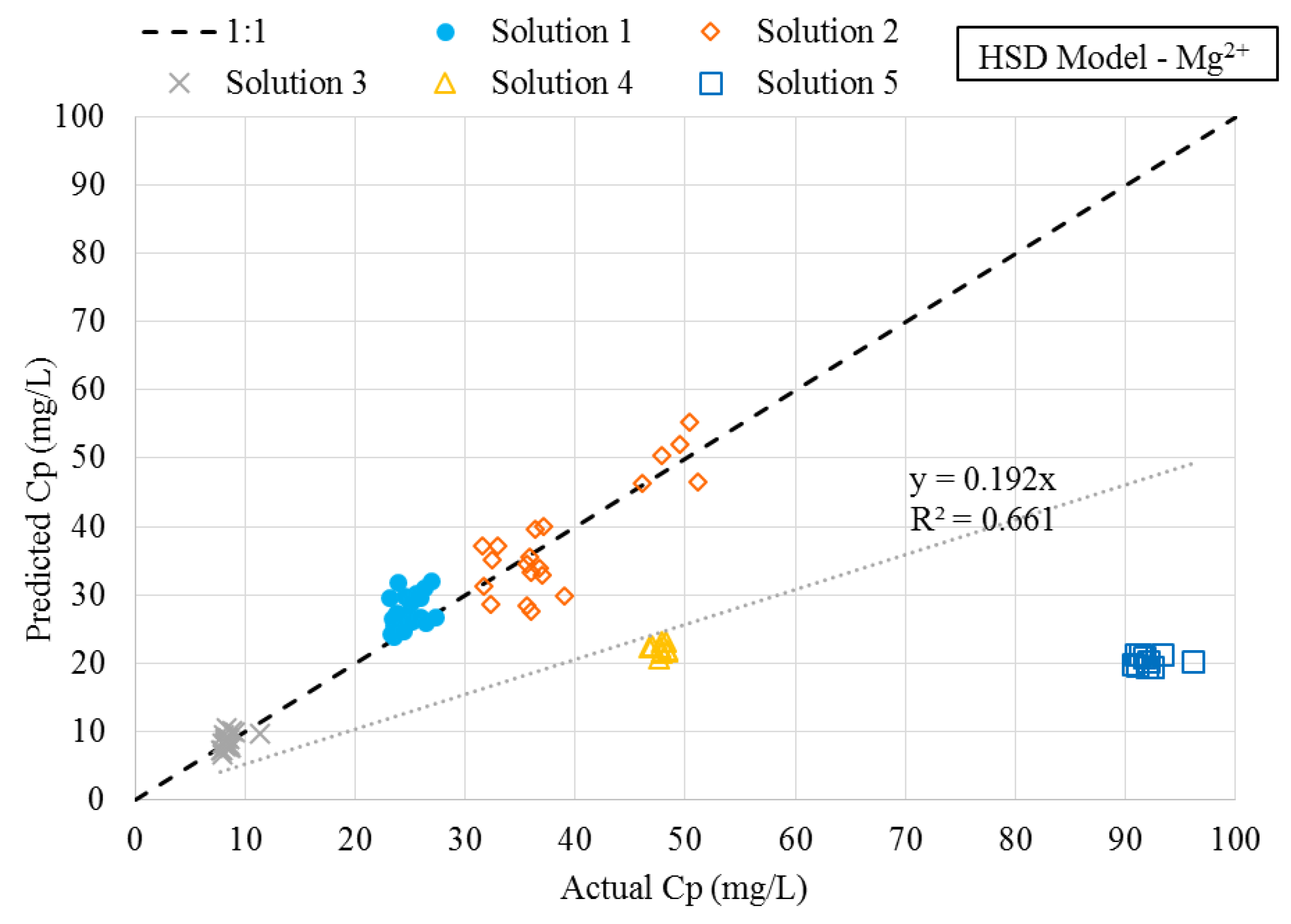
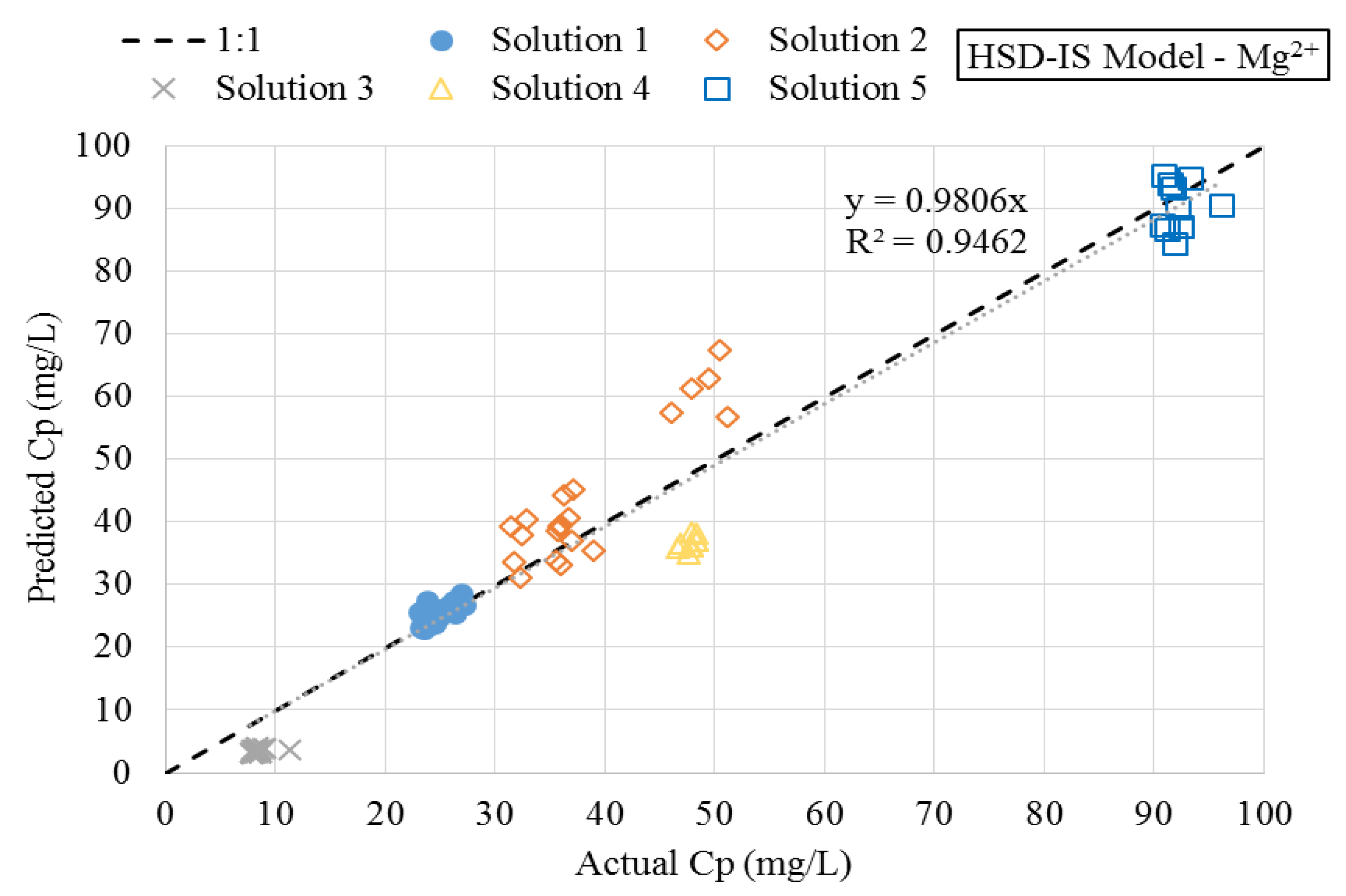
3.3. Numerical Simulations
4. Conclusions
- Feedwater ionic strength was observed to have a nonlinear impact on the diffusion of magnesium during a NF process.
- A modification of the HSD model was developed and proposed, which incorporated an empirical term related to the effect of feedwater ionic strength on diffusion of magnesium. This model was referred to as the HSD-IS model.
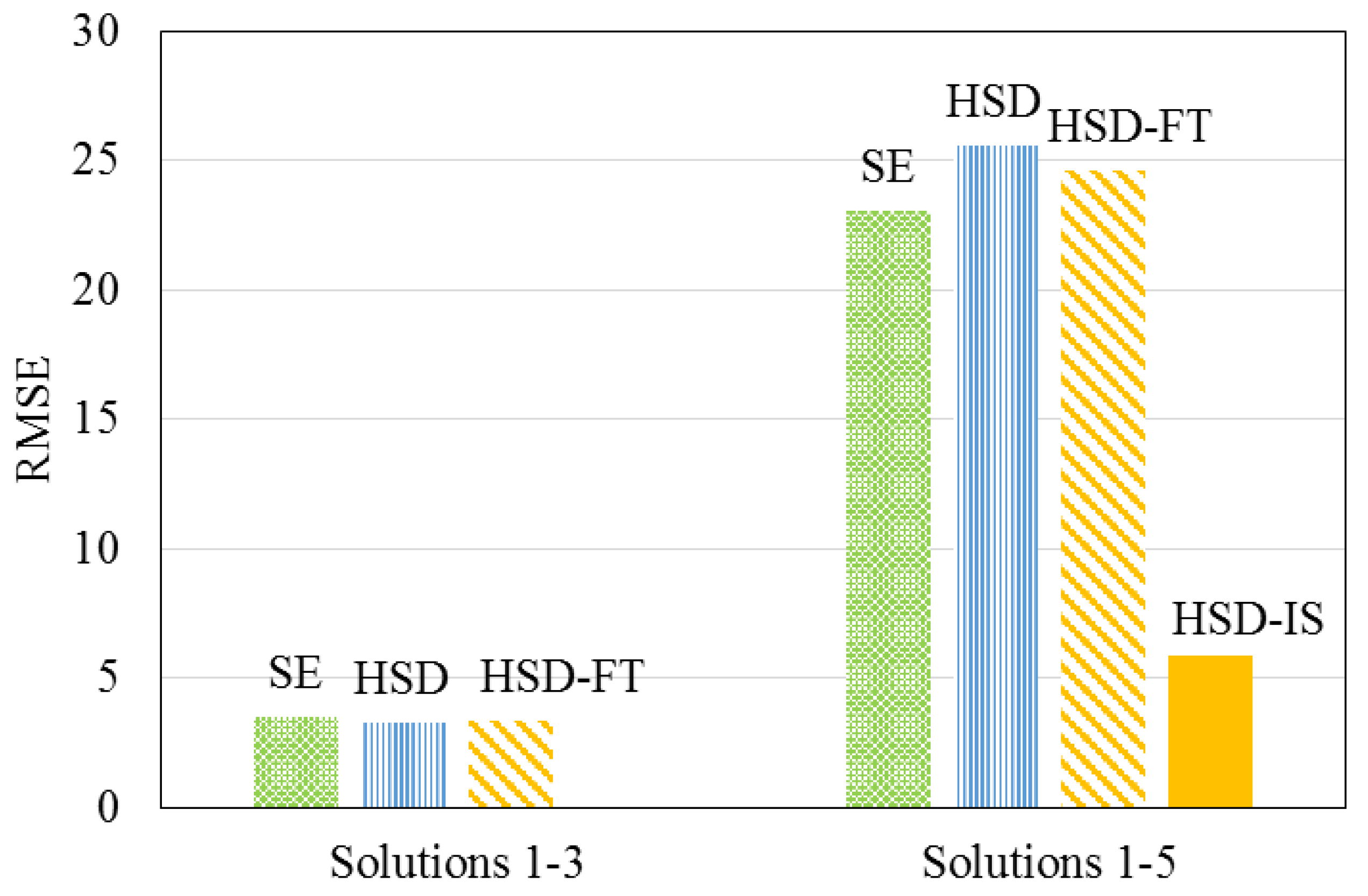
- The RMSE of the HSD-IS model was improved by 75%, as compared to existing models that do not incorporate a term related to feedwater ionic strength. This improvement, in turn, suggested that feedwater ionic strength should be considered when modeling hardness removal during nanofiltration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lonsdale, H.K.; Merten, U.; Riley, R.L. Transport properties of cellulose acetate osmotic membranes. J. Appl. Polym. Sci. 1965, 9, 1341–1362. [Google Scholar] [CrossRef]
- Duranceau, S.J. Modeling of Mass Transfer and Synthetic Organic Compound Removal in a Membrane Softening Process. Ph.D. Dissertation, University of Central Florida, Orlando, FL, USA, 1990. [Google Scholar]
- AWWA. Reverse Osmosis and Nanofiltration (M46), 2nd ed.; American Water Works Association: Denver, CO, USA, 2007. [Google Scholar]
- Zhao, Y.; Taylor, J.S.; Chellam, S. Predicting RO/NF water quality by modified solution diffusion model and artificial neural networks. J. Membr. Sci. 2005, 263, 38–46. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Gupta, S.K. Estimation of mass transfer coefficient using a combined nonlinear membrane transport and film theory model. Desalination 1997, 109, 39–49. [Google Scholar] [CrossRef]
- Fang, Y.; Duranceau, S.J. Comparison of nonhomogeneous and homogeneous mass transfer in reverse osmosis membrane processes. Desalin. Water Treat. 2013, 51, 6444–6458. [Google Scholar] [CrossRef]
- Sung, L. Film Theory and Ion Coupling Models for a Diffusion Controlled Membrane Process. Ph.D. Dissertation, University of Central Florida, Orlando, FL, USA, 1993. [Google Scholar]
- Cussler, E.L. Diffusion Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Zhao, Y.; Taylor, J.S. Modeling membrane performance over time. J. Am. Water Works Assoc. 2004, 96, 90–97. [Google Scholar] [CrossRef]
- Laisure, D.; Taylor, J. Modeling mass transfer in nanofiltration membrane systems. In Proceedings of the AWWA Membrane Technology Conference, Baltimore, MD, USA, 1–4 August 1993. [Google Scholar]
- Mulford, L.A.; Taylor, J.S.; Nickerson, D.M.; Chen, S.-S. NF performance at full and pilot scale. Am. Water Works Assoc. J. 1999, 91, 64. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Maung, H.; Song, L. Effect of pH and ionic strength on boron removal by RO membranes. Desalination 2009, 246, 605–612. [Google Scholar]
- Jones, K.L.; O’Melia, C.R. Protein and humic acid adsorption onto hydrophilic membrane surfaces: Effects of pH and ionic strength. J. Membr. Sci. 2000, 165, 31–46. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Sanchez, V. Model for colloidal fouling of membranes. Am. Inst. Chem. Eng. J. 1995, 41, 368–376. [Google Scholar] [CrossRef]
- Braghetta, A.; DiGiano, F.; Ball, W. Nanofiltration of natural organic matter: pH and ionic strength effects. J. Environ. Eng. 1997, 123, 628–641. [Google Scholar] [CrossRef]
- Levenstein, R.; Hasson, D.; Semiat, R. Utilization of the donnan effect for improving electrolyte separation with nanofiltration membranes. J. Membr. Sci. 1996, 116, 77–92. [Google Scholar] [CrossRef]
- Bartels, C.; Franks, R.; Rybar, S.; Schierach, M.; Wilf, M. The effect of feed ionic strength on salt passage through reverse osmosis membranes. Desalination 2005, 184, 185–195. [Google Scholar] [CrossRef]
- Donnan, F.G. Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J. Membr. Sci. 1995, 100, 45–55. [Google Scholar] [CrossRef]
- Nanda, D.; Tung, K.-L.; Hsiung, C.-C.; Chuang, C.-J.; Ruaan, R.-C.; Chiang, Y.-C.; Chen, C.-S.; Wu, T.-H. Effect of solution chemistry on water softening using charged nanofiltration membranes. Desalination 2008, 234, 344–353. [Google Scholar] [CrossRef]
- Zha, S.; Gusnawan, P.; Zhang, G.; Liu, N.; Lee, R.; Yu, J. Experimental study of PES/SiO2 based TFC hollow fiber membrane modules for oilfield produced water desalination with low-pressure nanofiltration process. J. Ind. Eng. Chem. 2016, 44, 118–125. [Google Scholar] [CrossRef]
- Yuan, Y.; Kilduff, J.E. Hydrodynamic modeling of NOM transport in UF: Effects of charge density and ionic strength on effective size and sieving. Environ. Sci. Technol. 2009, 43, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Wilf, M.; Awerbuch, L.; Bartels, C. The Guidebook to Membrane Desalination Technology Reverse Osmosis, Nanofiltration, and Hybrid Systems Process, Design, Applications and Economics; Balaban Desalination Publications: Hopkinton, MA, USA, 2007. [Google Scholar]
- Briggs, J.C.; Ficke, J.F. Quality of Rivers of the United States, 1975 Water Year—Based on the National Stream Quality Accounting Network (NASQAN); U.S. Geological Survey: Reston, VA, USA, 1977; p. 436.
- Nakatsuka, S.; Nakate, I.; Miyano, T. Drinking water treatment by using ultrafiltration hollow fiber membranes. Desalination 1996, 106, 55–61. [Google Scholar] [CrossRef]
- Ferry, J.D. Statistical evaluation of sieve constants in ultrafiltration. J. Gen. Physiol. 1936, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zeman, L.; Wales, M. Steric rejection of polymeric solutes by membranes with uniform pore size distribution. Sep. Sci. Technol. 1981, 16, 275–290. [Google Scholar] [CrossRef]
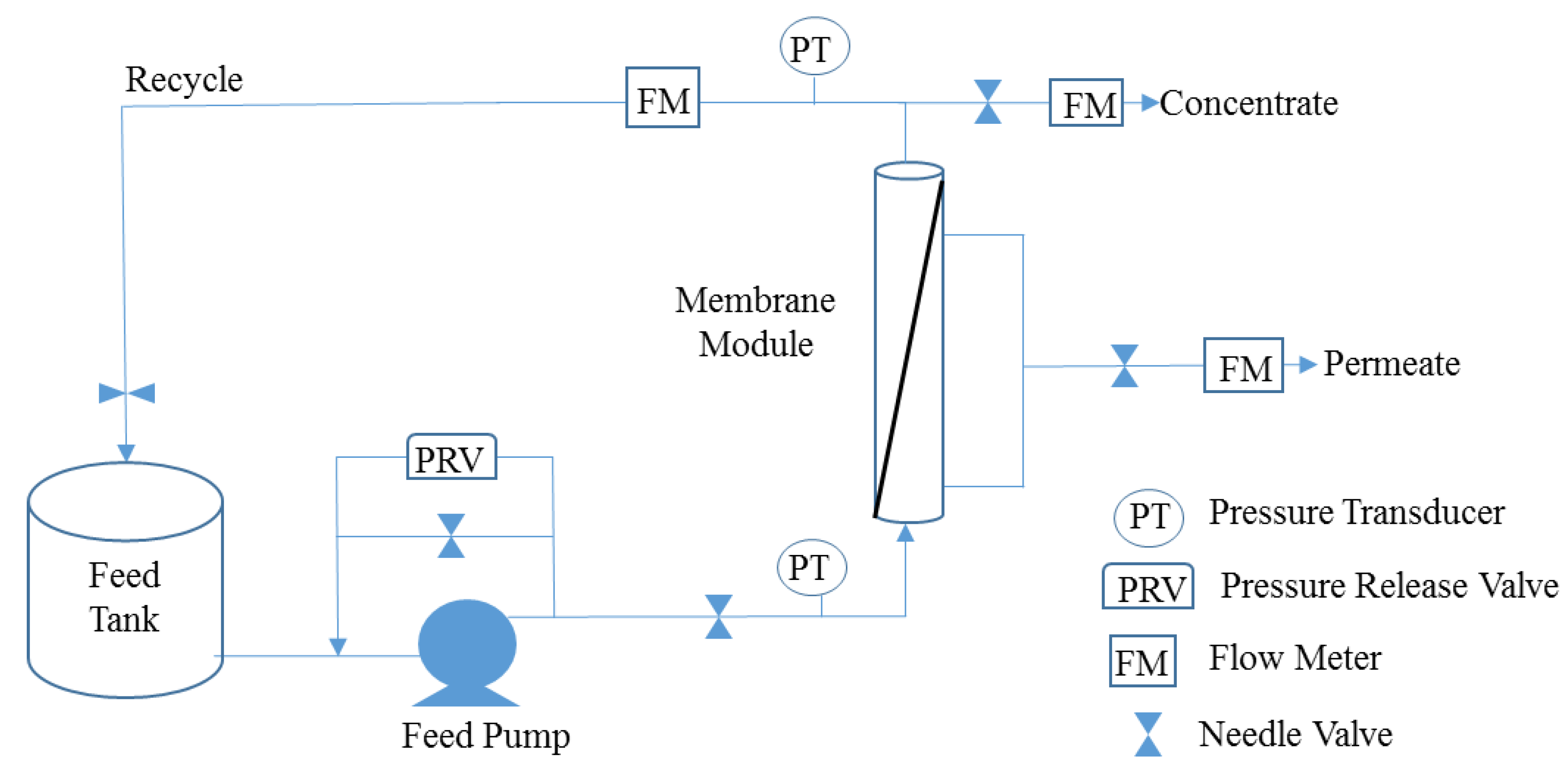
| Parameter | Value |
|---|---|
| Operating Flux (lmh) | 10–25 |
| Cross Flow Velocity (m/s) | 0.2–2.0 |
| MWCO (Da) | 700 |
| TOC Rejection % | 93–97 |
| Divalent Ion Rejection % | 30–60 |
| Solution | MgSO4•7H2O Concentration mg/L (mM) | NaCl Concentration mg/L (mM) | TDS (mg/L) | Ionic Strength (IS) | Hardness Class |
|---|---|---|---|---|---|
| Solution 1 | 600 (5) | <1 (<1) | 700 | 0.023 | Hard |
| Solution 2 | 960 (8) | <1 (<1) | 960 | 0.032 | Very Hard |
| Solution 3 | 240 (2) | <1 (<1) | 260 | 0.009 | Soft |
| Solution 4 | 600 (5) | 730 (12.5) | 1200 | 0.030 | Hard |
| Solution 5 | 600 (5) | 2340 (40) | 2600 | 0.054 | Hard |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonge, D.T.; Biscardi, P.G.; Duranceau, S.J. Modeling Ionic Strength Effects on Hollow-Fiber Nanofiltration Membrane Mass Transfer. Membranes 2018, 8, 37. https://doi.org/10.3390/membranes8030037
Yonge DT, Biscardi PG, Duranceau SJ. Modeling Ionic Strength Effects on Hollow-Fiber Nanofiltration Membrane Mass Transfer. Membranes. 2018; 8(3):37. https://doi.org/10.3390/membranes8030037
Chicago/Turabian StyleYonge, David T., Paul G. Biscardi, and Steven J. Duranceau. 2018. "Modeling Ionic Strength Effects on Hollow-Fiber Nanofiltration Membrane Mass Transfer" Membranes 8, no. 3: 37. https://doi.org/10.3390/membranes8030037
APA StyleYonge, D. T., Biscardi, P. G., & Duranceau, S. J. (2018). Modeling Ionic Strength Effects on Hollow-Fiber Nanofiltration Membrane Mass Transfer. Membranes, 8(3), 37. https://doi.org/10.3390/membranes8030037






