3.1. Membrane Functionalization and Stabilization
An electrostatic layer-by-layer assembly has been chosen to deposit hybrid responsive materials onto the porous surface of commercial PE hollow fibers. A colloidal dispersion of TiO2 nanoparticles in PAA aqueous solutions has been prepared at pH 6.75 without adding salts and has been successively patterned with a counter solution of PDDA. During the LBL built up the ionic charges that are distributed along the segment polymer chains of the ionic polyelectrolytes pair has been shielded.
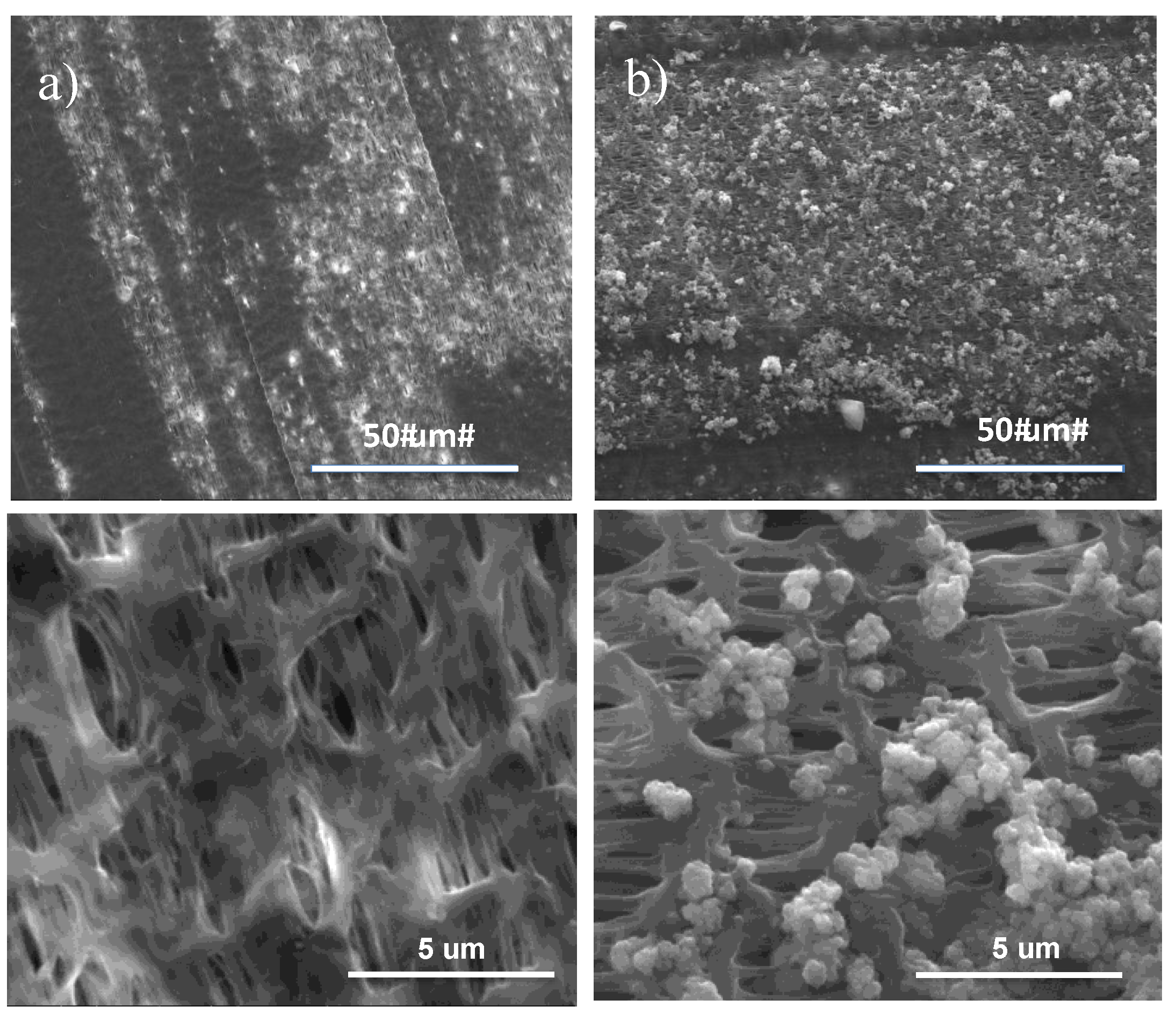
SEM micrographs reveal the formation of uniform particulate-like structure with size of 1 to 2 μm, thus leaving open pores over the PE shell side (
Figure 1).
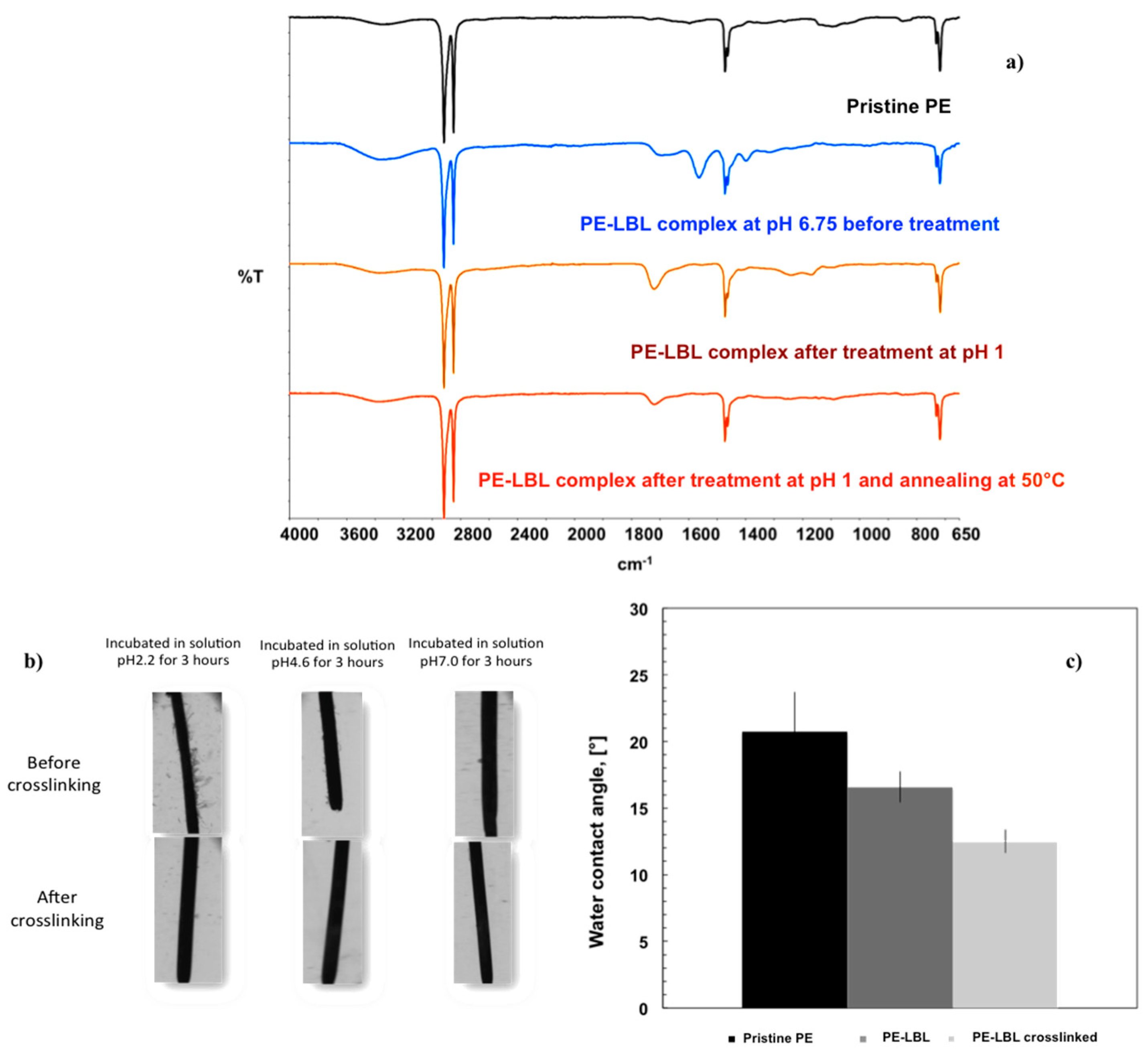
Infrared analyses confirm the occurrence of chemical changes through the surface due to the formation of the LBL complex (
Figure 2a). From ATR spectra, it is discernable a small broad stretching of carboxylic C=O group, while the appearance of two strong bands at 1563 and 1399 cm
−1 clearly indicate the presence of a large number of carboxylate anion groups of PAA onto the membrane surface. These two last vibrational modes are associated to asymmetrical and symmetrical stretching bands of the carboxylate anion, respectively.
At pH 6.75, the polyacid reaches an almost full-ionized state, thus causing the polymer chains to elongate in a flat conformation due to the high density of repulsive negative charge along the segments [
20]. The largest availability of the carboxylate anion enables PAA to shield the oppositely charged sub-layer (PDDA) leading to a major saturation of the oppositely charged sites with a reduced amount of material. Accordingly, PE pore occlusion has been successfully prevented.
The chemical stability of the LBL complex has been also examined within a broad range of pH; a certain tendency to leach has been observed with time, especially at the lowest pH value. Initially, the LBL is in a swelled state and the access of additional water molecules into the bulk is facilitated, thus yielding the polymer assembly more exposed to competitive intermolecular interactions that can affect the stability of the assembly locally. With this concern, the LBL complex has been, therefore, dipped in a buffer solution at pH 1.0 for a few seconds in order to promote cross-linking via hydrogen bonding; successively, the membranes have been annealed at 50°C for 3 h and the excess of adsorbed water has been removed so that compactness and chemical stability of LBL assembly have been achieved through strengthened H-bonding and electrostatic interactions between PDDA and PAA (
Figure 2).
The chemical cross-link has been confirmed by the disappearance of two bands at 1563 and 1399 cm
−1 (νCOO
−) and the concomitant appearance of the infrared mode associated to the COOH group at ν1720 cm
−1 (
Figure 2a). Optical microscopic observations have confirmed further the stability of LBL complex after 3 h of incubation in buffer solutions at various pH values (
Figure 2b).
At this stage, typical signature markers of TiO
2, that is associated to the stretching vibration of Ti-O-Ti and falling in the fingerprint region of 900–600 cm
−1, have been not easily discernable from ATR spectra due to the overlapping of two strong rocking vibrations of PE chains (ρCH
2) at 730 and 718 cm
−1, respectively. However, the broadness of the bands centered at 3364 and 1717 cm
−1 together with the appearance of a shoulder around 1409 cm
−1 suggest the occurrence of different molecular interactions due to the establishment of heterogeneous chemical environments that receive also the contribution of O–H bond of the surface-adsorbed species of particles (Ti–OH) [
25], plausibly. It is further interesting to observe that the cross-linked LBL surfaces confer hydrophilic character to the membranes due to a higher availability of hydrogen acceptor/donor groups. A reduction of 8° for contact angle values can be appreciated moving from pristine to cross-linked PE-LBL membranes (
Figure 3b).
3.2. Experiments in Batch
Raw OMW solutions deriving from the squeeze of olive fruits are somewhat complex mixtures. They contain acids, pectinase, and phenols, but also suspended solids and particles. So, pretreatments have been necessary before processing. The solutions have been filtered with a metal mesh having a cut off of 35 μm in order to remove larger suspended particles. The filtrate has been centrifuged at 6000 rmp for 45 min and the suspension has been examined by UV-Vis in order to estimate the content and composition in phenols: 2678 mg L
−1 against 2727 mg L
−1 valued for no-centrifuged solutions. The original OMW solution, which looks like turbid, exhibits a pH of 4.6 and a dynamic viscosity value of 0.00181 Pas. After adjusting the pH value to 2.2, a further clarification of the solution reduces the dynamic viscosity to 0.00168 Pas (
Figure 3c).
As reported in a recent study [
26], the further acidification causes a destabilization of the suspension by modifying the zeta potential with subsequent easier flocculation of suspended solids.
Nevertheless, both the OMW solutions have been used to explore the foulants adsorption on pristine PE and PE-LBL membrane surfaces in order to detect the role of the density of charge in the control of interfacial phenomena. Accordingly, pristine PE membranes have been dipped for 1, 7, and 24 h under stirring in both the OMW solutions at two different pH values.
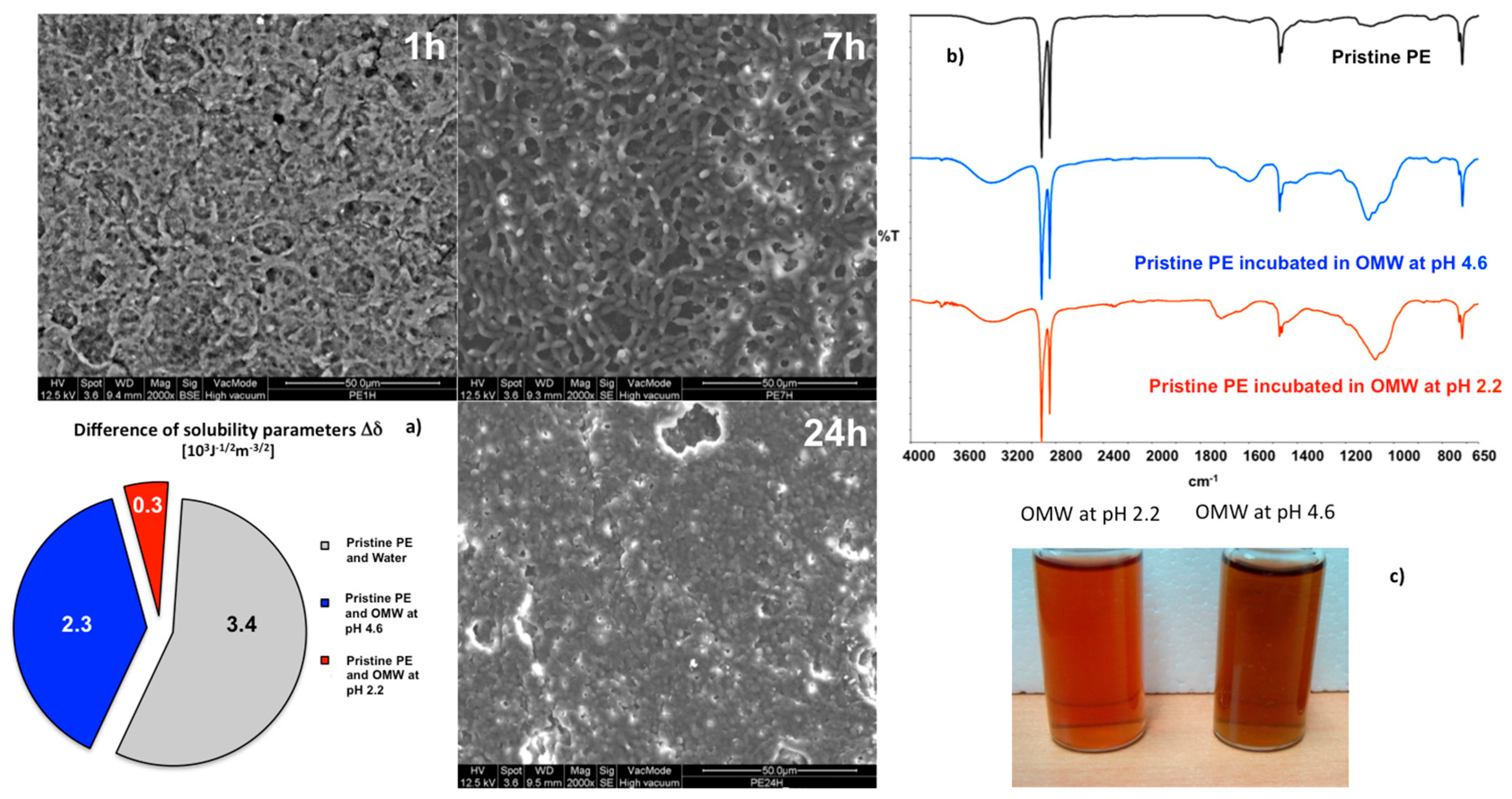
SEM images collected onto the surfaces reveal the formation of a film, which grows with time occluding increasingly the pores of the membrane. This large susceptibility to the foulants adsorption can be regarded as the result of an extensive affinity between membrane and solutions (
Figure 3a). At the lowest pH conditions, the smallest difference can be estimated between the parameters of solubility (Δ
δ) of PE membranes and OMWs. As explained hereafter, this parameter is analyzed through the calculation of overall surface free tensions and related parameters of solubility [
23], and is a good indicator of the power of interaction between interfaced systems. ATR spectra confirm further the occurrence of significant interactions between two systems (
Figure 3b). Stretching infrared modes falling in the regions of 3000–3500 cm
−1, 1550–1750 cm
−1, and 1000–1230 cm
−1, ascribable to νO-H, νC=O(OH), νC=O(O
−), and νC(C=O)C groups of species dissolved in OMWs can be easily detected. At pH 4.6, the νC=O bands is significantly shifted towards 1600–1550 cm
−1 vibrational region, suggesting a higher density of negative charge over the surface due to the increased number of carboxylate anions.
After functionalization, the nanostructured membranes have been again dipped in the dark at two different pH values under continuous stirring in order to reach sorption-desorption equilibrium. SEM micrographs (
Figure 4a’), collected onto the PE-LBL membrane surfaces, have showed a discernable formation of a dense film on the surface treated for 24 h at pH 2.2. Although marginally reduced, this adsorption of pollutant continues to be detectable on the surface dipped at pH 4.6 as well.
Once more, the degree of affinity of the PE-LBL complex to OMW solutions and pure water has been estimated.
Figure 4c shows the difference between the parameters of solubility of the membrane surface and water, as well as between the membrane and OMW
2.2 and OMW
4.6 solutions, respectively. Provided that this difference is regarded as a quantitative indicator of attraction between two systems coming in contact, small differences mean high affinity, while large differences indicate poor interactions between two systems. The lowest difference value (0.7 × 10
3 J
−1/2m
−3/2) of the solubility parameters can be estimated for the PE-LBL/OMW
2.2 pair, confirming the strongest interaction between the PE-LBL surface and acidified pollutant solution. Indeed, a strong accumulation of foulants has been detectable on the membrane surface by SEM and infrared analyses (
Figure 4a,b). At pH 2.2, a strong and broad vibrational absorption centered at 1708 cm
−1 (νC=O) and associated with carboxyl acid groups is visible, while a very broad absorption attributed to infrared modes νC=O(O-), νC-C(=O)-O, and νO-C-C can be observed in the region 900–1250 cm
−1.
At pH 4.6, a certain affinity continues to be observable, and, even if less intense, broader infrared absorptions (1760–1500, 1263, and 1100 cm
−1) are still noticed. It is relevant to observe that at a higher pH value (4.6), there is a major density of negative charge in OMW solution but also along the polyelectrolyte PAA segment chains forming the LBL complex. The increase in the density of negative charge is expected to cause higher repulsion between ionic species with similar charge, thus contrasting the formation of heavy foulants adsorption over the membrane surface, differently from what was observed at lower pH conditions (
Figure 4a). However, the contrast to the foulants adhesion seems to be not yet satisfactory.
3.3. Experiments in Batch under Irradiation
Samples of PE-LBL membranes have been also incubated in OMW solutions for 24 h under continuous irradiation and stirring. After dipping, SEM analysis reveal significantly reduced adsorption of pollutants onto both of the membrane surfaces dipped at pH 2.2 and 4.6 (
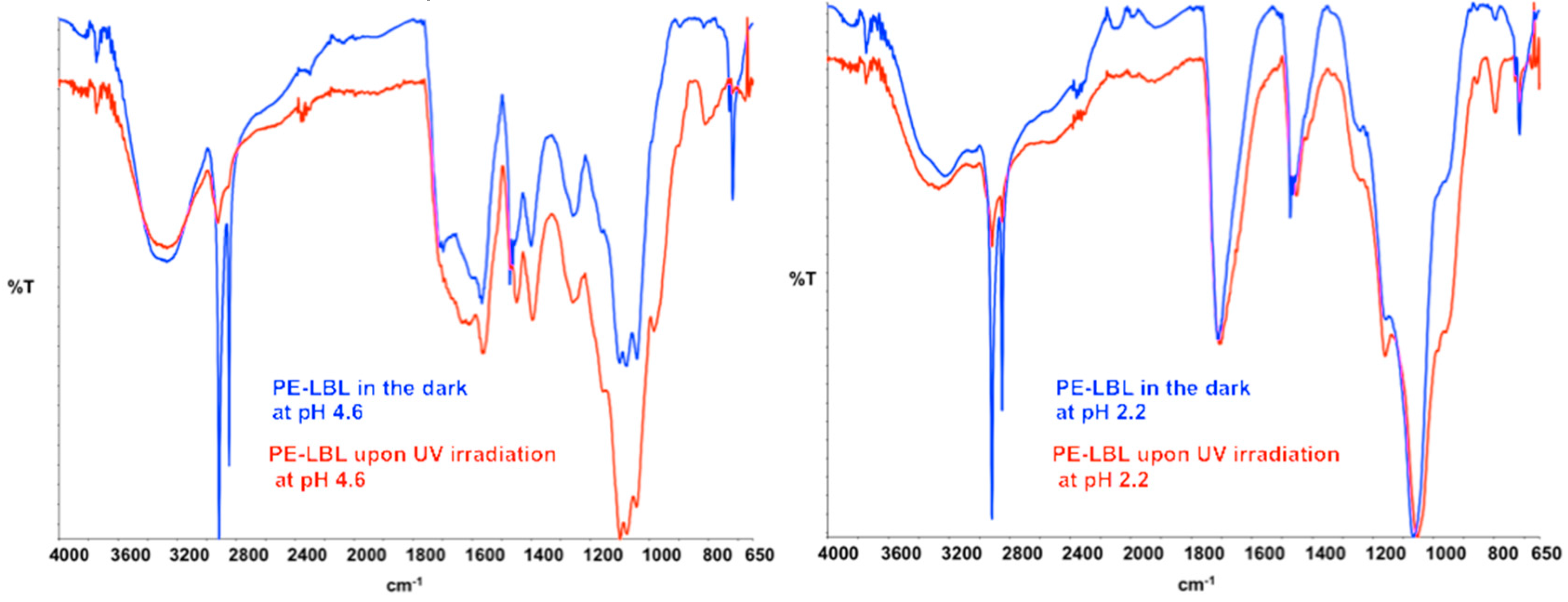
Figure 4a”), while ATR spectra show interesting variations in the absorption infrared bands (
Figure 5). In both of the cases, the irradiation causes broadness, together with the appearance of additional shoulders in the identified infrared bands νC-OH, νC=O, νC=O(OH), νC=O(O-), νC-C(=O)-O, and νO-C-C. Instead, aliphatic stretching modes νCH
2, νCH
3 (2917, 2849 cm
−1), as well as aliphatic bending and rocking modes δCH
2, δCH
3, ρCH
2 (1473, 1463, 1456, 730, 718 cm
−1) of the segment chains appear to be somewhat reduced in intensity and resolution, while a distinctive broad band looks around 800 cm
−1.
Concerning samples that are dipped at pH 4.6, the band centered at 1396 cm
−1 and attributed to symmetrical stretching of carboxylate anion appears to be much more intense, broader, and shifted to lower wavenumbers (Δν, 5 cm
−1). It should be noted that this wavenumber is also assigned to the O-H bond vibration of the surface-adsorbed species of NPs (Ti–OH) [
25]. This important shift suggests, hence, changes in the chemistry of the surface when irradiated. Also, the bands that are assigned to νO-H, νC=O, νCOO,
− and νC-O bonds, which are able to coordinate O-H groups of activated TiO
2 NPs, are shifted towards lower wavenumbers, thus providing a clear indication about light activation. Interestingly, the strong intensification of the band at 800 cm
−1 could be ascribed to a distortion due to coordination between TiO
2 NPs and aromatic pollutants.
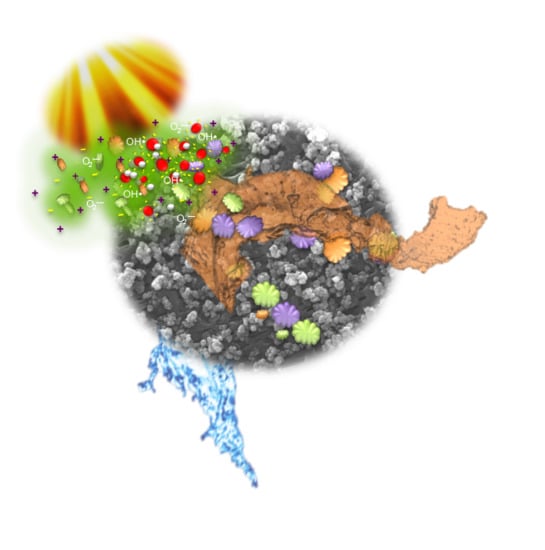
In this context, it would be reminded that once excitation occurs across the band gap of NPs, an electron-hole pair is generated, while a charge transfer takes place toward adsorbed species on the NPs surface from solution [
27]. The electron is predictable to reduce an electron acceptor, such as dissolved oxygen, whereas the hole migrates to the surface, oxidizing donor species such as hydroxyl species. As a result, superoxide anion (O
2•−) and hydroxyl (OH
•) radicals are generated and photo-degradation takes place reducing the foulants adsorption. Comparing the results achieved in batch conditions, enhanced resistance to contamination can be appreciated at higher pH values because favorable environmental conditions would be formed.
3.4. Water Flux Testing
PE-LBL membranes have been assembled in tubular modules and processed in a submerged microfiltration reactor (
Figure 6). This takes advantage over a traditional cross-flow microfiltration reactor of reduced power consumption and limited foulants deposition onto the membrane due to low operative pressures [
28,
29], even if lower fluxes are unfortunately obtained. One side of PE-LBL membranes has been sealed with glue, while the other side has been fixed with glue but left free without further set, as shown in
Figure 6a. The shell side of the membranes has been put in direct contact with OMW solution (feed side), while a slight vacuum has been applied to the free side of the fibers in order to cause a lower pressure at the lumen side of the membrane where the permeate has been collected. The absolute trans-membrane pressure (ΔP) has been monitored by a vacuum gauge kept between modulus and vacuum pump.
Figure 6b shows a comparison of pure water flux measured through PE-LBL membranes at 50 kPa under different pH conditions. Higher water flux has been measured at pH 2.2 due to the fact that the LBL complex is in a shrunk state. Because the polymer chains have been interlinked via hydrogen bonding interactions, the volumetric space in proximity to the PE pores is reduced and the resistance to the transport is limited further.
On the contrary, lower fluxes have been estimated at higher pH values due to a higher degree of ionization, which yields increased density of the negative charge. This brings about the polymer chains to expand and swell due to repulsive interactions; as a consequence, additional resistance to the transport is observed.
Before every experiment, the membranes modules have been, hence, compacted with a buffer at pH 2.2 within an absolute pressure range of 10 to 60 kPa at 20 °C in order to keep chemical, mechanical, and structural stability of the membranes during operation. Three running cycles have been necessary until constant pure water flux (7.1 ± 0.7 Lm−2h−1) has been reached.
3.5. Controlled Fouling during Submerged Microfiltration
Based on experiments carried out in-batch, submerged microfiltration testing has been run under different pH conditions, and UV light irradiation. The membranes have been worked for six hours. No middle cleaning steps or flux of air bubbles have been supplied during the tests with the precise intent to explore the efficiency of the photoactive gel without supplementary assistance.
Figure 7a,b show the average flux measured through pristine PE and PE-LBL membranes coming in contact with water and OMW streams, respectively.
It is relevant to note that the LBL complex causes an expected reduction of the pure water flux due to additional resistance that is induced by the gel deposited on the shell side of the membranes.
When comparing the performance of each single membrane, it is however observed that the flux falls heavily from 36 ± 3 Lm
−2h
−1 (
Jw, pure water) to 3.5–3.0 ± 0.2 Lm
−2h
−1 (
J, OMW
4.6–2.2) for pristine PE membranes, resulting in a relative flux of 0.11 to 0.09 after two first running hours, respectively (
Figure 7c). Functionalized membranes show almost halved flux, yielding after 2 h a relative flux of 0.57 at pH 4.6 and 0.48 at pH 2.2.
When considering that a larger affinity to OMW solutions is observed for pristine membranes (
Figure 3a), it is also pertinent to examine the changes in the resistance to the flux for each type of membrane (
Figure 7d). The latter are, in fact, good indicators of the capability of the membrane to contrast foulants adsorption.
At pH 2.2, both the pristine and functionalized PE membranes exhibit the highest resistance values. A certain propensity of the LBL complex to contrast foulants adsorption is just perceivable within the first two running hours. Given that the density of the negative charge of the LBL complex is almost negligible at pH 2.2, this initial hard effort to resist fouling could plausibly be ascribed to photodegradation activity, which tends to extinguish with time quickly.
At pH 4.6, the PE-LBL complex shows a slightly higher resistance value in the first part of the process, due to its tendency to swell. As expected, an inevitable slowing down on the fluid permeation is observed during the first hours of the experiments.
Conversely, after 3 h the increase in the resistance is quite contained and almost constant for LBL complex, whereas it becomes much more marked for PE pristine membranes (
Figure 7d). This reversal of behavior can be regarded as an undeniable indicator of the capability of the LBL complex to contrast the decline of the flux with time.
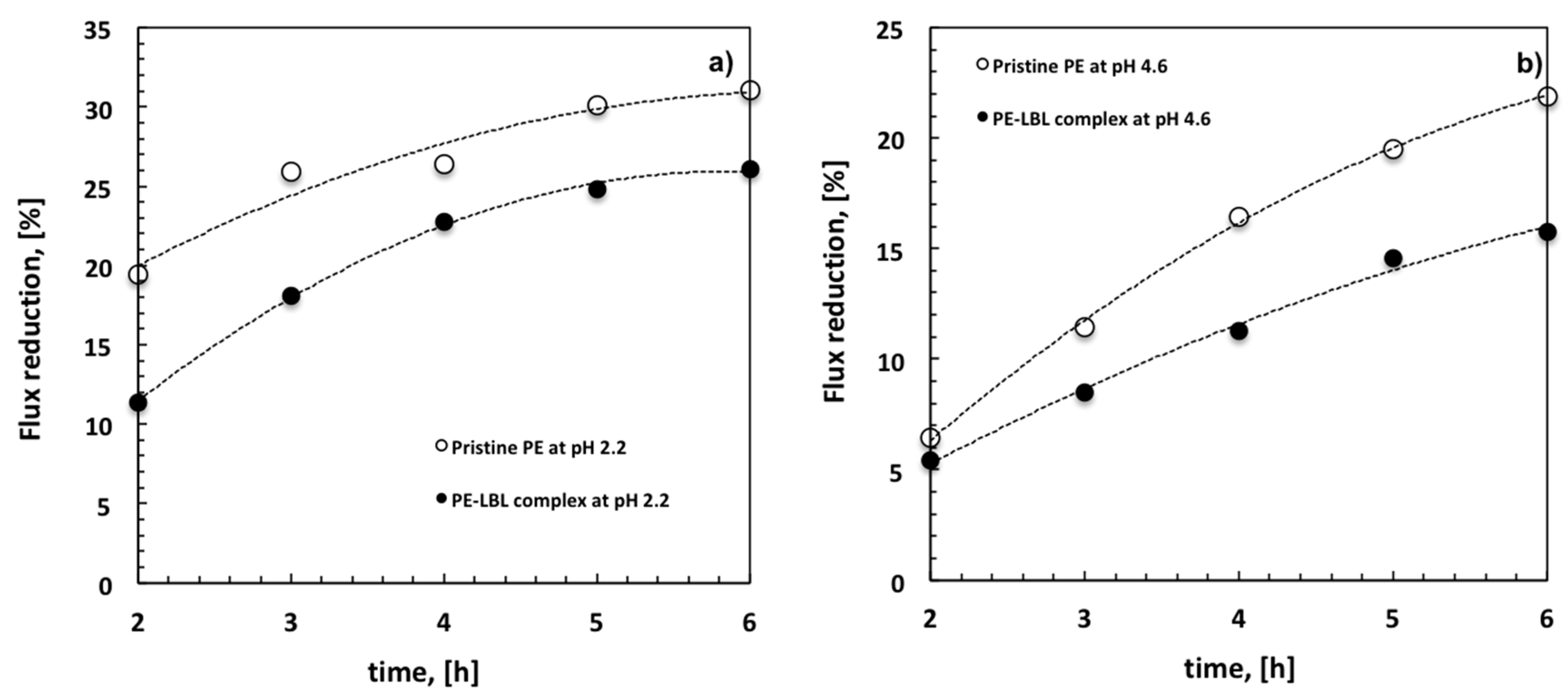
With this regard, it is also interesting to analyze the decline of the average flux (FD) with time for all of the membranes under different pH conditions (
Figure 8). At pH 2.2, a FD of 19% is detectable for pristine PE membranes against the 11% for LBL membranes after two hours. The decline reaches the 31% for unmodified membranes and a 26% for the modified ones after six hours (
Figure 8a).
Otherwise, when the module is irradiated at pH 4.6, the difference between pristine PE and PE-LBL membranes becomes much more discernable throughout the overall experiment. After six hours, the average flux looks to reduce with time more quickly for pristine PE (22%), while resulting in a slower decrease for PE-LBL membranes (15%) (
Figure 8b). This is in full agreement with the resistance behavior.
This datum becomes much more significant if compared to the decline of the average flux (63%) that is observed after 2 h for microfiltration of OMW with a hydrophilic cellulose acetate membrane (pore size: 0.2 μm, TMP, 18 kPa, T = 25 °C, pH= 5.02) [
30].
In full accordance with the experiments carried out in batch, the photocatalysis becomes effective when repulsive interfacial forces are well established between solution and membrane surface, according to the concept of photoactive gel assisted cleaning [
31].
At pH 4.6, the density of negative charge causes a partial elongation of the polymer chains; TiO
2 NPs are pulled out while electrostatic repulsive interactions contrast excessive adsorption of foulants onto the surface. Also, the polyelectrolytes act as dispersing agents and electrostatic stabilizers for TiO
2, preventing agglomeration and making a larger specific surface area. Further, they assist the transfer to NPs surface, wherein undesired species are more quickly degraded due to the formation of peroxide anion and hydroxyl radicals [
32,
33,
34]. This cooperative mechanism is expected to contrast the accumulation of foulants onto the membrane surface more efficiently, also preserving the action of the photocatalyst with time.
At pH 2.2, a significant loss of photodegradation efficiency is instead observed with time due to a larger accumulation of foulants onto the surface. Generally, it is found that the photocatalytic activity decreases with increasing reactant concentration, because the active sizes on the catalyst surface are limited [
35,
36]. Under these conditions, the path length of the photon through a thicker layer of pollutants is decreased, thereby causing quicker hole-electron pair recombination and reduced formation of the charge carrier [
37]. The result is a progressive decrease in photodegradation efficiency until a full deactivation of the photocatalyst.
Based on this premise, it is reasonable to assume that positive charge promote the establishment of attractive interactions between feed solution and membrane surface, causing rapid accumulation of foulants and, as a consequence, quicker hole-electron pair recombination. As a result, a lower resistance to fouling can be observed. Instead, negative charge assists TiO2 NPs with a reduced likelihood of energized holes and free electron recombination causing a longer and efficient in-situ cleaning.
3.6. Ionic Charge Switch to Wash Further Membranes
The pH responsiveness of the LBL complex takes the further advantage of removing over residues away from the membrane surface without using additional chemical reagents. At the end of the filtration process, the polymer ionic charge can be, in fact, switched causing swelling-deswelling of the hydrogel (
Figure 9).
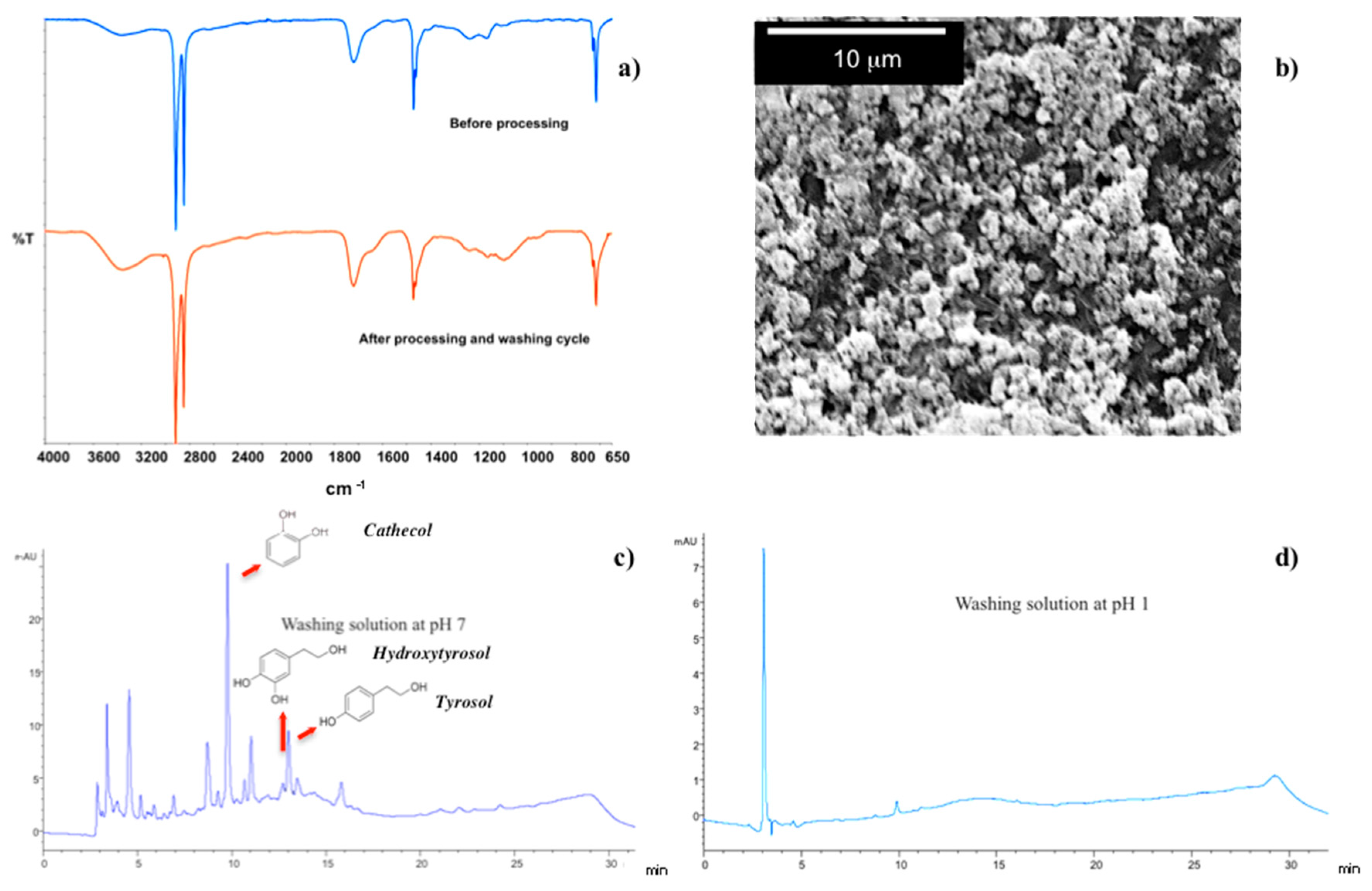
Two successive washing cycles at pH 7 (10 min) and pH 1 (10 s) result enough to clean fully and restore the membrane surface.
ATR spectra collected before processing and after final cleaning steps result indeed to be comparable as well as the SEM micrograph, collected onto membrane surface after washing, confirms the integrity of the LBL complex (
Figure 9a,b). Also, HPLC analyses reveal the presence of removed residues, such as phenols, i.e., catechol, hydroxtyrosol and tyrosol, in washing streams at pH 7 (
Figure 9b), whereas no contaminants are detectable in solution at pH 1 (
Figure 9c).
Once more, the high density of negative charge at pH 7 causes the polymer chains to swell and pull out adsorbed-surface contaminants, thereby facilitating the removal of over foulants from the surface. Successively, the full protonation-taking place in acid environment has ability to get PAA segments in a shrunk state and chemically compactness, resulting in a reversible fouling and water recovery of 99.6%.